HBsAg水平对ALT正常的HBeAg阳性慢性HBV感染者肝脏炎症发生的预测价值
DOI: 10.3969/j.issn.1001-5256.2022.05.011
Value of HBsAg level in predicting liver inflammation in patients with HBeAg-positive chronic hepatitis B virus infection and normal alanine aminotransferase
-
摘要:
目的 探讨ALT正常且高病毒载量的HBeAg阳性慢性HBV感染者人群的肝炎发生情况及预测因素。 方法 本研究临床注册号为NCT04032275。回顾性选取2008年10月—2015年5月ALT正常、高病毒载量、HBeAg阳性的慢性HBV感染者183例,根据其肝穿刺活检结果分为肝炎组和无肝炎组。计量资料两组间比较采用t检验或Mann-Whitney U检验。计数资料两组间比较采用χ2检验。使用单因素二元logistics回归分析预测因素,使用逐步后退法进行多因素二元logistics回归,使用受试者工作特征曲线(ROC)和约登指数分析截断值。 结果 肝炎组37例(20.2%),无肝炎组146例(79.8%),肝炎组的男性比例低于无肝炎组(45.9% vs 68.5%, χ2=6.508, P=0.011)、AST高于无肝炎组[24(21.25~35.55) U/L vs 21.2(18.08~24.65) U/L, Z=-3.344, P=0.001]、HBsAg log值低于无肝炎组[4.4(4.28~4.49) vs 4.46(4.4~4.74), Z=-2.184,P=0.029]。HBsAg log值是肝炎发生的预测因素(OR=0.077, P=0.017)。HBsAg的截断值为33 884.4 IU/mL。 结论 ALT正常、高病毒载量的HBeAg阳性患者中有20.2%肝脏存在炎症,HBsAg或许是肝脏炎症发生的预测因素。 Abstract:Objective To investigate the onset of liver inflammation and related predictive factors in patients with HBeAg-positive chronic hepatitis B virus (HBV) infection who have normal alanine aminotransferase (ALT) and a high viral load. Methods A retrospective analysis was performed for the clinical data of 183 patients with HBeAg-positive chronic HBV infection who had normal ALT and a high viral load and were treated from October 2008 to May 2015, and according to the results of liver biopsy, they were divided into hepatitis group and non- hepatitis group. The t-test or Mann-Whitney U testwas used for comparison of normally distributed continuous data between groups, the chi-square test was used for comparison of categorical data. The predictive factors were analyzed by univariate binary logistic regression, the multivariate binary logistic regression was carried out by stepback method, and the cut-off values were analyzed by receiver operating characteristic curve (ROC) and Jordan index. Results There were 37 patients (20.2%) in the hepatitis group and 146 patients (79.8%) in the non-hepatitis group. Compared with the non-hepatitis group, the hepatitis group had a significantly lower proportion of male patients (45.9% vs 68.5%, χ2=6.508, P=0.011), a significantly higher level of aspartate aminotransferase [24 (21.25~35.55) U/L vs 21.2 (18.08~ 24.65) U/L, Z=-3.344, P=0.001], and a significantly lower log(HBsAg) value [4.4(4.28~4.49) vs 4.46(4.4~4.74), Z=-2.184, P=0.029]. Log(HBsAg) value was a predictive factor for hepatitis (odds ratio=0.077, P=0.017), and the cutoff value of HBsAg was 33884.4I U/mL. Conclusion Among the patients with HBeAg-positive chronic HBV infection who have normal ALT and a high viral load, 20.2% have liver inflammation, and HBsAg may be a predictive factor for liver inflammation. -
据2018年全球癌症统计数据[1]显示,肝细胞癌(HCC)发病率在全球最常见的癌症中排名第六,在与癌症相关的死亡率中居第四位。全世界每年诊断出超过70万例HCC新病例,并且其发病率和死亡率仍在逐年上升[2-3]。尽管外科手术被认为是目前HCC最有效的治疗手段,但是大多数HCC患者在确诊时已处于中晚期,此时手术的治疗效果有限,且此类患者常常伴有较差的肝功能、肝内转移以及血管侵犯,已失去手术机会。经肝动脉化疗栓塞术(TACE)被认为是有效介入的治疗方法,可明显提高患者的生存率[4]。目前国内普遍将肝功能为Child-Pugh A级或B级,PS评分为0~2分的Ⅱb、Ⅲa和部分Ⅲb期HCC作为TACE的首选适应证[5]。众所周知,HCC患者的预后主要根据肝功能、肿瘤负荷及患者状态等多种因素进行评估。通常认为,当TACE用于无法切除的HCC患者时,更好的肝功能与良好的预后有关。肝功能主要由CTP等级定义,而国外的巴塞罗那临床肝癌分期(BCLC)系统经常被用作HCC治疗指南[6]。数十年来,CTP分级已被广泛用于评估肝功能障碍的严重程度,然而一些指标(如白蛋白和腹水)是相互关联的,而其他指标(如腹水和肝性脑病)的判定存在主观性[7]。2015年,Johnson等[8]引入了一种评估肝功能的新工具,即白蛋白-胆红素(ALBI)分级,并证明了ALBI也是预测HCC患者长期生存的预后因素。随着研究[9-11]的不断深入,ALBI已被证实与HCC患者的预后密切相关,然而目前ALBI在预测TACE治疗HCC预后方面的可用发表结果仍然有限,关于ALBI对于TACE术后预后的临床价值仍未达成共识。同时,许多失去手术机会的HCC患者在进一步治疗前都经历了重复TACE治疗,但很少有报道评估重复TACE治疗对这类患者肝功能的影响。因此,本研究旨在通过Meta分析探讨ALBI对TACE术后预后及慢加急性肝衰竭(ACLF)发生风险的评估价值, 并阐明重复TACE治疗对ALBI肝功能的影响,以期为今后的研究及临床治疗提供循证医学依据。
1. 资料与方法
1.1 检索策略
计算机检索PubMed、The Cochrane Library、EMbase、Web of Science、OVID、知网、万方、维普及中国生物医学文献服务系统数据库(CBM),收集所有关于ALBI分级对TACE术后患者肝功能变化及其预后评估的相关性研究。检索时间为数据库建立至2020年12月。英文检索词:ALBI,albumin-bilirubin,hepatocellular carcinoma,TACE,transarterial chemoembolization,hepatic function,prognostic;中文检索词:ALBI、白蛋白-胆红素、肝细胞癌、经肝动脉化疗栓塞术、肝功能、预后。必要时追溯纳入文献的参考文献。
1.2 纳入与排除标准
纳入标准:(1)研究对象按照《原发性肝癌诊疗规范(2019年版)》[5]的临床诊断及病理诊断标准确诊为HCC;(2)报道了ALBI对HCC患者TACE术后总体生存的评估价值;(3)生存结果通过HR及其95%CI或Kaplan-Meier曲线衡量;(4)报道了ALBI与TACE术后发生ACLF相关的OR及其95%CI;(5)报道了TACE治疗前患者肝功能以及1次、2次或多次TACE累积导致的ALBI肝功能等级变化;(6)重复TACE后肝功能恶化定义为ALBI 1级恶化为2级和(或)2级恶化为3级。排除标准:重复文献、综述、信函、社论、会议摘要、述评或无足够数据可供分析的文献。
1.3 文献筛选及资料提取
由两位研究者按照纳入及排除标准独立进行文献筛选,无法通过标题及摘要明确纳入或排除的,进行全文浏览,核对各自筛选后文献,若遇分歧,采取第三方协助或讨论形式解决。采用标准化的数据提取表从每个研究中提取数据,从每篇文章中提取以下信息:第一作者、发表年份、研究合作、国家、年龄、性别、样本量、肝病病因、ALBI等级、结局指标、临床病理特征、HR、OR及其95%CI、随访时间。在这项研究中,首选多变量分析的HR或OR,因为它们比单变量分析更可靠,其次是从Kaplan-Meier曲线中提取的HR或通过相关数据估算的OR。
1.4 文献质量评价
采用《系统评价中预后研究质量的评估》[12]提出的“基于潜在偏倚框架的预后研究质量评估指南”进行评价。标准包括:(1)研究样本能代表被关注人群的关键特征,足以限制结果的潜在风险;(2)失访(从样本到研究人群)未影响关键特征(即研究数据足以代表样本),足以限制潜在偏倚发生;(3)对目标预后因素有足够的测量,足以限制潜在偏倚发生;(4)对研究对象的目标结局测量充分,足以限制潜在偏倚发生;(5)应恰当考虑重要的混杂因素,限制与目标预后因素相关的潜在偏倚;(6)针对该研究设计的统计分析恰当,限制出现不可信的结果。以上6条采用“是”(低度偏倚)、“否”(高度偏倚)、“不清楚”(缺乏相关信息或偏倚情况不确定)进行评价,并将文献质量分为A、B、C 3级。18篇文献中有6篇文献未对研究的纳入排除标准作充分的说明,7篇文献的失访数据可能导致偏倚,2篇文献未恰当考虑混杂因素的影响。
1.5 统计学方法
采用RevMan 5.3软件进行Meta分析。各研究间的异质性采用χ2检验判断,若P≥0.10,I2≤50%,则认为无明显异质性,采用固定效应模型分析;若P<0.10,I2>50%,则认为有明显异质性,采用随机效应模型分析。分析结果以森林图表示,用HR或OR及其95%CI评价结局指标。采用漏斗图分析是否存在发表偏倚。P<0.05为差异有统计学意义。
2. 结果
2.1 文献筛选流程及纳入文献基本特征
共筛选出相关文献679篇,根据纳入及排除标准进行剔除,再经质量评价,最终纳入18篇文献[13-30],文献检索流程见图 1。纳入文献的基本特征见表 1。
表 1 纳入文献基本特征表第一作者及年份 国家 研究合作 样本量(例) 男/女(例) ALBI分级 结局指标 HR/OR(95%CI) 提取方式 质量等级 Ho等[13]2017 中国 单中心 881 673/208 Ⅰ~Ⅲ OS 2nd vs 1st:1.531(1.285~1.823),3rd vs 1st:1.525(0.976~2.382) R(M) A Waked等[14]2017 埃及 多中心 3030 2769/261 Ⅰ~Ⅲ OS Europe 2nd vs 1st:1.48(1.28~1.71),3rd vs1st:2.59 (1.99~3.38),3rd vs 2nd:1.63(1.28~2.06)
Japan 2nd vs 1st:1.37(1.01~1.87),3rd vs 1st:2.66 (1.77~4.00),3rdvs2nd:1.99(1.58~2.51)
Egypt 2nd vs 1st:1.43(1.10~1.86),3rd vs 1st:2.77 (2.00~3.84),3rd vs 2nd:2.15(1.74~2.65)
China 2nd vs 1st:1.21(0.71~2.05),3rd vs 1st:4.54 (1.71~12.05),3rd vs 2nd:2.90(1.51~5.57)E(U) B Zhao等[15]2020 中国 单中心 221 194/27 Ⅰ~Ⅱ OS 2nd vs 1st:1.83(1.22~2.74) E(U) B Kim等[16]2018 韩国 单中心 476 381/95 Ⅰ~Ⅲ OS 2nd vs 1st:1.77(1.37~2.30),3rd vs 1st:7.11 (3.98~12.7) R(M) B Na等[17]2018 韩国 单中心 905 - Ⅰ~Ⅲ OS 2nd vs 1st:1.40(1.15~1.71),3rd vs 1st:2.02 (1.48~2.75),3rd vs 2nd:1.64(1.26~2.14) E(U) B Zhong等[18]2019 中国 多中心 548 448/100 Ⅰ~Ⅲ OS 2nd vs 1st:1.193(0.964~1.476),3rd vs 1st:1.922(1.180~3.132) R(M) B Carling等[19]2019 挪威 单中心 49 38/11 I~II OS 2nd vs 1st:1.13(0.52~2.46) E(U) B 王哲轩等[20]2020 中国 单中心 185 159/26 Ⅰ~Ⅱ OS 2nd vs 1st:1.82(1.29~2.59) R(M) A 刘芳等[21]2020 中国 单中心 129 104/25 I~III OS 2nd vs 1st:1.54(0.49~4.80),3rd vs 1st:4.74 (1.14~19.71),3rd vs 2nd:3.36(1.27~8.89) R(M) B 黄海等[22]2019 中国 单中心 119 88/31 I~III OS 2nd vs 1st:1.67(1.06~2.63),3rd vs 1st:2.47 (1.53~3.99) R(M) A 张争运等[23]2019 中国 单中心 233 198/35 I~II OS 2nd vs 1st:1.29(0.94~1.77) E(U) B Lee等[24]2019 韩国 单中心 1715 1470/440 Ⅰ~Ⅲ OS 2nd vs 1st:1.55(1.34~1.79),3rd vs 1st:1.39 (1.02~1.89) R(M) B Hansmann等[25]2017 美国 单中心 180 142/38 Ⅱ~Ⅲ OS 3rd vs 2nd:1.82(1.19~2.79) E(U) A Hiraoka等[26]2017 日本 单中心 212 163/49 Ⅰ~Ⅱ 肝功能恶化 2次TACE vs 1次TACE:2.08(1.06~4.05) 3次TACE vs 1次TACE:3.32(1.72~6.40) 3次TACE vs 2次TACE:1.60(0.87~2.92) E(U) B Saito等[27]2020 日本 单中心 113 84/29 Ⅰ~Ⅱ 肝功能恶化 2次TACE vs 1次TACE:1.66(0.76~3.64) 3次TACE vs 1次TACE:3.08(1.44~6.57) 3次TACE vs 2次TACE:1.85(0.90~3.80) E(U) A 魏庭丰等[28]2020 中国 单中心 110 72/18 Ⅰ~Ⅱ 肝功能恶化 2次TACE vs 1次TACE:1.23(0.35~4.35)(Long-term) 2次TACE vs 1次TACE:2.38(1.03~5.45)(Short-term) 谢雪焜等[29]2020 中国 单中心 711 628/83 Ⅰ~Ⅱ ACLF 4.919(2.633~9.188) R(M) A Mohammed等[30]2018 德国 单中心 123 81/42 Ⅱ~Ⅲ ACLF 3.99(1.70~9.40) R(M) B 注:E(U),估计值(单变量);R(M),报告值(多变量);OS,总生存期;2nd vs 1st,ALBI 2 vs ALBI 1;3rd vs 1st,ALBI 3 vs ALBI1;3rd vs 2nd,ALBI 3 vs ALBI 2。 2.2 Meta分析结果
2.2.1 ALBI与OS的关系
2.2.1.1 2nd vs 1st
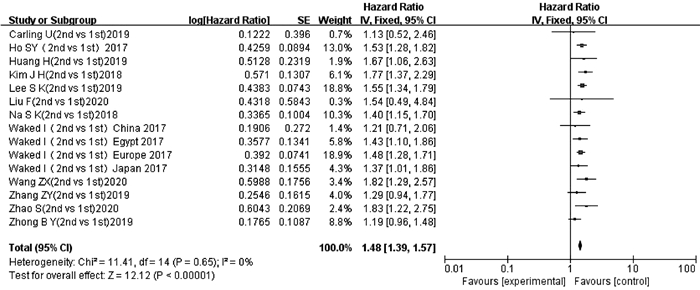
纳入文献中有12项研究[13-24]的15项数据报道了ALBI 2级和ALBI 1级与TACE后OS的多因素分析结果或生存曲线。异质性检验显示不存在异质性(P=0.65,I2=0),采用固定效应模型进行分析。结果显示,ALBI 2级患者TACE后OS短于ALBI 1级患者,差异有统计学意义(HR=1.48,95%CI:1.39~1.57,P<0.000 01)(图 2)。
2.2.1.2 3rd vs 1st
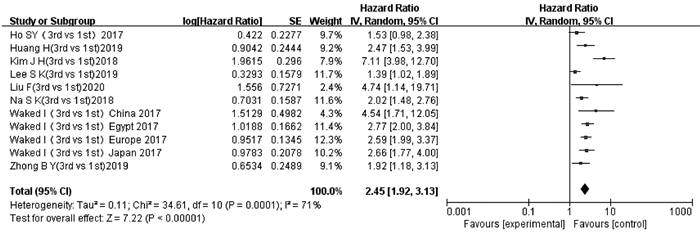
纳入文献中有8项研究[13-14, 16-18, 21-22, 24]的11项数据报道了ALBI3级和ALBI 1级与TACE后OS的多因素分析结果或生存曲线。异质性检验显示存在显著异质性(P=0.000 1,I2=71%),采用随机效应模型进行分析。结果显示,ALBI 3级患者TACE后OS短于ALBI 1级患者,差异有统计学意义(HR=2.45,95%CI:1.92~3.13,P<0.000 01)(图 3)。
2.2.1.3 3rd vs 2nd
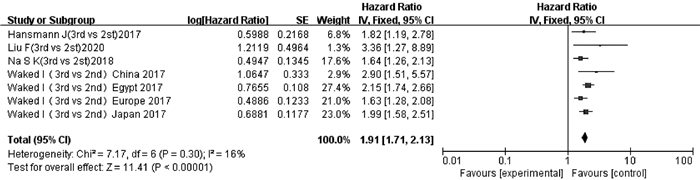
纳入文献中有4项研究[14, 17, 21, 25]的7项数据报道了ALBI 3级和ALBI 2级与TACE后OS的多因素分析结果或生存曲线。异质性检验显示无明显异质性(P=0.30,I2=16%),采用固定效应模型分析。结果显示,ALBI 3级患者TACE后OS短于ALBI 2级患者,差异有统计学意义(HR=1.91,95%CI:1.71~2.13,P<0.000 01)(图 4)。
2.2.2 ALBI变化与重复TACE次数的关系
2.2.2.1 2次vs 1次
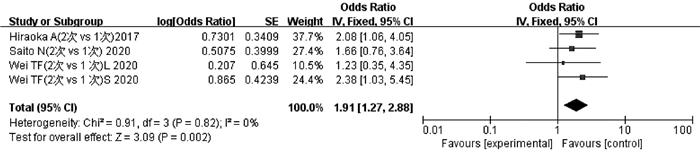
纳入文献中有3项研究[26-28]的4项数据报道了2次TACE与1次TACE后ALBI 1级恶化为2级和/或2级恶化为3级的程度。异质性检验显示不存在异质性(P=0.82,I2=0),采用固定效应模型分析。结果显示,2次TACE累积导致的ALBI恶化程度高于1次TACE,差异有统计学意义(OR=1.91,95%CI:1.27~2.88,P<0.05)(图 5)。
2.2.2.2 3次vs 1次
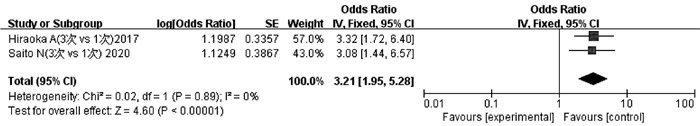
纳入文献中有2项研究[26-27]报道了3次TACE与1次TACE后ALBI 1级恶化为2级和/或2级恶化为3级的程度。异质性检验显示不存在异质性(P=0.89,I2=0),采用固定效应模型分析。结果显示,3次TACE累积导致的ALBI恶化程度高于1次TACE,差异有统计学意义(OR=3.21,95%CI:1.95~5.28,P<0.05)(图 6)。
2.2.2.3 3次vs 2次
纳入文献中有2项研究[26-27]报道了3次TACE与2次TACE后ALBI 1级恶化为2级和/或2级恶化为3级的程度。异质性检验显示不存在异质性(P=0.76,I2=0),采用固定效应模型分析。结果显示,3次TACE累积导致的ALBI恶化度高于2次TACE,差异有统计学意义(OR=1.70,95%CI:1.07~2.70,P<0.05)(图 7)。
2.2.3 ALBI与TACE后发生ACLF的关系
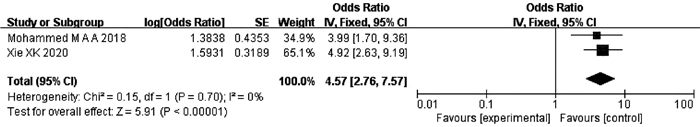
纳入文献中有2项研究[29-30]报道了ALBI分级与TACE后发生ACLF的关系。异质性检验显示不存在异质性(P=0.70,I2=0),采用固定效应模型分析。结果显示,ALBI分级可以预测HCC患者TACE术后ACLF的发生,差异有统计学意义(OR=4.57,95%CI:2.76~7.57,P<0.000 01)(图 8)。
2.3 根据潜在混杂因素进行亚组分析
在比较ALBI 3级与1级患者TACE术后OS时发现严重异质性,根据研究区域、样本量及HR提取方式进行了亚组分析(表 2)。在这些亚组中,较高的ALBI等级与TACE后较差的总体生存相关的结果没有改变。
表 2 Meta分析结果分析 研究数目 合并的HR(95%CI) P值 异质性 I2值 P值 研究区域 China 5 2.09(1.62~2.70) <0.001 35% 0.190 Korean 3 2.01(1.63~2.46) <0.001 92% <0.001 Japan 1 2.66(1.77~4.00) <0.001 - - Egypt 1 2.77(2.00~3.84) <0.001 - - Europe 1 2.59(1.99~3.37) <0.001 - - 样本量 <400 3 2.90(1.90~4.43) <0.001 0 0.370 >400 6 2.24(1.97~2.54) <0.001 78% <0.001 HR提取方式 E(U) 6 2.54(2.17~2.96) <0.001 0 0.480 R(M) 5 1.95(1.61~2.37) <0.001 84% <0.001 2.4 发表偏倚
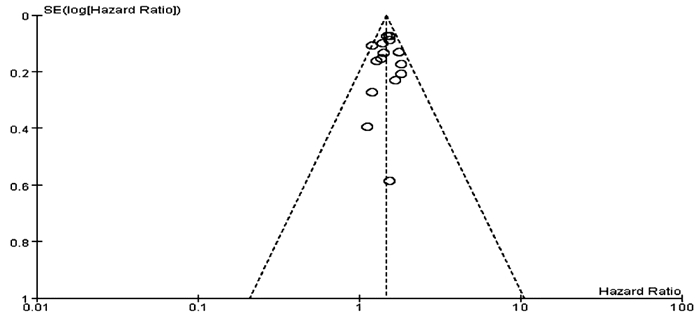
本研究中,有12篇文献[13-24]报道了ALBI 2级和1级患者TACE后总体生存情况,以OS为结局指标,对这12篇文献进行漏斗图分析,结果显示漏斗图呈不对称分布(图 9),提示存在一定的发表偏倚。其余研究纳入文献较少(文献数量<10),则不进行发表偏倚评价。
2.5 敏感性分析
该Meta分析关于ALBI 3rd vs 1st的分析结果异质性较高,对高异质性的结局指标进行敏感性分析,为了揭示单个研究数据对结果的影响,对纳入文献进行逐一剔除,以检验相关结果的稳定性。所幸的是,对纳入文献进行逐一剔除后,结果未发生实质性变化,说明敏感性低,结论稳定性高。
3. 讨论
先前的研究已经提出了许多HCC分期系统,其中最广为人知的是BCLC分期系统。该系统基于随机对照试验和队列研究的结果,将肿瘤分期、患者表现状态以及潜在的肝功能与当前基于证据的治疗算法联系起来,为临床医生的治疗决策提供指导[6]。除了肿瘤本身,潜在的肝功能也是影响HCC患者生存的另一个重要因素[31-32]。肝功能是BCLC分期系统的关键组成部分,使用CTP分级进行评估。起初,CTP仅被用于评估静脉曲张破裂出血的肝硬化手术患者的预后[33-34],数十年来,其已被广泛用于评估肝功能障碍的严重程度。但是这种评分系统在实际应用中存在缺陷[7]。实际上,许多HCC患者不存在肝硬化,而是从轻度异常到晚期纤维化的一系列病理过程[35]。此外,CTP包含的白蛋白、胆红素、凝血酶原时间、腹水和肝性脑病五个指标中[36],白蛋白和腹水是相互关联的,而腹水和肝性脑病的判定存在主观性。因此,基于上述局限性,Johnson等[8]于2015年引入了一种评估肝功能的新工具,即ALBI分级,并首次证明ALBI是整个BCLC疾病阶段的有效预后指标。临床上,胆红素升高通常提示肝损伤;同时,白蛋白减少也可提示肝损伤,并且也与机体较差的营养状况相关,而机体的营养状况差导致的免疫功能下降则是肿瘤发生、发展的重要因素。有基础研究[37]证明,白蛋白可通过调节AFP或通过生长控制激酶的作用直接抑制HCC的生长,因此白蛋白与肿瘤的大小及侵袭性密切相关。较高ALBI等级的HCC患者具有较差的肝功能储备、较差的肿瘤生物学行为及较差的全身情况,因此具有更差的预后。
自引入ALBI以来,已有多个研究[38-39]证明其可作为总体生存的有效预测指标。Chan等[40]进行了一项针对3696例患者的大型国际多中心队列研究,并比较了基于CTP和基于ALBI的BCLC系统的预后能力,结果表明,无论病因、地理区域或者治疗方式如何,基于ALBI的BCLC系统可提供与CTP分级类似的预后判断。Shao等[41]研究了142例接受抗血管生成治疗的晚期HCC患者,并使用ALBI评估肝功能储备,然后将其合并到用ALBI修改的CLIP评分中,结果可将患者分为5个不同的预后分层,证明基于ALBI的CLIP评分可客观评估肝功能储备,保留并可能改善晚期HCC的预后预测。本Meta分析结果显示,不同ALBI等级患者在TACE后总体生存上表现出明显的差别。正常肝组织具有双重血供系统,而HCC的血供主要来自肝动脉,TACE的作用机制就是利用了HCC的这一特点针对性地将高浓度化疗药物集中于肿瘤细胞区域内,同时利用明胶海绵颗粒、碘化油等栓塞肿瘤供血动脉,从而使肿瘤供血减少而发生缩小、坏死[42]。但在实际操作过程中,非肿瘤区域的肝损伤及药物的全身副作用依然不可避免,栓塞次数增多、化疗药物毒性作用可导致肝功能受损[43]。Lencioni等[44]通过对1980年—2013年有关碘化油TACE治疗HCC疗效与安全性的文献进行系统回顾,发现TACE后最常见的不良反应之一是肝转氨酶异常,提示TACE可造成肝损伤。张成佳等[45]报道了118例HCC患者首次TACE后1周内肝功能呈一过性损伤。另据报道[46-47],重复TACE可导致ALBI肝功能持续恶化,本研究比较了1次与多次TACE累积后ALBI变化的差异,结果表明重复TACE次数与ALBI肝功能恶化呈正相关。
由于大部分HCC患者发现时已处于中晚期,此时手术切除已失去意义,TACE因此成为较为安全有效的治疗手段[5]。TACE通常可导致患者肝功能受损,当患者自身肝功能储备较差如合并中慢性肝硬化疾病时,术后肝功能迅速恶化,严重时可能出现ACLF。ACLF是在慢性肝病基础上发生(通常≥4周)的急性肝衰竭失代偿性疾病[48],其作为TACE后主要并发症之一,由于进展迅速,短期病死率高[49],不利于TACE后患者的预后。一项研究[50]在长期随访98例TACE治疗的腹水患者中,17例(17.3%)发生了TACE后肝衰竭,其中16例(94.1%)在1年内发生死亡。多数HCC患者存在慢性肝病基础,如HBV、酒精性肝病、HCV等,因此在TACE前评估ACLF发生风险对于改善患者预后及提高生存质量具有重要指导意义。已有多项研究[51-52]显示ALBI在某些肝病中具有较好的预测能力。本研究提示ALBI能够用于评估TACE后ACLF的发生风险。
本研究纳入的18篇文献中,13篇报道了ALBI与总体生存的关系,3篇文献研究了ALBI变化与重复TACE次数的关系,2篇文献探讨了ALBI与TACE后发生ACLF的关系。在生存分析中,ALBI等级将接受TACE的患者分为三个不同的肝功能风险类别,并分别从各项研究中提取了多变量分析的HR或从Kaplan-Meier曲线中得到了估计值HR及其相应的95%CI,Meta分析的结果一致表明,较高ALBI等级的HCC患者TACE后OS较短。提示ALBI是影响TACE后预后的独立危险因素之一。就研究区域而言,本研究还提供了来自不同地区(包括中国、美国、日本、韩国、欧洲和埃及)的人群生存数据。在比较不同地区人群的OS时,采用ALBI分级观察到了相同的结果。这表明本研究结论同时适用于不同种族背景和生活方式的人群。在ALBI肝功能恶化程度与重复TACE次数的分析中,研究结果表明重复TACE治疗可导致ALBI肝功能持续恶化。同时,本研究确认了ALBI对于HCC患者TACE后发生ACLF的评估能力,这进一步拓宽了ALBI的潜在作用。
本研究存在局限性。第一,在比较ALBI 3级与1级患者TACE后总体生存时发现了高度异质性,根据亚组分析,研究区域、样本量及HR提取方式是异质性的三个来源,但是其他因素如治疗细节、研究人群的基线特征以及所有纳入研究随访信息的变化也可能导致当前研究的高度异质性;第二,所有纳入的研究均为回顾性分析,论证强度低于随机对照研究,排除了综述、摘要、信函、会议、述评等文献(其中12篇摘要因无法获得全文及有效数据被排除在外),且一些结局指标符合纳入标准的文献较少,研究可能存在一定的偏倚;第三,18篇文献中有6篇文献的HR是从Kaplan-Meier曲线中提取的,3篇文献的OR是通过相关数据得出的估计值,这与报告多因素分析的研究相比准确性可能较低;第四,本研究仅限于经TACE治疗的HCC患者,其结论是否适用于其他治疗方式(如手术切除、射频消融、靶向药物治疗或免疫治疗等)有待进一步研究。
这项Meta分析表明,重复TACE治疗可导致ALBI肝功能持续恶化,并且ALBI对于评估TACE术后预后及预测ACLF发生风险具有重要临床价值。ALBI分级仅利用单纯的血液肝功能检查指标,具有简便、经济、实用的特点,有望成为从肝功能角度评估HCC患者预后的全球工具。未来应该进行大样本前瞻性研究,以进一步确定重复TACE治疗对HCC患者肝功能的影响及ALBI对TACE术后评估的临床价值。
-
表 1 肝炎组与无肝炎组基线情况对比
Table 1. Comparisons of baseline between hepatitis group and non-hepatitis group
指标 总体(n=183) 无肝炎组(n=146) 肝炎组(n=37) 统计值 P值 年龄(岁) 31.99±9.08 31.30±8.53 34.73±10.72 t=-1.806 0.077 男性[例(%)] 117(63.9) 100(68.5) 17(45.9) χ2=6.508 0.011 ALT(U/L) 28.20(22.00~33.70) 28.20(21.50~33.05) 28.30(23.10~35.10) Z=-0.836 0.403 ALT分层比较[例(%)] <20 U/L 25(13.7) 21(14.4) 4(10.8) χ2=-0.185 0.853 20~29.9 U/L 82(44.8) 65(44.5) 17(46.0) χ2=-0.498 0.619 30~40 U/L 76(41.5) 60(41.1) 16(43.2) χ2=-1.230 0.219 AST(U/L) 22.10(18.80~25.50) 21.20(18.08~24.65) 24.00(21.25~35.55) Z=-3.344 0.001 TBil(μmol/L) 12.85(9.50~17.43) 12.60(9.40~17.75) 13.20(9.55~16.25) Z=-0.142 0.887 Alb(g/L) 46.40(44.18~48.90) 46.40(44.30~48.85) 46.40(43.70~49.10) Z=-0.122 0.903 WBC(×109/L) 5.91±1.34 5.94±1.28 5.76±1.58 t=0.698 0.486 PLT(×109/L) 212.15±50.41 212.81±51.53 209.38±46.07 t=0.346 0.730 PTA(%) 88.08±9.29 88.47±9.85 86.54±6.58 t=0.929 0.355 HBV DNA(logIU/mL) 7.72(7.52~7.99) 7.72(7.5~8.01) 7.73(7.59~7.99) Z=-0.608 0.543 HBsAg(logU/mL) 4.43(4.40~4.71) 4.46(4.40~4.74) 4.40(4.28~4.49) Z=-2.184 0.029 HBeAg(S/CO) 1400.56(1246.11~1550.96) 1411.00(1269.70~1553.30) 1323.60(995.70~1519.30) Z=-1.899 0.058 表 2 单因素logistic回归分析肝炎危险因素
Table 2. Single factor logistic regression analysis of risk factors for hepatitis
项目 B值 Wald P值 OR 95%CI 性别(男性) -0.939 6.273 0.012 0.391 0.188~0.815 年龄(岁) 0.040 4.111 0.043 1.041 1.001~1.082 AST(U/L) 0.047 7.140 0.008 1.048 1.013~1.085 HBsAg(logU/mL) -2.566 5.716 0.017 0.077 0.009~0.630 HBeAg(S/CO) -0.002 7.349 0.007 0.998 0.997~1.000 -
[1] TAN M, BHADORIA AS, CUI F, et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: A systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2021, 6(2): 106-119. DOI: 10.1016/S2468-1253(20)30307-1. [2] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. [3] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [4] KUMAR M, SARIN SK, HISSAR S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT[J]. Gastroenterology, 2008, 134(5): 1376-1384. DOI: 10.1053/j.gastro.2008.02.075. [5] IANNACONE M, GUIDOTTI LG. Immunobiology and pathogenesis of hepatitis B virus infection[J]. Nat Rev Immunol, 2022, 22(1): 19-32. DOI: 10.1038/s41577-021-00549-4. [6] CAI Y, PAN L, LIN B, et al. Relationship between Treg/Th17 balance and changes in HBeAg among HBeAg-positive chronic hepatitis B patients receiving entecavir therapy[J]. Int J Virol, 2020, 27(5): 407-411. DOI: 10.3760/cma.j.issn.1673-4092.2020.05.013.蔡云, 潘良, 林斌, 等. 恩替卡韦治疗HBeAg阳性慢性乙型肝炎患者外周血Treg/Th17平衡与HBeAg的变化[J]. 国际病毒学杂志, 2020, 27(5): 407-411. DOI: 10.3760/cma.j.issn.1673-4092.2020.05.013. [7] VLACHOGIANNAKOS J, PAPATHEODORIDIS GV. Hepatitis B: Who and when to treat?[J]. Liver Int, 2018, 38 Suppl 1: 71-78. DOI: 10.1111/liv.13631. [8] GAO P, LUO Y, CHEN L, et al. The effect of hepatitis B virus on T lymphocyte and its subsets in chronic hepatitis B patients in different ALT stages: A new concept ALT in HBV infection[J]. Int Immunopharmacol, 2021, 101(Pt A): 108182. DOI: 10.1016/j.intimp.2021.108182. [9] SEONG G, SINN DH, KANG W, et al. Age and fibrosis index for the prediction of hepatocellular carcinoma risk in patients with high hepatitis B virus DNA but normal alanine aminotransferase[J]. Eur J Gastroenterol Hepatol, 2022, 34(1): 69-75. DOI: 10.1097/MEG.0000000000001915. [10] KIM GA, LIM YS, HAN S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B[J]. Gut, 2018, 67(5): 945-952. DOI: 10.1136/gutjnl-2017-314904. [11] LI MH, XIE Y. Antiviral therapy is not recommended for patients in the immune-tolerant phase of hepatitis B virus infection[J]. J Clin Hepatol, 2021, 37(4): 783-784. DOI: 10.3969/j.issn.1001-5256.2021.04.011.李明慧, 谢尧. HBV感染免疫耐受期患者不建议抗病毒治疗[J]. 临床肝胆病杂志, 2021, 37(4): 783-784. DOI: 10.3969/j.issn.1001-5256.2021.04.011. [12] LEE HA, LEE HW, KIM IH, et al. Extremely low risk of hepatocellular carcinoma development in patients with chronic hepatitis B in immune-tolerant phase[J]. Aliment Pharmacol Ther, 2020, 52(1): 196-204. DOI: 10.1111/apt.15741. [13] JIANG XY, HUANG B, HUANG DP, et al. Long-term follow-up of cumulative incidence of hepatocellular carcinoma in hepatitis B virus patients without antiviral therapy[J]. World J Gastroenterol, 2021, 27(11): 1101-1116. DOI: 10.3748/wjg.v27.i11.1101. [14] CHI Z, ZHAO W, LI JW, et al. Combination of quantitative hepatitis B core antibody (qHBcAb) and aspartate aminotransferase (AST) can accurately diagnose immune tolerance of chronic hepatitis B virus infection based on liver biopsy[J]. Clin Res Hepatol Gastroenterol, 2021, 45(6): 101563. DOI: 10.1016/j.clinre.2020.10.008. [15] TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. [16] LU J. A study on the influence of ALT, AST and ALP on the occurrence and development of viral hepatitis[J]. Labeled Immunoassays Clin Med, 2020, 27(1): 67-69, 95. DOI: 10.11748/bjmy.issn.1006-1703.2020.01.015.卢瑾. ALT、AST、ALP对病毒性肝炎患者病情发生发展的影响研究[J]. 标记免疫分析与临床, 2020, 27(1): 67-69, 95. DOI: 10.11748/bjmy.issn.1006-1703.2020.01.015. [17] VAILLANT A. HBsAg, subviral particles, and their clearance in establishing a functional cure of chronic hepatitis B virus infection[J]. ACS Infect Dis, 2021, 7(6): 1351-1368. DOI: 10.1021/acsinfecdis.0c00638. [18] LI MH, CHEN QQ, ZHANG L, et al. Association of cytokines with hepatitis B virus and its antigen[J]. J Med Virol, 2020. DOI: 10.1002/jmv.26301. [19] LIN YJ, SUN FF, ZENG Z, et al. Combination and intermittent therapy based on pegylated interferon Alfa-2a for chronic hepatitis B with Nucleoside (Nucleotide) analog-experienced resulting in hepatitis B surface antigen clearance: A case report[J]. Viral Immunol, 2022, 35(1): 71-75. DOI: 10.1089/vim.2021.0112. [20] LI MH, ZHANG L, QU XJ, et al. The predictive value of baseline HBsAg level and early response for HBsAg loss in patients with HBeAg-positive Chronic Hepatitis B during pegylated interferon alpha-2a treatment[J]. Biomed Environ Sci, 2017, 30(3): 177-184. DOI: 10.3967/bes2017.025. [21] PAN CQ, LI MH, YI W, et al. Outcome of Chinese patients with hepatitis B at 96 weeks after functional cure with IFN versus combination regimens[J]. Liver Int, 2021, 41(7): 1498-1508. DOI: 10.1111/liv.14801. [22] LI M, ZHANG L, LU Y, et al. Early serum HBsAg kinetics as predictor of HBsAg loss in patients with HBeAg-Negative chronic hepatitis B after treatment with pegylated interferonα-2a[J]. Virol Sin, 2021, 36(2): 311-320. DOI: 10.1007/s12250-020-00290-7. [23] CHAN HL, WONG VW, WONG GL, et al. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B[J]. Hepatology, 2010, 52(4): 1232-1241. DOI: 10.1002/hep.23803. [24] LIU J, WANG J, YAN X, et al. Presence of liver inflammation in Asian patients with chronic hepatitis B with normal ALT and detectable HBV DNA in absence of liver fibrosis[J]. Hepatol Commun, 2021. DOI: 10.1002/hep4.1859. 期刊类型引用(8)
1. 刘朝辉,张晓晓,赵朵,刘腊腊. 甲苯磺酸索拉非尼片联合奥沙利铂治疗肝癌化疗患者的疗效及对肝功能的影响. 检验医学与临床. 2024(07): 976-979 .  百度学术
百度学术2. 梁锡天,杨威,陈宇欣,陈余,韩雪,程文. 可视化瞬时弹性成像联合脂肪变定量分析评估消融术后肝功能损伤. 中华超声影像学杂志. 2024(03): 209-215 .  百度学术
百度学术3. 胡碧娟,黄黎银,杨贻金,赖蓁,邱素玲,张梦龙. 二维剪切波弹性成像对经肝动脉化疗栓塞术治疗肝癌患者肝脏储备功能变化的评估价值. 现代医用影像学. 2024(08): 1481-1484 .  百度学术
百度学术4. 李佳清,徐啸阳,胡泽鑫,张申,仲斌演,朱晓黎. 改良白蛋白-胆红素分级对经导管动脉化疗栓塞术联合免疫及抗血管生成药物治疗的Child-Pugh A级不可切除肝细胞癌患者预后的预测价值. 临床肝胆病杂志. 2024(12): 2450-2456 .  本站查看
本站查看5. 刘臣臣,谢军,王洪剑. 白蛋白-胆红素分级对经肝动脉插管化疗栓塞治疗的老年中期原发性肝癌预后价值. 中国老年学杂志. 2023(05): 1057-1061 .  百度学术
百度学术6. 张天澜,路营,雷进,董政,千年松. 多次微波消融对原发性肝癌患者术后肝功能的影响. 解放军医学院学报. 2023(07): 733-738+799 .  百度学术
百度学术7. 徐道辉,林靓宇. 血清TBIL、CCL18与非小细胞肺癌临床病理特征的相关性. 医学理论与实践. 2023(20): 3430-3432+3439 .  百度学术
百度学术8. 王天赐,李弋飞,王田蔚. 联合靶免治疗与单纯TACE术后肝功能的中期评估. 中国实验诊断学. 2022(10): 1484-1487 .  百度学术
百度学术其他类型引用(7)
-




 PDF下载 ( 1912 KB)
PDF下载 ( 1912 KB)


 下载:
下载:









 下载:
下载:

 百度学术
百度学术