人工肝治疗HBV相关慢加急性肝衰竭的血小板计数变化及其影响因素
DOI: 10.3969/j.issn.1001-5256.2022.05.015
Influence of artificial liver support system therapy on platelet in treatment of hepatitis B virus-related acute-on-chronic liver failure
-
摘要:
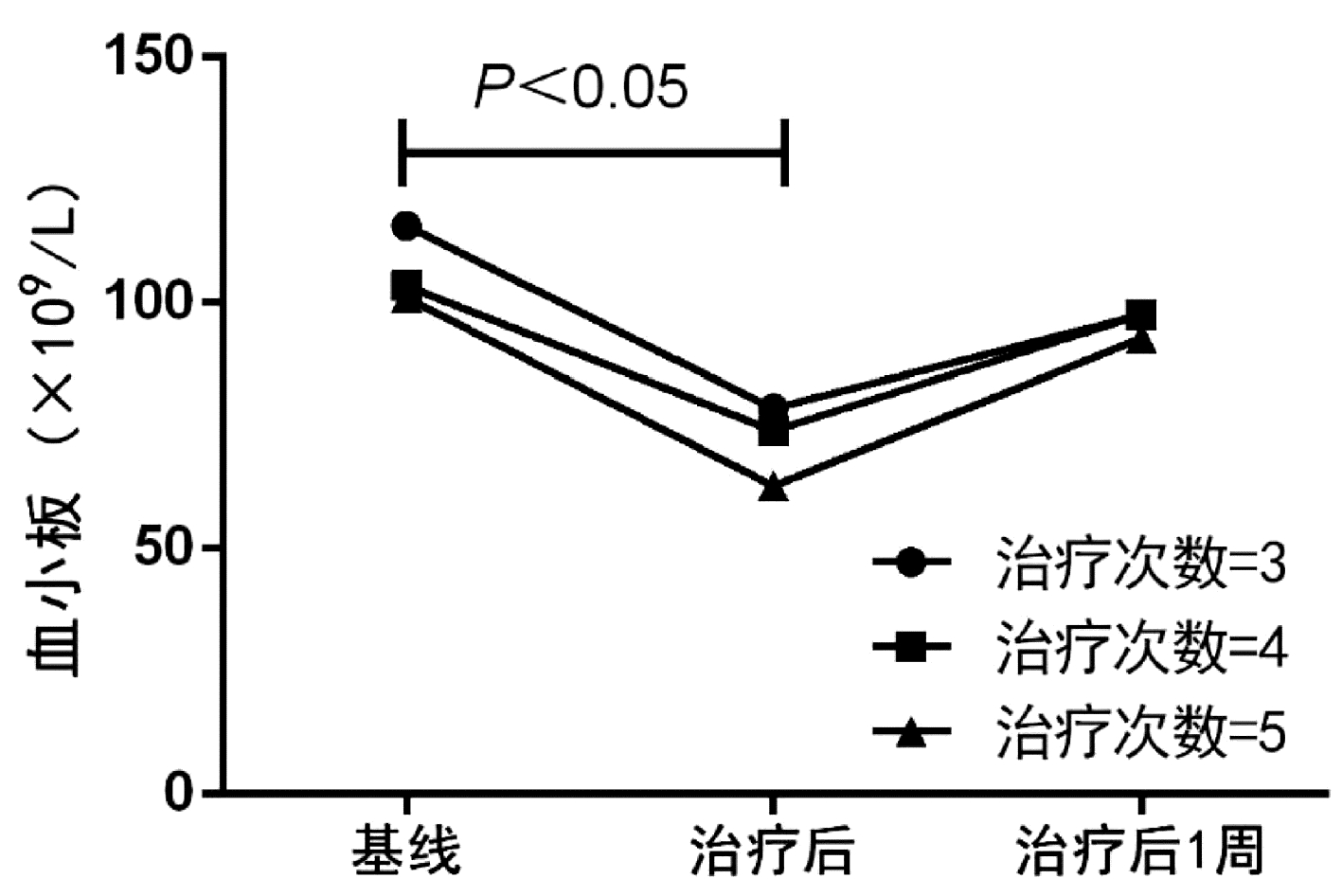
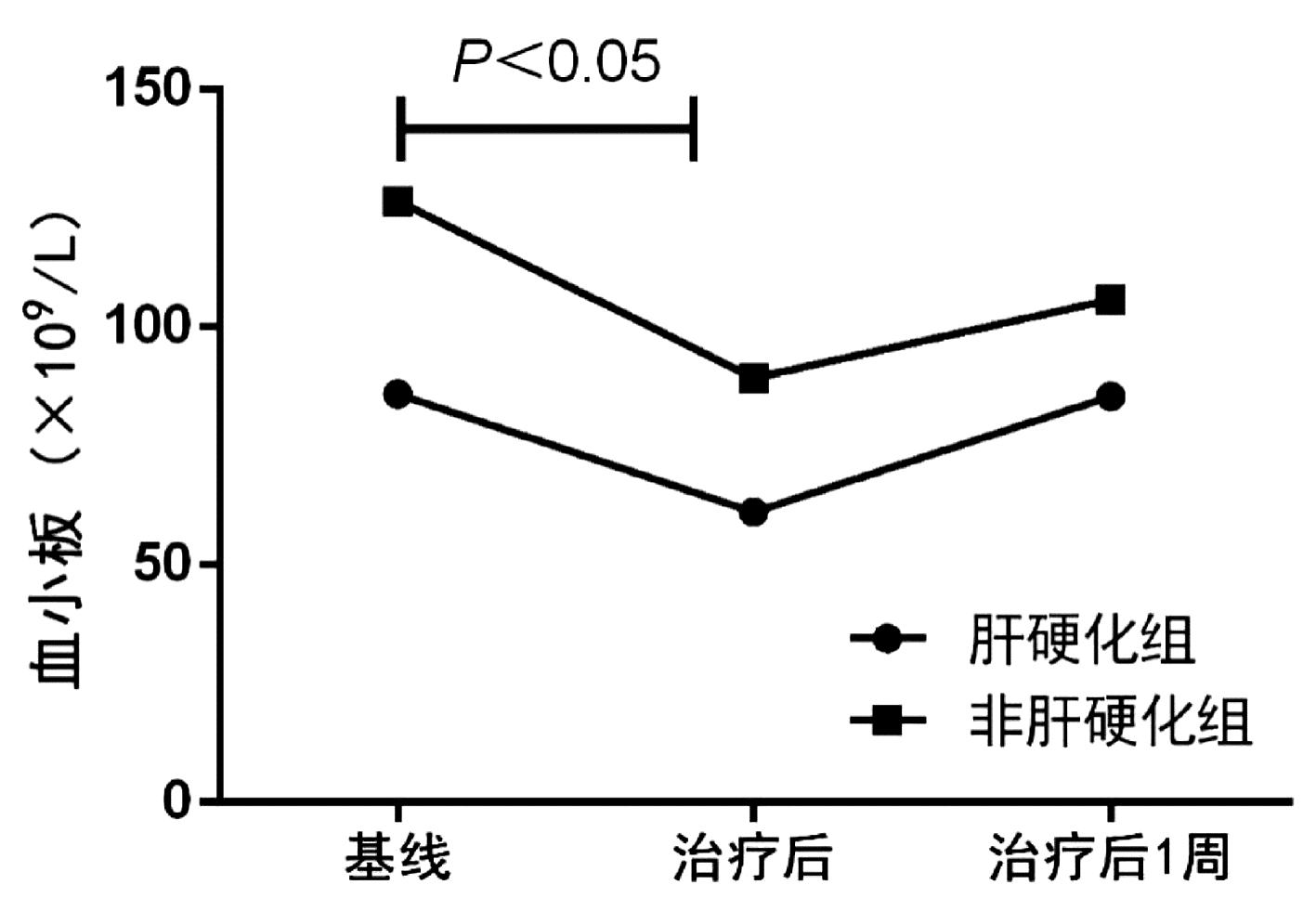
目的 探究乙型肝炎相关慢加急性肝衰竭(HBV-ACLF)患者接受人工肝治疗后PLT计数的变化趋势和影响因素。 方法 选取2018年1月—2021年11月在中山大学附属第三医院住院接受血浆置换治疗(n=102)和双重血浆分子吸附系统联合低剂量血浆置换(n=50)的152例HBV-ACLF患者,分析患者的临床资料和实验室指标。计量资料两组间比较采用独立样本t检验或Mann-Whitney U检验,计数资料两组间比较采用χ2检验;采用logisitic多因素分析影响人工肝治疗后PLT>50×109/L的危险因素, 采用ROC曲线分析基线PLT计数对人工肝治疗后PLT>50×109/L的预测价值。 结果 纳入患者以中年男性为主,70例(46.1%)患者在入院时合并肝硬化,114例(75.0%)患者接受3次人工肝治疗,基线PLT>50×109/L患者占比为88%。总体患者人工肝治疗后PLT计数较基线水平显著下降(79.5±47.7 vs 112.5±64.1, t=4.965, P<0.001),治疗后1周PLT计数升高至基线水平(97.2±50.7 vs 112.5±64.1, t=1.787, P=0.075)。进一步比较人工肝治疗后1周PLT计数较基线的变化量,发现肝硬化组PLT下降幅度显著高于非肝硬化组(U=1986.5,P=0.026),而在不同人工肝术式、治疗次数(3~5次)之间无显著差异(P值均>0.05)。通过logisitic多因素分析发现,合并肝硬化(OR=3.097,95%CI:1.255~7.645,P=0.014)和基线PLT>50×109/L(OR=0.019,95%CI:0.002~0.154,P<0.001)是影响人工肝治疗后PLT>50×109/L的独立危险因素。对基线PLT计数进行ROC曲线分析,发现基线PLT>80.5×109/L是影响治疗后PLT>50×109/L的最佳截断值,ROC曲线下面积为0.818。 结论 人工肝治疗对PLT的影响是暂时性的,但肝硬化患者的PLT生长能力弱于非肝硬化患者; 基线PLT>80.5×109/L是降低人工肝治疗后出血风险的最佳界值。 Abstract:Objective To investigate the changing trend of platelet count (PLT) and related influencing factors in patients with hepatitis B virus-related chronic-on-acute liver failure (HBV-ACLF) after artificial liver support system (ALSS) therapy. Methods A total of 152 patients with HBV-ACLF who were hospitalized and treated in The Third Affiliated Hospital of Sun Yat-Sen University from January 2018 to November 2021 were included in the study, among whom 102 patients received plasma exchange (PE) and 50 patients received double plasma molecular absorption system combined with low-dose PE, and their clinical data and laboratory marker were measured. The independent samples t-test or the Mann-Whitney U test was used for the comparison of continuous data between two groups, and the chi-square test was used for the comparison of categorical data between two groups; a multivariate logistic regression analysis was used to investigate the risk factors for PLT > 50×109/L after ALSS therapy; the receiver operating characteristic (ROC) curve was used to investigate the value of baseline PLT in predicting PLT > 50×109/L after ALSS therapy. Results The patients were mostly middle-aged male adults; among the 152 patients, 70 (46.1%) had liver cirrhosis on admission, 114 (75.0%) received three sessions of ALSS therapy, and 88% had a baseline PLT count of > 50×109/L. There was a significant reduction in PLT from baseline to after ALSS therapy (79.5±47.7 vs 112.5±64.1, t=4.965, P < 0.001), and at 1 week after treatment, PLT increased to the baseline level (97.2±50.7 vs 112.5±64.1, t=1.787, P=0.075). As for the change in PLT from baseline to 1 week after ALSS therapy, the liver cirrhosis group had a significantly greater reduction in PLT than the non-liver cirrhosis group (U=1986.5, P=0.026), while there was no significant difference between different procedures of ALSS therapy and different sessions of treatment (3-5 sessions) (all P > 0.05). The multivariate logistic regression analysis showed that cirrhosis (odds ratio [OR]=3.097, 95% confidence interval [CI]: 1.255-7.645, P=0.014) and PLT > 50×109/L at baseline (OR=0.019, 95%CI: 0.002-0.154, P < 0.001) were independent risk factors for PLT > 50×109/L after ALSS therapy. The ROC curve analysis of baseline PLT showed that PLT > 80.5×109/L at baseline was the optimal cut-off value affecting PLT > 50×109/L after treatment, with an area under the ROC curve of 0.818. Conclusion The influence of ALSS therapy on PLT is temporary, but cirrhotic patients have a weaker PLT generation ability than non-cirrhotic patients. PLT > 80.5×109/L at baseline is the optimal cut-off value to reduce the risk of bleeding after ALSS therapy. -
Key words:
- Hepatitis B virus /
- Liver Failure /
- Liver, Artificial /
- Platelet
-
表 1 HBV-ACLF患者临床基本资料分析
Table 1. Baseline characteristics of study patients
指标 数值 年龄(岁) 43±10 性别[例(%)] 男 143(94.1) 女 9(5.9) ACLF分级[例(%)] ACLF-1 94(61.8) ACLF-2 57(37.5) ACLF-3 1(0.7) 肝性脑病[例(%)] 14(9.2) 肝硬化[例(%)] 70(46.1) 人工肝术式[例(%)] PE 102(67.1) DPMAS+LPE 50(32.9) 人工肝治疗次数[例(%)] 3次 114(75.0) 4次 23(15.1) 5次 15(9.9) AST(U/L) 112(75~183) ALT(U/L) 108(59~268) Alb(g/L) 35.6±4.8 TBil(μmol/L) 422.6±129.2 TBA(μmol/L) 258.2±97.0 BUN(μmol/L) 3.6±1.8 Cr(μmol/L) 69.3±18.1 PT(s) 26.4±8.3 INR 2.5±1.0 K(mmol/L) 3.6±0.5 Na(mmol/L) 136.0±9.2 WBC(×109/L) 7.0±2.7 NEU(%) 0.68±0.10 PLT(×109/L) 112.5±64.1 PLT>50×109/L[例(%)] 135(88.8) MELD评分 25.0±4.7 COSSH-ACLF Ⅱ评分 7.0±0.8 表 2 人工肝治疗后PLT较基线变化量的比较
Table 2. The changes of PLT from baseline after ALSS treatment
组别 例数 ΔPLT(×109/L) ΔPLT(1周) (×109/L) 是否肝硬化 肝硬化组 70 22(9~48) 3(-17~ 26) 非肝硬化组 82 31(13~ 49) 18(-5~ 45)1) 人工肝治疗方式 PE组 102 23(7~ 48) 14(-17~ 37) DPMAS+LPE组 50 33(17~ 52) 13(-11~44) 人工肝治疗次数 治疗3次 114 28(12~48) 12(-14~36) 治疗4次 23 27(9~48) 15(-12~39) 治疗5次 15 22(8~55) 17(-15~51) 注:与肝硬化组比较,1)P<0.05。 表 3 不同PLT水平的短期死亡率或肝移植率比较
Table 3. Short-term mortality or liver transplantation rates in patients with different levels of PLT
组别 例数 28 d死亡或肝移植率 90 d死亡或肝移植率 基线PLT[例(%)] >50×109/L 135 18(13.3) 26(19.3) ≤50×109/L 17 8(47.1)1) 10(58.8)1) 治疗后PLT[例(%)] >50×109/L 110 13(11.8) 19(17.3) ≤50×109/L 42 13(31.0)2) 17(40.5)2) 治疗后1周PLT[例(%)] >50×109/L 135 23(17.0) 30(22.2) ≤50×109/L 17 3(17.6) 6(35.3) 注:与基线PLT>50×109/L比较,1)P<0.05; 与治疗后PLT>50×109/L比较,2)P<0.05。 表 4 影响人工肝治疗后PLT>50×109/L的危险因素分析
Table 4. Risk factors for PLT > 50×109/L in patients after ALSS treatment
指标 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 年龄 1.048(1.009~1.088) 0.015 ACLF分级 3.294(1.567~6.926) 0.002 肝硬化 3.238(1.533~6.842) 0.002 3.097(1.255~7.645) 0.014 INR 1.912(1.313~2.783) 0.001 WBC 0.838(0.713~0.985) 0.032 基线PLT>50×109/L 0.033(0.007~0.155) <0.001 0.019(0.002~0.154) <0.001 -
[1] SARIN SK, KEDARISETTY CK, ABBAS Z, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014[J]. Hepatol Int, 2014, 8(4): 453-471. DOI: 10.1007/s12072-014-9580-2. [2] BERNAL W, JALAN R, QUAGLIA A, et al. Acute-on-chronic liver failure[J]. Lancet, 2015, 386(10003): 1576-1587. DOI: 10.1016/S0140-6736(15)00309-8. [3] YOU S, RONG Y, ZHU B, et al. Changing etiology of liver failure in 3, 916 patients from northern China: A 10-year survey[J]. Hepatol Int, 2013, 7(2): 714-720. DOI: 10.1007/s12072-013-9424-5. [4] Liver Failure and Artificial Liver Group, Branch of Infectious Diseases, Chinese Medical Association. Guideline for non-bioartificial liver support systems in treatment of liver failure: 2016 update[J]. Chin J Clin Infect Dis, 2016, 9(2): 97-103. DOI: 10.3760/cma. j. issn. 1674-2397. 2016.02.001.中华医学会感染病学分会肝衰竭与人工肝学组. 非生物型人工肝治疗肝衰竭指南(2016年版)[J]. 中华临床感染病杂志, 2016, 9(2): 97-103. DOI: 10.3760/cma. j.issn. 1674-2397. 2016.02.001. [5] XU KL, LEI M, YUAN WF, et al. Effect of dual plasma molecular adsorption system in the treatment of hyperbilirubinemia in patients with liver failure[J]. Traum Crit Med, 2020, 8(2): 91-93, 96. DOI: 10.16048/j.issn.2095-5561.2020.02.08.许开亮, 雷鸣, 袁维方, 等. 双重血浆分子吸附系统治疗肝衰竭高胆红素患者疗效研究[J]. 创伤与急危重病医学, 2020, 8(2): 91-93, 96. DOI: 10.16048/j.issn.2095-5561.2020.02.08. [6] DONG JX, ZHANG XY, SUN YH. Changes of blood routine and electrolyte in plasma exchange for chronic severe hepatitis[J]. J Math Med, 2007, 20(1): 38. DOI: 10.3969/j.issn.1004- 4337.2007.01.017.董九香, 张细云, 孙友华. 血浆置换治疗慢性重型肝炎血常规及电解质的变化[J]. 数理医药学杂志, 2007, 20(1): 38. DOI: 10.3969/j.issn.1004-4337.2007.01.017. [7] NU RY, XIANG DD, WANG YM, et al. Effect of different anticoagulant on the activity of platelets during treatment of severe hepatitis with artificial liver(ALSS)[J]. Prac J Med Pharm, 2005, 22(10): 865-867. DOI: 10.3969/j.issn.1671-4008.2005.10.001.牛润章, 向德栋, 王宇明, 等. 肝素对接受人工肝治疗重型肝炎患者血小板的影响[J]. 实用医药杂志, 2005, 22(10): 865-867. DOI: 10.3969/j.issn.1671-4008.2005.10.001. [8] HUANG BB, NING L, LI WY, et al. Establishment of a predictive model for the prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure treated with plasma exchange and double plasma molecular adsorption system alone or in combination[J]. J Clin Hepatol, 2021, 37(12): 2802-2807. DOI: 10.3969/j.issn.1001-5256.2021.12.015.黄贝贝, 宁玲, 李文渊, 等. 血浆置换与双重血浆分子吸附系统单用或联合使用治疗HBV相关慢加急性肝衰竭患者预后预测模型的建立与评估[J]. 临床肝胆病杂志, 2021, 37(12): 2802-2807. DOI: 10.3969/j.issn.1001-5256.2021.12.015. [9] WU T, LI J, SHAO L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure[J]. Gut, 2018, 67(12): 2181-2191. DOI: 10.1136/gutjnl-2017-314641. [10] WANG BQ. Interpretation of 2015 guidelines on prevention and treatment of chronic hepatitis B in Chin[J/CD]. Chin J Front Med Sci (electronic edition), 2016, 8(3): 1-4. DOI: 10.3969/j.issn.1674-7372.2016.03.001.王贵强. 2015年中国慢性乙型肝炎防治指南解读[J/CD]. 中国医学前沿杂志(电子版), 2016, 8(3): 1-4. DOI: 10.3969/j.issn.1674-7372.2016.03.001. [11] KAMATH PS, KIM WR, Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD)[J]. Hepatology, 2007, 45(3): 797-805. DOI: 10.1002/hep.21563. [12] LI J, LIANG X, YOU S, et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure[J]. J Hepatol, 2021, 75(5): 1104-1115. DOI: 10.1016/j.jhep.2021.05.026. [13] CHEN ZL, ZHANG J, TANG G. Clinical significance of platelet parameter detection before and after artificial liver therapy in patients with severe liver disease[J]. Chin J Clin Lab Sci, 2004, 22(4): 292. DOI: 10.13602/j.cnki.jcls.2004.04.032.陈正林, 张杰, 唐格. 重症肝病患者人工肝治疗前后血小板参数检测的临床意义[J]. 临床检验杂志, 2004, 22(4): 292. DOI: 10.13602/j.cnki.jcls.2004.04.032. [14] XIONG ML, HE JQ, YANG LL, et al. Effect of plasma exchange with two different apertures on blood cells[J]. Chin J Gerontol, 2014, 34(13): 3573-3574. DOI: 10.3969/j.issn. 1005-9202.2014.13.029.熊墨龙, 何金秋, 杨玲玲, 等. 两种不同孔径分离器血浆置换后对血细胞的影响[J]. 中国老年学杂志, 2014, 34(13): 3573-3574. DOI: 10.3969/j.issn.1005-9202.2014.13.029. [15] GAO YY, ZHAO YQ, WANG SJ. Incidence of the thrombocytopenia and analysis of the causes in hospitalized patients treated with heparin preparations[J]. J China-Japan Friendship Hosp, 2010, 24(4): 198-201. DOI: 10.3969/j.issn.1001-0025.2010.04.002.高亚玥, 赵永强, 王书杰. 肝素制剂应用患者中血小板减少症的发病率及病因分析[J]. 中日友好医院学报, 2010, 24(4): 198-201. DOI: 10.3969/j.issn.1001-0025.2010.04.002. [16] YUAN S, QIAN Y, TAN D, et al. Therapeutic plasma exchange: A prospective randomized trial to evaluate 2 strategies in patients with liver failure[J]. Transfus Apher Sci, 2018, 57(2): 253-258. DOI: 10.1016/j.transci.2018.02.001. [17] PECK-RADOSAVLJEVIC M. Thrombocytopenia in chronic liver disease[J]. Liver Int, 2017, 37(6): 778-793. DOI: 10.1111/liv.13317. [18] van der MEIJDEN P, HEEMSKERK J. Platelet biology and functions: new concepts and clinical perspectives[J]. Nat Rev Cardiol, 2019, 16(3): 166-179. DOI: 10.1038/s41569-018-0110-0. [19] CUI L, MORAGA I, LERBS T, et al. Tuning MPL signaling to influence hematopoietic stem cell differentiation and inhibit essential thrombocythemia progenitors[J]. Proc Natl Acad Sci U S A, 2021, 118(2): e2017849118. DOI: 10.1073/pnas.2017849118. [20] HU DH, ZHAO K, LIU LM, et al. Study of significance of platelet consumption in patients with cirrhosis during the development of thrombocytopenia[J]. J Southeast Univ(Med Sci Edi), 2018, 37(1): 69-74. DOI: 10.3969/j.issn.1671-6264.2018.01.015.胡东辉, 赵康, 刘黎明, 等. 血小板消耗在肝硬化患者血小板减少症发展过程中的意义[J]. 东南大学学报(医学版), 2018, 37(1): 69-74. DOI: 10.3969/j.issn.1671-6264.2018.01.015. [21] SIMONETTO DA, SINGAL AK, GARCIA-TSAO G, et al. ACG clinical guideline: Disorders of the hepatic and mesenteric circulation[J]. Am J Gastroenterol, 2020, 115(1): 18-40. DOI: 10.14309/ajg.0000000000000486. [22] GISH RG, BROTHERS JM. Current observations in the management of hypo-and hypercoagulability in patients with acute or chronic liver failure[J]. Gastroenterol Hepatol (N Y), 2021, 17(1 Suppl 1): 23-26. [23] XU SS, WEI XH, LIN W, et al. Clinical significance of platelet count and its dynamic change in patients with acute-on-chronic liver failure[J]. J Clin Hepatol, 2018, 34(4): 810-813. DOI: 10.3969/j.issn.1001-5256.2018.04.023.许姗姗, 韦新焕, 林伟, 等. 慢加急性肝衰竭患者血小板计数及其动态变化的临床意义[J]. 临床肝胆病杂志, 2018, 34(4): 810-813. DOI: 10.3969/j.issn.1001-5256.2018.04.023. [24] LIANG C, TAKAHASHI K, FURUYA K, et al. Platelets stimulate liver regeneration in a rat model of partial liver transplantation[J]. Liver Transpl, 2021, 27(5): 719-734. DOI: 10.1002/lt.25962. -



 PDF下载 ( 2341 KB)
PDF下载 ( 2341 KB)

 下载:
下载: