甘油三酯葡萄糖乘积指数与BMI对2型糖尿病合并非酒精性脂肪性肝病的预测价值
DOI: 10.3969/j.issn.1001-5256.2022.05.017
Value of triglyceride-glucose index and body mass index in predicting nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus
-
摘要:
目的 探讨甘油三酯葡萄糖乘积指数(TyG)与BMI对2型糖尿病(T2DM)合并非酒精性脂肪性肝病(NAFLD)的预测价值。 方法 回顾性分析2020年5月—2021年7月中国医科大学附属盛京医院诊治349例T2DM患者的临床资料,按照有无NAFLD分为T2DM合并NAFLD组(n=213)和单纯T2DM组(n=136)。计量资料两组间比较采用t检验或Mann-Whitney U检验;计数资料两组间比较采用χ2检验。采用logistic回归分析TyG及BMI与T2DM合并NAFLD的关系,受试者工作特征曲线(ROC曲线)评价TyG、BMI及TyG联合BMI对T2DM合并NAFLD的预测效能。采用Kappa系数分析预测结果的一致性。 结果 T2DM合并NAFLD组的BMI、舒张压、空腹血糖、糖化血红蛋白、ALT、AST、GGT、TG、TC、LDL-C、TyG均高于单纯T2DM组(P值均<0.05),T2DM合并NAFLD组的HDL-C低于单纯T2DM组(P<0.05),两组在收缩压、总胆红素、直接胆红素、间接胆红素间的差异均无统计学意义(P值均>0.05)。Logistic分析显示TyG(OR=6.513,95%CI:1.884~22.517,P=0.003)和BMI(OR=1.369,95%CI:1.191~1.575,P<0.001)为T2DM合并NAFLD的独立危险因素。ROC曲线显示,TyG预测T2DM合并NAFLD的曲线下面积(AUC)为0.875,最佳截断点9.41,敏感度、特异度、阳性预测值和阴性预测值分别为80.3%、80.1%、86.36%、72.19%;BMI预测T2DM合并NAFLD的AUC为0.787,最佳截断点24.22,敏感度、特异度、阳性预测值和阴性预测值分别为78.9%、64.0%、77.36%、64.23%;TyG联合BMI预测T2DM合并NAFLD的AUC、敏感度、特异度、阳性预测值和阴性预测值分别为0.910、81.2%、88.2%、91.53%、75.00%。TyG、BMI及TyG联合BMI预测T2DM合并NAFLD的Kappa系数分别为0.592、0.416、0.673。 结论 TyG和BMI可用来预测T2DM合并NAFLD的发生,二者联合可提高预测价值。 -
关键词:
- 非酒精性脂肪性肝病 /
- 糖尿病, 2型 /
- 甘油三酯葡萄糖乘积指数 /
- 人体质量指数
Abstract:Objective To investigate the value of triglyceride-glucose index (TyG) and body mass index (BMI) in predicting nonalcoholic fatty liver disease (NAFLD) in type 2 diabetes mellitus (T2DM). Methods A retrospective analysis was performed for the clinical data of 349 patients with T2DM who were treated in Shengjing Hospital of China Medical University from May 2020 to July 2021, and according to the presence or absence of NAFLD, they were divided into T2DM+NAFLD group with 213 patients and simple T2DM group with 136 patients. The t-test or the Mann Whitney U test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. A logistic regression analysis was used to investigate the association of TyG and BMI with T2DM+NAFLD, and the receiver operating characteristic (ROC) curve was plotted to evaluate the prediction efficiency of TyG alone, BMI alone, and TYG combined with BMI for NAFLD in T2DM. The Kappa coefficient was used to analyze the consistency of prediction results. Results Compared with the simple T2DM group, the T2DM+NAFLD group had significantly higher BMI, diastolic pressure, fasting blood glucose, HbA1c, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transpeptidase, triglyceride, total cholesterol, low-density lipoprotein cholesterol, and TyG (all P < 0.05) and a significantly lower high-density lipoprotein cholesterol (P < 0.05), while there were no significant differences between the two groups in systolic pressure, total bilirubin, direct bilirubin, and indirect bilirubin (all P > 0.05). The logistic regression analysis showed that TyG (odds ratio [OR]=6.513, 95% confidence interval [CI]: 1.884-22.517, P < 0.001) and BMI (OR=1.369, 95% CI: 1.191-1.575, P < 0.001) were independent risk factors for NAFLD in T2DM. The ROC curve analysis showed that TyG had an area under the ROC curve (AUC) of 0.875 in predicting NAFLD in T2DM, with a sensitivity of 80.3%, a specificity of 80.1%, a positive predictive value of 86.36%, and a negative predictive value of 72.19% at the optimal cut-off value of 9.41; BMI had an AUC of 0.787, with a sensitivity of 78.9%, a specificity of 64.0%, a positive predictive value of 77.36%, and a negative predictive value of 64.23% at the optimal cut-off value of 24.22; TyG combined with BMI had an AUC of 0.910, a sensitivity of 81.2%, a specificity of 88.2%, a positive predictive value of 91.53%, and a negative predictive value of 75.00% in predicting NAFLD in T2DM. TyG alone, BMI alone, and TyG combined with BMI had a Kappa coefficient of 0.592, 0.416, and 0.673, respectively, in predicting NAFLD in T2DM. Conclusion TyG and BMI can be used to predict the onset of NAFLD in T2DM, and the combination of TyG and BMI can improve the predictive value. -
随着经济水平的发展和膳食结构的改变,我国2型糖尿病(T2DM)的患病率一直处于上升趋势,流行病学调查显示我国18岁及以上成年人中T2DM的患病率已达11.2%[1]。非酒精性脂肪性肝病(NAFLD)是肝细胞内脂质的过度沉积[2],T2DM与NAFLD的关系已得到充分认可。T2DM合并NAFLD的全球患病率为55.5%[3],对于T2DM合并NAFLD的预测具有重要的意义。肥胖和胰岛素抵抗(IR)是T2DM合并NAFLD的危险因素[4]。BMI是判断肥胖的常用指标,甘油三酯葡萄糖乘积指数(triglyceride-glucose index,TyG)可反应体内的IR。因此,本研究通过回顾性分析中国医科大学附属盛京医院T2DM合并NAFLD患者的临床资料,探讨TyG联合BMI对T2DM合并NAFLD的预测价值。
1. 资料与方法
1.1 研究对象
收集2020年5月—2021年7月经本院诊治为T2DM患者的临床资料,T2DM的诊断符合《中国2型糖尿病防治指南(2020年版)》[5],按照有无NAFLD分为T2DM合并NAFLD组和单纯T2DM组,NAFLD的诊断符合《非酒精性脂肪性肝病防治指南(2018更新版)》[6]。
排除标准:(1)年龄<18岁或>65岁的患者;(2)1型糖尿病及其他类型糖尿病患者,2型糖尿病并发酮症酸中毒或高渗昏迷等急性并发症的患者;(3)酒精性肝病、病毒性肝炎、药物性肝损伤、自身免疫性肝病、遗传代谢性肝病等其他肝病患者;(4)恶性肿瘤患者;(5)近3个月口服保肝药物的患者。
1.2 研究指标
1.2.1 一般资料测量
(1) 测量患者的身高和晨起体质量,计算BMI;(2)测量晨起静息状态下右上臂收缩压及舒张压。
1.2.2 生化指标检测
清晨空腹采肘静脉血,采用日立7600全自动生化分析仪检测AST、ALT、GGT、TBil、IBil、DBil、TG、TC、HDL-C、LDL-C、空腹血糖(FBG)、糖化血红蛋白(HbA1c)。计算TyG指数,TyG=ln[TG(mg/dL)× FBG(mg/dL)/2]。
1.2.3 肝脏彩超检测
使用TOSHIBA Aplio500彩色多普勒超声于空腹状态下检查肝脏,NAFLD表现包括肝脏近场回声增强、肝内管道结构显示不清、远场回声衰减等特征。
1.3 统计学方法
采用SPSS 25.0统计学软件进行分析。符合正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验;不符合正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验;计数资料两组间比较采用χ2检验。采用logistic回归分析TyG及BMI与T2DM合并NAFLD的关系;绘制受试者工作特征曲线(ROC曲线)评价TyG、BMI及TyG联合BMI对T2DM合并NAFLD的预测效能,测量曲线下面积(AUC),根据约登指数最大原则确定最佳截断点,并计算敏感度、特异度、阳性预测值、阴性预测值,采用Kappa系数分析预测结果的一致性程度。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入T2DM患者349例。T2DM合并NAFLD组213例,其中男139例,女74例,年龄21~65岁,平均(45.59±12.53) 岁;单纯T2DM组136例,其中男95例,女41例,年龄19~64岁,平均(43.82±12.95)岁。两组的年龄(t=1.265)、性别(χ2=0.793)差异均无统计学意义(P值均>0.05)。
2.2 两组BMI、血压及生化指标的比较
T2DM合并NAFLD组的BMI、舒张压、FBG、HbA1c、ALT、AST、GGT、TG、TC、LDL-C、TyG均高于单纯T2DM组(P值均<0.05),T2DM合并NAFLD组的HDL-C低于单纯T2DM组(P<0.05),两组在收缩压、TBil、DBil、IBil间的差异均无统计学意义(P值均>0.05)(表 1)。
表 1 两组一般资料的比较Table 1. Comparison of general data between two groups指标 T2DM合并NAFLD组(n=213) 单纯T2DM组(n=136) 统计值 P值 BMI(kg/m2) 26.33(24.52~29.37) 23.23(21.92~25.39) Z=-9.044 <0.001 收缩压(mmHg) 130.00(120.00~140.00) 130.00(120.00~140.00) Z=-1.729 0.084 舒张压(mmHg) 80.00(80.00~90.00) 80.00(75.00~84.00) Z=-3.251 0.001 FBG(mmol/L) 9.85(8.20~12.29) 7.96(6.36~9.68) Z=-6.126 <0.001 HbA1c(%) 8.90(7.45~10.25) 7.35(6.50~9.05) Z=-5.075 <0.001 ALT(U/L) 31.00(22.00~44.00) 21.00(13.00~26.00) Z=-7.820 <0.001 AST(U/L) 22.00(17.00~29.00) 18.00(15.00~21.00) Z=-5.447 <0.001 GGT(U/L) 36.00(24.00~56.00) 18.00(13.00~25.25) Z=-9.402 <0.001 TBil(μmol/L) 10.25(8.30~13.40) 10.40(7.80~13.20) Z=-0.098 0.922 DBil(μmol/L) 3.20(2.50~4.18) 3.30(2.53~4.38) Z=-1.129 0.259 IBil(μmol/L) 7.25(5.40~9.20) 7.05(5.25~9.00) Z=-0.226 0.821 TG(mmol/L) 2.42(1.74~3.63) 1.07(0.81~1.50) Z=-11.831 <0.001 TC(mmol/L) 5.04(4.28~5.96) 4.21(3.86~4.70) Z=-7.188 <0.001 HDL-C(mmol/L) 1.02(0.88~1.25) 1.16(1.02~1.37) Z=-4.897 <0.001 LDL-C(mmol/L) 3.28(2.63~3.97) 2.87(2.63~3.87) Z=-4.042 <0.001 TyG 9.98±0.79 8.87±0.61 t=13.832 <0.001 2.3 TyG、BMI与T2DM合并NAFLD的logistic回归分析
以是否合并NAFLD为因变量,TyG和BMI为自变量纳入logistic回归方程,提示TyG和BMI为T2DM合并NAFLD的危险因素。校正单因素分析具有统计学差异的舒张压、FBG、HbA1c、ALT、AST、GGT、TG、TC、LDL-C、HDL-C后,仍具有统计学意义,表示TyG和BMI均为T2DM合并NAFLD的独立危险因素(表 2)。
表 2 TyG、BMI与T2DM合并NAFLD的logistic回归分析Table 2. Logistic regression analysis of TyG, BMI in T2DM combined with NAFLD指标 β值 标准误 Wald值 OR值 95%CI P值 校正前BMI 0.392 0.051 59.377 1.479 1.339~1.634 <0.001 校正前TyG 2.439 0.269 82.237 11.459 6.764~19.411 <0.001 校正后BMI 0.314 0.071 19.390 1.369 1.191~1.575 <0.001 校正后TyG 1.874 0.633 8.766 6.513 1.884~22.517 0.003 2.4 TyG、BMI及TyG联合BMI对T2DM合并NAFLD的预测价值
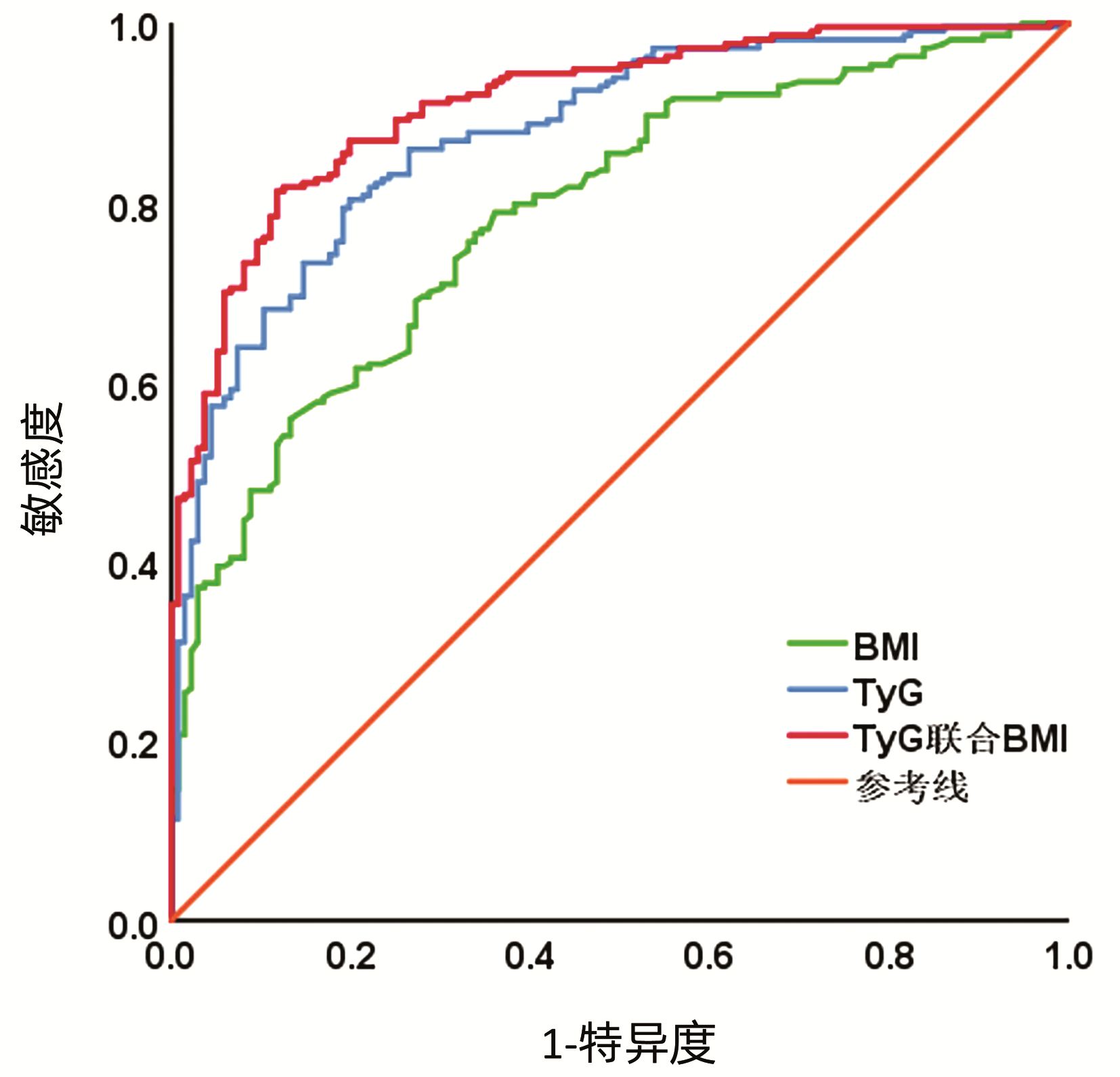
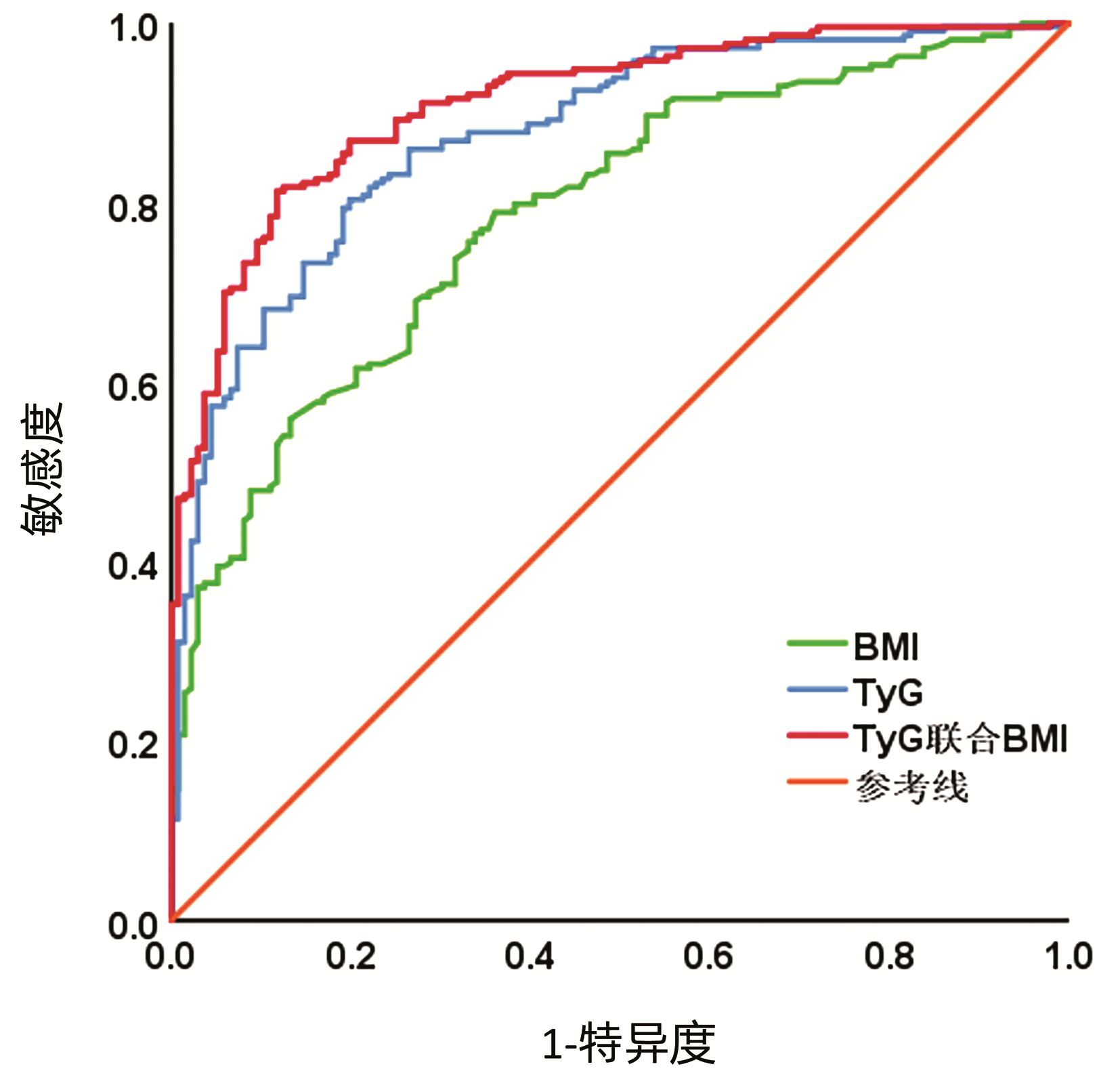
根据ROC曲线结果可见,TyG和BMI预测T2DM合并NAFLD的最佳截断点分别为9.41、24.22,TyG预测的AUC、特异度、敏感度、阳性预测值、阴性预测值均高于BMI,二者联合可以进一步提高预测效能。Kappa系数显示TyG联合BMI预测T2DM合并NAFLD具有更好的一致性(图 1,表 3)。
表 3 TyG、BMI及TyG联合BMI对T2DM合并NAFLD的预测价值Table 3. Predictive value of TyG, BMI and TyG combined BMI for T2DM combined with NAFLD指标 最佳截断点 AUC 95%CI 敏感度(%) 特异度(%) 阳性预测值(%) 阴性预测值(%) Kappa系数 BMI 24.22 0.787 0.740~0.834 78.9 64.0 77.36 64.23 0.416 TyG 9.41 0.875 0.839~0.911 80.3 80.1 86.36 72.19 0.592 TyG联合BMI 0.910 0.881~0.940 81.2 88.2 91.53 75.00 0.673 3. 讨论
超声作为最常用的影像学检查方法,广泛用于临床上对于脂肪肝的诊断,但超声对于轻度脂肪肝的检出率及敏感性较低[7],且超声在诊断脂肪肝过程中易受操作者主观判断及患者皮下脂肪厚度、脾肾回声、肝纤维化的影响,使其有一定的局限性[8]。因此,寻找一个简单可行的,不受操作者主观影响的无创指标,对于NAFLD的预测具有重要的临床意义。BMI包含身高和体质量,TyG包含TG和FBG,均为日常工作中最基础的指标,构成简单,应用方便,因此具有用于预测T2DM合并NAFLD的基本要求。
BMI升高反映着超重和肥胖的存在,本研究结果显示BMI是T2DM合并NAFLD的独立危险因素,原因在于:(1)超重或肥胖时出现脂肪营养不良,肝细胞则会储存多余的脂质[9];(2)脂肪细胞肥大、增生的过程中产生细胞因子,释放脂肪酸,在肝脏内积聚后诱发肝脏炎症反应、细胞损伤及凋亡[10];(3)肥胖时分泌的脂肪因子向更具脂肪及纤维生成性、炎症性的方向转移[11],加重肝损伤。超重或肥胖与NAFLD有着密切的关系,因此BMI可用于预测T2DM合并NAFLD的发生。
IR是T2DM和NAFLD的发病基础。TyG指数最早由Simental-Mendía于2008年提出[12],与评估IR金标准的高胰岛素-正葡萄糖钳夹试验显著相关[13],因此也被看作是IR的标志物[14]。IR在T2DM合并NAFLD中的影响在于:(1)IR时外周组织对胰岛素的敏感性下降,胰岛素对脂解的抑制作用也减弱,导致游离脂肪酸异位沉积于肝脏中[15-16]。(2)IR状态下的高胰岛素和高血糖上调SREBP1c和ChEBP活性,激活糖分解和脂肪生成酶,刺激肝脏脂肪的从头合成[17]。(3)IR活化巨噬细胞,介导肝巨噬细胞和肝星状细胞关联,促进NAFLD发展为肝纤维化甚至肝癌[18]。因此,作为IR标志物的TyG可预测T2DM中的NAFLD。
本研究结果指出TyG联合BMI对T2DM合并NAFLD的预测效能和一致性水平上高于单一指标检测,考虑在于二者联合综合了IR和肥胖对T2DM合并NAFLD的影响,所以提高了预测价值。目前对于NAFLD的筛查要求,也指出在明确NAFLD的同时应评估患者是否同时存在其他代谢综合征组分[19],所以,TyG联合BMI在预测NAFLD的同时,也能对肥胖、IR、血糖血脂等代谢组分的评估进行指导,因此具有较高的应用价值。
综上所述,应重视T2DM合并NAFLD的预测,TyG和BMI可作为预测T2DM合并NAFLD的指标,联合用于T2DM合并NAFLD的筛查与管理。
-
表 1 两组一般资料的比较
Table 1. Comparison of general data between two groups
指标 T2DM合并NAFLD组(n=213) 单纯T2DM组(n=136) 统计值 P值 BMI(kg/m2) 26.33(24.52~29.37) 23.23(21.92~25.39) Z=-9.044 <0.001 收缩压(mmHg) 130.00(120.00~140.00) 130.00(120.00~140.00) Z=-1.729 0.084 舒张压(mmHg) 80.00(80.00~90.00) 80.00(75.00~84.00) Z=-3.251 0.001 FBG(mmol/L) 9.85(8.20~12.29) 7.96(6.36~9.68) Z=-6.126 <0.001 HbA1c(%) 8.90(7.45~10.25) 7.35(6.50~9.05) Z=-5.075 <0.001 ALT(U/L) 31.00(22.00~44.00) 21.00(13.00~26.00) Z=-7.820 <0.001 AST(U/L) 22.00(17.00~29.00) 18.00(15.00~21.00) Z=-5.447 <0.001 GGT(U/L) 36.00(24.00~56.00) 18.00(13.00~25.25) Z=-9.402 <0.001 TBil(μmol/L) 10.25(8.30~13.40) 10.40(7.80~13.20) Z=-0.098 0.922 DBil(μmol/L) 3.20(2.50~4.18) 3.30(2.53~4.38) Z=-1.129 0.259 IBil(μmol/L) 7.25(5.40~9.20) 7.05(5.25~9.00) Z=-0.226 0.821 TG(mmol/L) 2.42(1.74~3.63) 1.07(0.81~1.50) Z=-11.831 <0.001 TC(mmol/L) 5.04(4.28~5.96) 4.21(3.86~4.70) Z=-7.188 <0.001 HDL-C(mmol/L) 1.02(0.88~1.25) 1.16(1.02~1.37) Z=-4.897 <0.001 LDL-C(mmol/L) 3.28(2.63~3.97) 2.87(2.63~3.87) Z=-4.042 <0.001 TyG 9.98±0.79 8.87±0.61 t=13.832 <0.001 表 2 TyG、BMI与T2DM合并NAFLD的logistic回归分析
Table 2. Logistic regression analysis of TyG, BMI in T2DM combined with NAFLD
指标 β值 标准误 Wald值 OR值 95%CI P值 校正前BMI 0.392 0.051 59.377 1.479 1.339~1.634 <0.001 校正前TyG 2.439 0.269 82.237 11.459 6.764~19.411 <0.001 校正后BMI 0.314 0.071 19.390 1.369 1.191~1.575 <0.001 校正后TyG 1.874 0.633 8.766 6.513 1.884~22.517 0.003 表 3 TyG、BMI及TyG联合BMI对T2DM合并NAFLD的预测价值
Table 3. Predictive value of TyG, BMI and TyG combined BMI for T2DM combined with NAFLD
指标 最佳截断点 AUC 95%CI 敏感度(%) 特异度(%) 阳性预测值(%) 阴性预测值(%) Kappa系数 BMI 24.22 0.787 0.740~0.834 78.9 64.0 77.36 64.23 0.416 TyG 9.41 0.875 0.839~0.911 80.3 80.1 86.36 72.19 0.592 TyG联合BMI 0.910 0.881~0.940 81.2 88.2 91.53 75.00 0.673 -
[1] LI Y, TENG D, SHI X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study[J]. BMJ, 2020, 369: m997. DOI: 10.1136/bmj.m99. [2] TARIQ T, DESAI AP. Nonalcoholic fatty liver disease: Making the diagnosis[J]. Clin Liver Dis (Hoboken), 2020, 16(2): 53-57. DOI: 10.1002/cld.924. [3] YOUNOSSI ZM, GOLABI P, de AVILA L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis[J]. J Hepatol, 2019, 71(4): 793-801. DOI: 10.1016/j.jhep.2019.06.021. [4] ZHOU Q, WANG Y, WANG J, et al. Prevalence and risk factor analysis for the nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus[J]. Medicine (Baltimore), 2021, 100(10): e24940. DOI: 10.1097/MD.0000000000024940. [5] Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020edition)[J]. Chin J Diabetes, 2021, 13(4): 315-409. DOI: 10.3760/cma.j.cn115791-20210221-00095.中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 中华糖尿病杂志, 2021, 13(4): 315-409. DOI: 10.3760/cma.j.cn115791-20210221-00095. [6] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [7] HONG J, SHI YW, WU XN, et al. Application value of imaging diagnosis in nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2018, 34(12): 2698-2701. DOI: 10.3969/j.issn.1001-5256.2018.12.041.洪佳, 施漪雯, 吴晓宁, 等. 影像学诊断技术在非酒精性脂肪性肝病中的应用价值[J]. 临床肝胆病杂志, 2018, 34(12): 2698-2701. DOI: 10.3969/j.issn.1001-5256.2018.12.041. [8] DU J, YANG ZH. Application progress of imaging examination in liver fat quantification[J]. Radiol Pract, 2017, 32(5): 479-482. DOI: 10.13609/.cnki.1000-0213.2017.05.011.杜婧, 杨正汉. 影像学检查在肝脏脂肪定量中的应用进展[J]. 放射学实践, 2017, 32(5): 479-482. DOI: 10.13609/.cnki.1000-0213.2017.05.011. [9] POLYZOS SA, KOUNTOURAS J, MANTZOROS CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics[J]. Metabolism, 2019, 92: 82-97. DOI: 10.1016/j.metabol.2018.11.014. [10] APPARI M, CHANNON KM, MCNEILL E. Metabolic regulation of adipose tissue macrophage function in obesity and diabetes[J]. Antioxid Redox Signal, 2018, 29(3): 297-312. DOI: 10.1089/ars.2017.7060. [11] POLYZOS SA, KOUNTOURAS J, MANTZOROS CS. Adipose tissue, obesity and non-alcoholic fatty liver disease[J]. Minerva Endocrinol, 2017, 42(2): 92-108. DOI: 10.23736/S0391-1977.16.02563-3. [12] SIMENTAL-MENDÍA LE, RODRÍGUEZ-MORÁN M, GUERRERO-ROMERO F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects[J]. Metab Syndr Relat Disord, 2008, 6(4): 299-304. DOI: 10.1089/met.2008.0034. [13] TORO-HUAMANCHUMO CJ, URRUNAGA-PASTOR D, GUARNIZO-POMA M, et al. Triglycerides and glucose index as an insulin resistance marker in a sample of healthy adults[J]. Diabetes Metab Syndr, 2019, 13(1): 272-277. DOI: 10.1016/j.dsx.2018.09.010. [14] RODRÍGUEZ-MORÁN M, SIMENTAL-MENDÍA LE, GUERRERO-ROMERO F. The triglyceride and glucose index is useful for recognising insulin resistance in children[J]. Acta Paediatr, 2017, 106(6): 979-983. DOI: 10.1111/apa.13789. [15] BUZZETTI E, PINZANI M, TSOCHATZIS EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD)[J]. Metabolism, 2016, 65(8): 1038-1048. DOI: 10.1016/j.metabol.2015.12.012. [16] XIE J, YANG M, XING Y. Effects of liraglutide on glucose and lipid metabolism and insulin resistance in type 2 diabetes mellitus patients with non-alcoholic fatty liver disease[J/CD]. Chin J Liver Dis (Electronic Version), 2021, 13(4): 46-53. DOI: 10.3969/j.issn.1674-7380.2021.04.008.谢晶, 杨淼, 邢英. 利拉鲁肽对2型糖尿病合并非酒精性脂肪性肝病患者糖脂代谢及胰岛素抵抗的影响[J/CD]. 中国肝脏病杂志(电子版), 2021, 13(4): 46-53. DOI: 10.3969/j.issn.1674-7380.2021.04.008. [17] LI TT, BAI XP. Two-way relationship and pathogenesis of nonalcoholic fatty liver disease and type 2 diabetes mellitus[J]. Med Recapitulate, 2021, 27(1): 158-162, 168. DOI: 10.3969/j.issn.1006-2084.2021.01.030.李婷婷, 白秀平. 非酒精性脂肪性肝病与2型糖尿病的双向关系及发病机制[J]. 医学综述, 2021, 27(1): 158-162, 168. DOI: 10.3969/j.issn.1006-2084.2021.01.030. [18] LIU J, REN CQ, FENG YL, et al. Relationship between liver fibrosis and insulin resistance in patients with type 2 diabetes and nonalcoholic fatty liver disease[J]. Chin J Clin Res, 2021, 34(4): 443-448. DOI: 10.13429/j.cnki.cjcr.2021.04.003.刘佼, 任彩琴, 冯亚莉, 等. 2型糖尿病合并非酒精性脂肪性肝病患者肝纤维化与胰岛素抵抗的关系[J]. 中国临床研究, 2021, 34(4): 443-448. DOI: 10.13429/j.cnki.cjcr.2021.04.003. [19] HAN L, XIE H, SUN Y, et al. Diagnosis and evaluation of metabolic related fatty liver disease[J]. Chin Hepatol, 2021, 26(2): 205-210. DOI: 10.14000/j.cnki.issn.1008-1704.2021.02.029.韩琳, 谢欢, 孙颖, 等. 代谢相关脂肪性肝病的诊断与评估现状[J]. 肝脏, 2021, 26(2): 205-210. DOI: 10.14000/j.cnki.issn.1008-1704.2021.02.029. 期刊类型引用(5)
1. 白伟,李帅. TyG-BMI联合血尿酸对2型糖尿病合并非酒精性脂肪性肝病的预测价值. 罕少疾病杂志. 2024(07): 68-70 .  百度学术
百度学术2. 李梦婷,陆璧,张彦,蒋琳. 基于胰岛素抵抗替代指标评估非肥胖2型糖尿病患者NAFLD的价值. 海军医学杂志. 2024(07): 739-744 .  百度学术
百度学术3. 徐瑞君,薛一,周姣姣,杨奇超. 2型糖尿病患者甘油三酯葡萄糖乘积指数与非酒精性脂肪性肝病患病风险的相关性研究. 中国医药科学. 2024(23): 177-180 .  百度学术
百度学术4. 熊琦君,朱雯. 2型糖尿病合并非酒精性脂肪性肝病患者血尿酸水平变化及临床意义. 肝脏. 2023(12): 1476-1479 .  百度学术
百度学术5. 刘婧,彭松. 三酰甘油-葡萄糖指数对急性缺血性脑卒中合并代谢综合征患者短期预后的影响. 实用心脑肺血管病杂志. 2022(12): 35-39 .  百度学术
百度学术其他类型引用(3)
-




 PDF下载 ( 2162 KB)
PDF下载 ( 2162 KB)

 下载:
下载:

 下载:
下载:
 百度学术
百度学术