6-姜酮酚抑制肝内胆管癌细胞的增殖、迁移、侵袭作用及其机制探讨
DOI: 10.3969/j.issn.1001-5256.2022.04.022
Inhibitory effect of 6-paradol on the proliferation, migration, and invasion of intrahepatic cholangiocarcinoma cells and its mechanism
-
摘要:
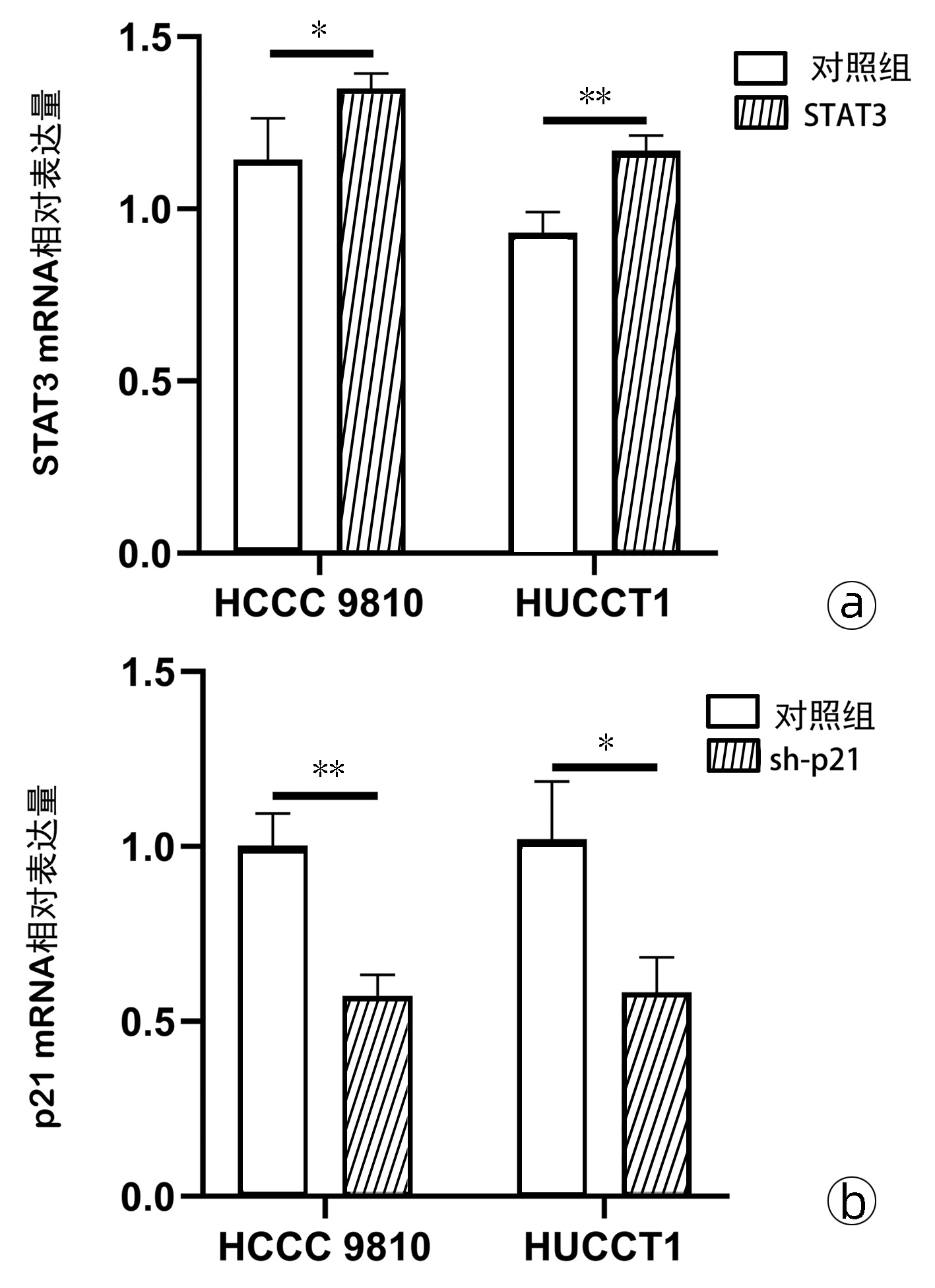
目的 研究6-姜酮酚(6-Paradol)对人肝内胆管癌细胞增殖、迁移、侵袭的作用及机制。 方法 人肝内胆管癌细胞系HCCC 9810和HUCCT1以不同浓度的6-Paradol及等体积的DMSO(对照组)处理后,采用CCK-8、平板克隆、划痕愈合及Transwell实验检测其增殖、迁移及侵袭能力。应用生物信息学软件Swiss Target Prediction预测6-Paradol作用的蛋白靶点,采用Western blot检测胆管癌细胞中STAT3、p-STAT3、SRC、p-mTOR、p21、Bcl-2以及p53的蛋白表达水平;采用药物亲和反应实验(DARTS)探究6-Paradol与STAT3的相互作用;胆管癌HCCC 9810和HUCCT1细胞分别转染STAT3过表达质粒或sh-p21质粒后,qRT-PCR检测各组细胞中STAT3和p21的mRNA水平,Western blot检测STAT3和p21的蛋白表达量;CCK-8、划痕愈合及Transwell实验检测各组细胞增殖及迁移侵袭能力。计量资料两组间比较采用t检验;多组间比较采用方差分析,进一步两两比较采用LSD-t检验。 结果 与对照组相比,6-Paradol处理组中各组细胞的增殖、迁移、侵袭能力明显减弱(P值均<0.05)。6-Paradol处理组细胞中STAT3、p-STAT3的表达水平较对照组显著下调(P值均<0.05),p21的表达水平显著增加(P值均<0.05),而Bcl-2、SRC和p-mTOR的表达水平无明显变化(P值均>0.05)。6-Paradol处理组中STAT3被蛋白酶水解的比例分别减少了48.66%、45.33%(t值分别为16.64、8.76, P值均<0.05);分别转染STAT3过表达质粒及沉默p21质粒后,胆管癌细胞中STAT3 mRNA水平均显著增加(tHCCC 9810=2.82,tHUCCT1=5.60,P值均<0.05),p21 mRNA水平均显著降低(tHCCC 9810=6.84,tHUCCT1=3.91, P值均<0.05)。CCK-8结果提示6-Paradol作用48 h及72 h后HCCC 9810细胞和HUCCT1细胞中STAT3过表达组的增殖率较单独给药组显著提高,p21沉默组增殖率较单独给药组也明显提高(P值均<0.05)。划痕愈合实验结果提示在过表达STAT3或沉默p21的HCCC 9810及HUCCT1细胞中,与单独给药组相比划痕愈合率明显提升(P值均<0.05)。Transwell实验结果表明,在过表达STAT3或者沉默p21表达的HCCC 9810及HUCCT1细胞中,与单独给药组相比迁移率均明显提升(P值均<0.05);在过表达STAT3或沉默p21表达的HCCC 9810及HUCCT1细胞中,与单独给药组相比侵袭率均显著提升(P值均<0.05)。 结论 6-Paradol通过靶向作用于STAT3-p21通路抑制胆管癌细胞的增殖、迁移和侵袭。 Abstract:Objective To investigate the effect of 6-paradol on the proliferation, migration, and invasion of human intrahepatic cholangiocarcinoma cells and its mechanism. Methods Human intrahepatic cholangiocarcinoma cell lines HCCC 9810 and HUCCT1 were treated with different concentrations of 6-paradol or an equal volume of DMSO (control group), and then CCK-8 assay, plate colony formation assay, wound healing assay, and Transwell assay were used to measure cell proliferation, migration, and invasion. The bioinformatics software Swiss Target Prediction was used to predict the protein targets of 6-paradol, and Western blot was used to measure the protein expression levels of STAT3, p-STAT3, SRC, p-mTOR, p21, Bcl-2, and p53; Drug Affinity Responsive Target Stability (DARTS) assay was used to investigate the interaction between 6-paradol and STAT3. After cholangiocarcinoma HCCC 9810 and HUCCT1 cells were transfected with STAT3 overexpression plasmid or sh-p21 plasmid, quantitative real-time PCR was used to measure the mRNA expression levels of STAT3 and p21, and Western blot was used to measure the protein expression levels of STAT3 and p21; CCK-8 assay, wound healing assay, and Transwell assay were used to measure cell proliferation, migration, and invasion. The t-test was used for comparison of data between two groups; an analysis of variance was used for comparison between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the control group, the 6-paradol treatment groups had significant reductions in cell proliferation, migration, and invasion (P < 0.05). Compared with the control group, the 6-paradol treatment groups had significant reductions in the expression levels of STAT3 and p-STAT3 (all P < 0.05) and a significant increase in the expression level of p21 (all P < 0.05), while there were no significant changes in the expression levels of Bcl-2, SRC, and p-mTOR (all P > 0.05). In the 6-paradol treatment groups, the proportion of STAT3 hydrolyzed by protease was reduced by 48.66% and 45.33%, respectively (t=16.64 and 8.76, both P < 0.05); after transfection with STAT3 overexpression plasmid or p21-silencing plasmid in cholangiocarcinoma cells, there was a significant increase in the mRNA expression level of STAT3 (tHCCC 9810=2.82, tHUCCT1=5.60, both P < 0.05) and a significant reduction in the mRNA expression level of p21 (tHCCC 9810=6.84, tHUCCT1=3.91, both P < 0.05). CCK-8 assay showed that for HCCC 9810 and HUCCT1 cells treated with 6-paradol for 48 and 72 hours, the STAT3 overexpression group had a significantly higher proliferation rate than the single administration group, and the p21 silencing group also had a significantly higher proliferation rate than the single administration group (P < 0.05). The wound healing assay showed that the HCCC 9810 and HUCCT1 cells with STAT3 overexpression or p21 silencing had a significantly higher wound healing rate than the single administration group (all P < 0.05). Transwell assay showed that the HCCC 9810 and HUCCT1 cells with STAT3 overexpression or p21 silencing had significant increases in migration rate and invasion rate compared with the single administration group (all P < 0.05). Conclusion 6-Paradol inhibits the proliferation, migration, and invasion of cholangiocarcinoma cells by targeting the STAT3-p21 pathway. -
注:a, 6-Paradol处理后HCCC 9810细胞中STAT3、p-mTOR蛋白表达量;b, 6-Paradol处理后HUCCT1细胞中STAT3、p-mTOR蛋白表达量;c, 6-Paradol处理后HCCC 9810细胞中p-STAT3、SRC蛋白表达量;d, 6-Paradol处理后HUCCT1细胞中p-STAT3、SRC蛋白表达量;e, 6-Paradol处理后HCCC 9810细胞中p21、Bcl-2、p53蛋白表达量;f, 6-Paradol处理后HUCCT1细胞中p21、Bcl-2、p53蛋白表达量。
图 4 Western blot检测不同浓度的6-Paradol对人HCCC 9810和HUCCT1细胞中相关蛋白表达的影响
Figure 4. Western blot was used to detect the effect of different concentrations of 6-Paradol on the expression of related proteins in HCCC 9810 and HUCCT1 cells
-
[1] RAZUMILAVA N, GORES GJ. Cholangiocarcinoma[J]. Lancet, 2014, 383(9935): 2168-2179. DOI: 10.1016/s0140-6736(13)61903-0. [2] MOEINI A, SIA D, BARDEESY N, et al. Molecular pathogenesis and targeted therapies for intrahepatic cholangiocarcinoma[J]. Clin Cancer Res, 2016, 22(2): 291-300. DOI: 10.1158/1078-0432.CCR-14-3296. [3] ANDERSEN JB. Molecular pathogenesis of intrahepatic cholangiocarcinoma[J]. J Hepatobiliary Pancreat Sci, 2015, 22(2): 101-113. DOI: 10.1002/jhbp.155. [4] KIM HJ, KIM IS, REHMAN SU, et al. Effects of 6-paradol, an unsaturated ketone from gingers, on cytochrome P450-mediated drug metabolism[J]. Bioorg Med Chem Lett, 2017, 27(8): 1826-1830. DOI: 10.1016/j.bmcl.2017.02.047. [5] SURH YJ, PARK KK, CHUN KS, et al. Anti-tumor-promoting activities of selected pungent phenolic substances present in ginger[J]. J Environ Pathol Toxicol Oncol, 1999, 18(2): 131-139. [6] MARIADOSS AV, KATHIRESAN S, MUTHUSAMY R, et al. Protective effects of[6]-paradol on histological lesions and immunohistochemical gene expression in DMBA induced hamster buccal pouch carcinogenesis[J]. Asian Pac J Cancer Prev, 2013, 14(5): 3123-3129. DOI: 10.7314/apjcp.2013.14.5.3123. [7] HUYNH J, CHAND A, GOUGH D, et al. Therapeutically exploiting STAT3 activity in cancer - using tissue repair as a road map[J]. Nat Rev Cancer, 2019, 19(2): 82-96. DOI: 10.1038/s41568-018-0090-8. [8] HARPER JW, ADAMI GR, WEI N, et al. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases[J]. Cell, 1993, 75(4): 805-816. DOI: 10.1016/0092-8674(93)90499-g. [9] LECHNER JF, STONER GD. Gingers and their purified components as cancer chemopreventative agents[J]. Molecules, 2019, 24(16): 2859. DOI: 10.3390/molecules24162859. [10] LI Q, WANG R, WANG LP, et al. Paradol inhibits proliferation and migration of human hepatocellular carcinoma cells[J]. Sci Adv Mater, 2019, 11(10): 1467-1473. DOI: 10.1166/sam.2019.3555. [11] YU H, JOVE R. The STATs of cancer-new molecular targets come of age[J]. Nat Rev Cancer, 2004, 4(2): 97-105. DOI: 10.1038/nrc1275. [12] HE G, KARIN M. NF-κB and STAT3 - key players in liver inflammation and cancer[J]. Cell Res, 2011, 21(1): 159-168. DOI: 10.1038/cr.2010.183. [13] FUKUDA A, WANG SC, MORRIS JPT, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression[J]. Cancer Cell, 2011, 19(4): 441-455. DOI: 10.1016/j.ccr.2011.03.002. [14] SAINI U, NAIDU S, ELNAGGAR AC, et al. Elevated STAT3 expression in ovarian cancer ascites promotes invasion and metastasis: A potential therapeutic target[J]. Oncogene, 2017, 36(2): 168-181. DOI: 10.1038/onc.2016.197. [15] GARTEL AL, RADHAKRISHNAN SK. Lost in transcription: p21 repression, mechanisms, and consequences[J]. Cancer Res, 2005, 65(10): 3980-3985. DOI: 10.1158/0008-5472.CAN-04-3995. [16] SHAMLOO B, USLUER S. p21 in cancer research[J]. Cancers (Basel), 2019, 11(8): 1178. DOI: 10.3390/cancers11081178. [17] GEORGAKILAS AG, MARTIN OA, BONNER WM. p21: A two- faced genome guardian[J]. Trends Mol Med, 2017, 23(4): 310-319. DOI: 10.1016/j.molmed.2017.02.001. 期刊类型引用(10)
1. 杭霞瑜,李益坤,胡俊,曹若琪,张琰,徐楠,宋宪强,孙向东. 不同铅门宽度对肝细胞癌立体定向放射治疗的剂量学影响研究. 医疗卫生装备. 2024(07): 51-55 .  百度学术
百度学术2. 颛瑞娟,闫九. 射波刀联合替吉奥治疗原发性肝癌疗效观察. 黑龙江医药科学. 2021(01): 53-54+56 .  百度学术
百度学术3. 郭学玲,贺晓东,赵宪芝,贾臻,代智涛,居小萍,刘永明,张火俊. 射波刀治疗肝癌的放射治疗计划参数的质量控制研究. 肿瘤. 2021(08): 559-564 .  百度学术
百度学术4. 王俊东,刘长珠,冯清华,张志平,欧志涛. 复发性肝癌诊疗中超声的运用价值分析. 现代医用影像学. 2020(06): 998-1000 .  百度学术
百度学术5. 赵毅峰,杨生虎,张萍. 射波刀与经肝动脉化疗栓塞治疗原发性肝癌. 肝胆外科杂志. 2019(01): 50-53 .  百度学术
百度学术6. 沈雅平,任育昕,杨熙园,张涛,周政,吕哲宇,赵立新. 肝癌介入治疗的远期疗效和影响因素分析. 临床医药文献电子杂志. 2019(63): 20 .  百度学术
百度学术7. 蔡秀军,金仁安. 复发性肝癌治疗方式合理选择. 中国实用外科杂志. 2019(10): 1015-1020 .  百度学术
百度学术8. 秦建民. 肝细胞癌切除术后复发的原因与防治策略. 世界华人消化杂志. 2019(23): 1407-1418 .  百度学术
百度学术9. 李甜甜,孙静,王权,丁俊强,王佳,薛慧,张弢,段学章. 小肝癌立体定向放射治疗的效果及预后分析. 临床肝胆病杂志. 2018(08): 1702-1706 .  本站查看
本站查看10. 刘城林,梁昆,于礼建. 复发性肝癌治疗的研究进展与展望. 世界最新医学信息文摘. 2018(84): 23-27 .  百度学术
百度学术其他类型引用(4)
-




 PDF下载 ( 9970 KB)
PDF下载 ( 9970 KB)


 下载:
下载:





 百度学术
百度学术

