赖氨酰氧化酶家族在肝细胞癌发生发展中的作用
DOI: 10.3969/j.issn.1001-5256.2022.03.039
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:毛德文、乐滢玉负责研究选题;曾胜澜、谭利婷参与收集数据,设计论文框架;覃小宾、李祖隆负责修订论文;毛德文、覃小宾负责拟定写作思路,指导撰写文章并最后定稿。
Role of lysyl oxidase family in the development and progression of hepatocellular carcinoma
-
摘要: 赖氨酰氧化酶(LOX)家族是由LOX和LOX样蛋白(LOXL1~4)组成的铜胺氧化酶,在肿瘤组织中过表达,通过细胞外基质的共价交联促进肿瘤转移,具有细胞生长控制、肿瘤抑制、衰老和趋化作用。近年来,越来越多的证据表明LOX家族成员在肝细胞癌(HCC)的发病机理中发挥着关键作用,提示它们作为治疗靶点的巨大潜力。本文就LOX家族成员在HCC发生发展中的作用及中药提取物通过调节LOX家族对HCC的干预作用进行综述,以期为防治HCC的深入研究提供参考。
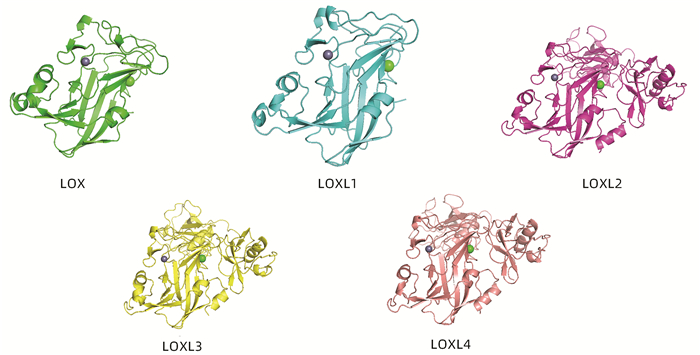
-
关键词:
- 癌, 肝细胞 /
- 蛋白赖氨酸6-氧化酶 /
- 中草药
Abstract: Lysyl oxidase (LOX) family is a group of copper-containing amine oxidases composed of LOX and LOX-like proteins (LOXL1, LOXL2, LOXL3, and LOXL4). It is overexpressed in tumor tissue and promotes tumor metastasis through covalent cross-linking of extracellular matrix, with the functions of cell growth control, tumor inhibition, senescence, and chemotaxis. In recent years, more and more evidence has shown that LOX family members play a key role in the pathogenesis of hepatocellular carcinoma (HCC), suggesting that they have great potential as therapeutic targets. This article reviews the role of LOX family members in the development and progression of HCC and the intervention effect of traditional Chinese medicine extracts on HCC by regulating LOX family, in order to provide a reference for further research on the prevention and treatment of HCC. -
表 1 LOX家族成员在HCC中的促进作用
作用 成员 机制 参考文献 促进血管生成 LOX 通过p38MAPK信号上调VEGF; [13] 通过整合素αV/β5/Akt-核Sp1通路上调VEGFR2 [15-16] LOXL1 增强FAK血管内皮细胞内的MAPK信号通路 [17] LOXL2 通过Snail信号上调VEGF;沉积Ⅳ型胶原,协调血管基底膜的支架 [18-19] LOXL4 经外泌体的介导转移到亲代HCC细胞和内皮细胞上,促进血管生成 [20] 促进EMT LOX 在HIF-1α介导下降低E-钙黏蛋白,增加波形蛋白、Slug蛋白以及TWIST蛋白 [23] LOXL2 以Snail依赖方式降低E-钙黏蛋白;
与E-钙黏蛋白启动子结合,激活FAK/Src信号[24-25] 重塑肿瘤微环境 LOX 诱导Ⅳ型胶原线性重排,重塑ECM使机械应力增加 [29] LOXL2 活化成纤维细胞,调节细胞骨架重组,募集骨髓来源细胞归巢到转移部位;增强M2巨噬细胞的极化,导致免疫抑制 [30-32] LOXL4 激活PD-L1的表达,诱导巨噬细胞免疫抑制; [33] 激活FAK/Src通路,增强ECM硬度; [20] 通过Smad依赖和JunB/Fra2途径参与血管基质重塑 [34] -
[1] Professional Committee for Prevention and Control of Hepatobiliary and Pancreatic Diseases of Chinese Preventive Medicine Association; Professional Committee for Hepatology, Chinese Research Hospital Association; Chinese Society of Hepatology, Chinese Medical Association, et al. Guideline for stratified screening and surveillance of primary liver cancer (2020 edition)[J]. J Clin Hepatol, 2021, 37(2): 286-295. DOI: 10.3969/j.issn.1001-5256.2021.02.009.中华预防医学会肝胆胰疾病预防与控制专业委员会, 中国研究型医院学会肝病专业委员会, 中华医学会肝病学分会, 等. 原发性肝癌的分层筛查与监测指南(2020版)[J]. 临床肝胆病杂志, 2021, 37(2): 286-295. DOI: 10.3969/j.issn.1001-5256.2021.02.009. [2] VANNUCCI L. Stroma as an active player in the development of the tumor microenvironment[J]. Cancer Microenviron, 2015, 8(3): 159-166. DOI: 10.1007/s12307-014-0150-x. [3] PINNELL SR, MARTIN GR. The cross-linking of collagen and elastin: Enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone[J]. Proc Natl Acad Sci U S A, 1968, 61(2): 708-716. DOI: 10.1073/pnas.61.2.708. [4] TENTI P, VANNUCCI L. Lysyl oxidases: Linking structures and immunity in the tumor microenvironment[J]. Cancer Immunol Immunother, 2020, 69(2): 223-235. DOI: 10.1007/s00262-019-02404-x. [5] JUNG ST, KIM MS, SEO JY, et al. Purification of enzymatically active human lysyl oxidase and lysyl oxidase-like protein from Escherichia coli inclusion bodies[J]. Protein Expr Purif, 2003, 31(2): 240-246. DOI: 10.1016/s1046-5928(03)00217-1. [6] GRIMSBY JL, LUCERO HA, TRACKMAN PC, et al. Role of lysyl oxidase propeptide in secretion and enzyme activity[J]. J Cell Biochem, 2010, 111(5): 1231-1243. DOI: 10.1002/jcb.22845. [7] MOLNAR J, FONG KS, HE QP, et al. Structural and functional diversity of lysyl oxidase and the LOX-like proteins[J]. Biochim Biophys Acta, 2003, 1647(1-2): 220-224. DOI: 10.1016/s1570-9639(03)00053-0. [8] WATERHOUSE A, BERTONI M, BIENERT S, et al. SWISS-MODEL: Homology modelling of protein structures and complexes[J]. Nucleic Acids Res, 2018, 46(W1): w296-w303. DOI: 10.1093/nar/gky427. [9] BIENERT S, WATERHOUSE A, de BEER TA, et al. The SWISS-MODEL Repository-new features and functionality[J]. Nucleic Acids Res, 2017, 45(D1): d313-d319. DOI: 10.1093/nar/gkw1132. [10] GUEX N, PEITSCH MC, SCHWEDE T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective[J]. Electrophoresis, 2009, 30 Suppl 1: s162-s173. DOI: 10.1002/elps.200900140. [11] STUDER G, REMPFER C, WATERHOUSE AM, et al. QMEANDisCo-distance constraints applied on model quality estimation[J]. Bioinformatics, 2020, 36(6): 1765-1771. DOI: 10.1093/bioinformatics/btz828. [12] BERTONI M, KIEFER F, BIASINI M, et al. Modeling protein quaternary structure of homo-and hetero-oligomers beyond binary interactions by homology[J]. Sci Rep, 2017, 7(1): 10480. DOI: 10.1038/s41598-017-09654-8. [13] ZHU J, HUANG S, WU G, et al. Lysyl oxidase is predictive of unfavorable outcomes and essential for regulation of vascular endothelial growth factor in hepatocellular carcinoma[J]. Dig Dis Sci, 2015, 60(10): 3019-3031. DOI: 10.1007/s10620-015-3734-5. [14] SALGADO R, BENOY I, BOGERS J, et al. Platelets and vascular endothelial growth factor (VEGF): A morphological and functional study[J]. Angiogenesis, 2001, 4(1): 37-43. DOI: 10.1023/a:1016611230747. [15] DONG Y, XIE X, WANG Z, et al. Increasing matrix stiffness upregulates vascular endothelial growth factor expression in hepatocellular carcinoma cells mediated by integrin β1[J]. Biochem Biophys Res Commun, 2014, 444(3): 427-432. DOI: 10.1016/j.bbrc.2014.01.079. [16] WANG Y, ZHANG X, WANG W, et al. Integrin αVβ5/Akt/Sp1 pathway participates in matrix stiffness-mediated effects on VEGFR2 upregulation in vascular endothelial cells[J]. Am J Cancer Res, 2020, 10(8): 2635-2648. [17] YUAN R, LI Y, YANG B, et al. LOXL1 exerts oncogenesis and stimulates angiogenesis through the LOXL1-FBLN5/αvβ3 integrin/FAK-MAPK axis in ICC[J]. Mol Ther Nucleic Acids, 2021, 23: 797-810. DOI: 10.1016/j.omtn.2021.01.001. [18] FAN Z, ZHENG W, LI H, et al. LOXL2 upregulates hypoxia-inducible factor-1α signaling through Snail-FBP1 axis in hepatocellular carcinoma cells[J]. Oncol Rep, 2020, 43(5): 1641-1649. DOI: 10.3892/or.2020.7541. [19] UMANA-DIAZ C, PICHOL-THIEVEND C, MARCHAND MF, et al. Scavenger Receptor Cysteine-Rich domains of Lysyl Oxidase-Like2 regulate endothelial ECM and angiogenesis through non-catalytic scaffolding mechanisms[J]. Matrix Biol, 2020, 88: 33-52. DOI: 10.1016/j.matbio.2019.11.003. [20] LI R, WANG Y, ZHANG X, et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis[J]. Mol Cancer, 2019, 18(1): 18. DOI: 10.1186/s12943-019-0948-8. [21] YAN L, XU F, DAI CL. Relationship between epithelial-to-mesenchymal transition and the inflammatory microenvironment of hepatocellular carcinoma[J]. J Exp Clin Cancer Res, 2018, 37(1): 203. DOI: 10.1186/s13046-018-0887-z. [22] HAPKE RY, HAAKE SM. Hypoxia-induced epithelial to mesenchymal transition in cancer[J]. Cancer Lett, 2020, 487: 10-20. DOI: 10.1016/j.canlet.2020.05.012. [23] UMEZAKI N, NAKAGAWA S, YAMASHITA YI, et al. Lysyl oxidase induces epithelial-mesenchymal transition and predicts intrahepatic metastasis of hepatocellular carcinoma[J]. Cancer Sci, 2019, 110(6): 2033-2043. DOI: 10.1111/cas.14010. [24] WANG M, ZHAO X, ZHU D, et al. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment[J]. J Exp Clin Cancer Res, 2017, 36(1): 60. DOI: 10.1186/s13046-017-0533-1. [25] CUEVAS EP, MORENO-BUENO G, CANESIN G, et al. LOXL2 catalytically inactive mutants mediate epithelial-to-mesenchymal transition[J]. Biol Open, 2014, 3(2): 129-137. DOI: 10.1242/bio.20146841. [26] HU Q, MASUDA T, KURAMITSU S, et al. Potential association of LOXL1 with peritoneal dissemination in gastric cancer possibly via promotion of EMT[J]. PLoS One, 2020, 15(10): e0241140. DOI: 10.1371/journal.pone.0241140. [27] LAURENTINO TS, SOARES R, MARIE S, et al. LOXL3 function beyond amino oxidase and role in pathologies, including cancer[J]. Int J Mol Sci, 2019, 20(14): 3587. DOI: 10.3390/ijms20143587. [28] WU Y, GE G. Complexity of type Ⅳ collagens: From network assembly to function[J]. Biol Chem, 2019, 400(5): 565-574. DOI: 10.1515/hsz-2018-0317. [29] FANG M, PENG CW, YUAN JP, et al. Coevolution of the tumor microenvironment revealed by quantum dot-based multiplexed imaging of hepatocellular carcinoma[J]. Future Oncol, 2013, 9(7): 1029-1037. DOI: 10.2217/fon.13.63. [30] WONG CC, TSE AP, HUANG YP, et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma[J]. Hepatology, 2014, 60(5): 1645-1658. DOI: 10.1002/hep.27320. [31] BARKER HE, BIRD D, LANG G, et al. Tumor-secreted LOXL2 activates fibroblasts through FAK signaling[J]. Mol Cancer Res, 2013, 11(11): 1425-1436. DOI: 10.1158/1541-7786.MCR-13-0033-T. [32] XING X, WANG Y, ZHANG X, et al. Matrix stiffness-mediated effects on macrophages polarization and their LOXL2 expression[J]. FEBS J, 2021, 288(11): 3465-3477. DOI: 10.1111/febs.15566. [33] TAN HY, WANG N, ZHANG C, et al. Lysyl oxidase-like 4 fosters an immunosuppressive microenvironment during hepatocarcinogenesis[J]. Hepatology, 2021, 73(6): 2326-2341. DOI: 10.1002/hep.31600. [34] BUSNADIEGO O, GONZÁLEZ-SANTAMARÍA J, LAGARES D, et al. LOXL4 is induced by transforming growth factor β1 through Smad and JunB/Fra2 and contributes to vascular matrix remodeling[J]. Mol Cell Biol, 2013, 33(12): 2388-2401. DOI: 10.1128/MCB.00036-13. [35] CONTENTE S, KENYON K, SRIRAMAN P, et al. Epigenetic inhibition of lysyl oxidase transcription after transformation by ras oncogene[J]. Mol Cell Biochem, 1999, 194(1-2): 79-91. DOI: 10.1023/a:1006913122261. [36] CSISZAR K, FONG SF, UJFALUSI A, et al. Somatic mutations of the lysyl oxidase gene on chromosome 5q23.1 in colorectal tumors[J]. Int J Cancer, 2002, 97(5): 636-642. DOI: 10.1002/ijc.10035. [37] KANEDA A, WAKAZONO K, TSUKAMOTO T, et al. Lysyl oxidase is a tumor suppressor gene inactivated by methylation and loss of heterozygosity in human gastric cancers[J]. Cancer Res, 2004, 64(18): 6410-6415. DOI: 10.1158/0008-5472.CAN-04-1543. [38] NILSSON M, ADAMO H, BERGH A, et al. Inhibition of lysyl oxidase and lysyl oxidase-like enzymes has tumour-promoting and tumour-suppressing roles in experimental prostate cancer[J]. Sci Rep, 2016, 6: 19608. DOI: 10.1038/srep19608. [39] ZHENG Y, WANG X, WANG H, et al. Expression of the lysyl oxidase propeptide in hepatocellular carcinoma and its clinical relevance[J]. Oncol Rep, 2014, 31(4): 1669-1676. DOI: 10.3892/or.2014.3044. [40] NARESHKUMAR RN, SULOCHANA KN, CORAL K. Inhibition of angiogenesis in endothelial cells by Human Lysyl oxidase propeptide[J]. Sci Rep, 2018, 8(1): 10426. DOI: 10.1038/s41598-018-28745-8. [41] WU G, GUO Z, CHANG X, et al. LOXL1 and LOXL4 are epigenetically silenced and can inhibit ras/extracellular signal-regulated kinase signaling pathway in human bladder cancer[J]. Cancer Res, 2007, 67(9): 4123-4129. DOI: 10.1158/0008-5472.CAN-07-0012. [42] CHOI SK, KIM HS, JIN T, et al. LOXL4 knockdown enhances tumor growth and lung metastasis through collagen-dependent extracellular matrix changes in triple-negative breast cancer[J]. Oncotarget, 2017, 8(7): 11977-11989. DOI: 10.18632/oncotarget.14450. [43] JOERGER AC, FERSHT AR. The p53 pathway: Origins, inactivation in cancer, and emerging therapeutic approaches[J]. Annu Rev Biochem, 2016, 85: 375-404. DOI: 10.1146/annurev-biochem-060815-014710. [44] SHAO J, LU J, ZHU W, et al. Derepression of LOXL4 inhibits liver cancer growth by reactivating compromised p53[J]. Cell Death Differ, 2019, 26(11): 2237-2252. DOI: 10.1038/s41418-019-0293-x. [45] BIE B, SUN J, GUO Y, et al. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma[J]. Biomed Pharmacother, 2017, 93: 1285-1291. DOI: 10.1016/j.biopha.2017.07.068. [46] NGUYEN LT, SONG YW, CHO SK. Baicalein inhibits epithelial to mesenchymal transition via downregulation of Cyr61 and LOXL-2 in MDA-MB231 breast cancer cells[J]. Mol Cells, 2016, 39(12): 909-914. DOI: 10.14348/molcells.2016.0243. [47] LI X, LI J, WANG L, et al. The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1α accumulation and fibrosis in hypoxic adipose tissue[J]. Br J Pharmacol, 2016, 173(12): 2001-2015. DOI: 10.1111/bph.13493. [48] MOHSENI R, ARAB SADEGHABADI Z, GOODARZI MT, et al. Co-administration of resveratrol and beta-aminopropionitrile attenuates liver fibrosis development via targeting lysyl oxidase in CCl4-induced liver fibrosis in rats[J]. Immunopharmacol Immunotoxicol, 2019, 41(6): 644-651. DOI: 10.1080/08923973.2019.1688829. [49] LU L, LIU S, DONG Q, et al. Salidroside suppresses the metastasis of hepatocellular carcinoma cells by inhibiting the activation of the Notch1 signaling pathway[J]. Mol Med Rep, 2019, 19(6): 4964-4972. DOI: 10.3892/mmr.2019.10115. [50] QIN Y, LIU HJ, LI M, et al. Salidroside improves the hypoxic tumor microenvironment and reverses the drug resistance of platinum drugs via HIF-1α signaling pathway[J]. EBioMedicine, 2018, 38: 25-36. DOI: 10.1016/j.ebiom.2018.10.069. [51] HU Q, YOU SP, LIU T, et al. An investigation on the anti-liver cancer effect of cistanche[J]. Carcinog Teratogenesis Mutagen, 2018, 30(3): 194-199. DOI: 10.3969/j.issn.1004-616x.2018.03.006.胡琼, 由淑萍, 刘涛, 等. 肉苁蓉苯乙醇总苷抗肝癌作用的实验研究[J]. 癌变·畸变·突变, 2018, 30(3): 194-199. DOI: 10.3969/j.issn.1004-616x.2018.03.006. [52] WEN L, HU J, ZHANG J, et al. Phenylethanol glycosides from Herba Cistanche improve the hypoxic tumor microenvironment and enhance the effects of oxaliplatin via the HIF-1α signaling pathway[J]. Mol Med Rep, 2021, 24(1): 517. DOI: 10.3892/mmr.2021.12156. -



 PDF下载 ( 3314 KB)
PDF下载 ( 3314 KB)

 下载:
下载: