成体人肝源性干细胞外泌体对刀豆蛋白A诱导的小鼠急性肝损伤的作用
DOI: 10.3969/j.issn.1001-5256.2022.05.024
Effect of exosomes from adult human liver-derived stem cells on concanavalin A-induced acute liver injury in mice
-
摘要:
目的 探讨成体人肝源性干细胞外泌体(HLSC-exo)不同时间静脉注射对刀豆蛋白A(ConA)诱导的小鼠急性肝损伤的保护作用。 方法 差速离心法提取HLSC-exo,Western blot检测其标志蛋白CD9、CD63的表达,纳米颗粒跟踪分析粒径分布。将56只C57BL/6雄性小鼠随机分为3组,空白对照组、ConA模型组和HLSC-exo治疗组。根据给予磷酸盐缓冲液或HLSC-exo后再注射ConA间隔时间不同,分为3 h、6 h、12 h亚组;检测血清中ALT、AST、TNFα、IL-10水平;比较各组小鼠肝脏大体形态及病理组织变化。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 HLSC-exo是直径为90~110 nm的囊泡体,电镜下可见清晰的“茶托样”结构,其特异性标志蛋白CD9和CD63均有明显的表达;空白对照组小鼠ALT、AST水平分别为(31.81±6.74)U/L、(69.75±8.30)U/L。相较于空白对照组,ConA 3 h、6 h、12 h模型组小鼠ALT、AST水平明显升高(P值均<0.001);HLSC-exo 3 h、6 h治疗组ALT、AST水平较ConA 3 h、6 h模型组明显下降[(225.58±115.59)U/L vs (1989.32±347.67)U/L、(1174.71±203.30)U/L vs (2208.33±349.96)U/L,(303.53±126.68)U/L vs (2534.27±644.72)U/L、(1340.70±262.56)U/L vs (2437.13±288.13)U/L,P值均<0.001];与HLSC-exo 6 h治疗组相比,HLSC-exo 3 h治疗组下降更明显(P<0.001)。空白对照组小鼠IL-10、TNFα水平分别为(313.51±10.97)pg/mL,(476.05±7.31)pg/mL。相较于空白对照组,ConA3 h、6 h、12 h模型组IL-10水平均明显下降(P值均<0.001);HLSC-exo 3 h、6 h治疗组IL-10水平相较于ConA 3 h、6 h模型组明显升高[(331.61±10.46)pg/mL vs (266.20±8.15)pg/mL、(288.13±10.74)pg/mL vs (264.41±9.12)pg/mL,P值均<0.001];且与HLSC-exo 6 h治疗组相比,HLSC-exo 3 h治疗组升高更明显(P<0.001)。相较于空白对照组,ConA 3 h、6 h、12 h模型组TNFα水平较正常组明显升高(P值均<0.001);HLSC-exo 3 h、6 h治疗组TNFα水平相较于ConA 3 h、6 h模型组明显下降[(478.26±12.99)pg/mL vs (551.31±17.70)pg/mL、(515.58±7.18)pg/mL vs (556.21±11.15)pg/mL,P值均<0.001];且与HLSC-exo 6 h治疗组相比,HLSC-exo 3 h治疗组下降更明显(P<0.001)。小鼠肝脏大体形态及病理显示HLSC-exo 3 h、6 h治疗组肝细胞坏死程度较ConA 3 h、6 h模型组明显减轻,HLSC-exo 3 h治疗组肝小叶结构基本完整,仅可见散在点状坏死;HLSC-exo 12 h治疗组与ConA 12 h模型组相比,肝细胞坏死无明显改善。 结论 成体人肝源性干细胞外泌体静脉注射可以减轻ConA诱导的小鼠急性肝损伤,以提前3 h注射保护作用最显著。通过调控细胞因子是HLSC-exo减轻肝损伤的重要机制之一。 Abstract:Objective To investigate the protective effect of adult human liver-derived stem cell exosomes (HLSC-exo) intravenously injected at different time points against acute liver injury induced by concanavalin A (ConA) in mice. Methods HLSC-exo was extracted by differential centrifugation. Western blot was used to measure the expression of the marker proteins CD9 and CD63, and nanoparticle tracking analysis was used to investigate particle size distribution. A total of 56 male C57BL/6 mice were randomly divided into blank control group, ConA model group, and HLSC-exo treatment group. The ConA model group and the HLSC-exo treatment group were further divided into 3-, 6-, and 12-hour subgroups according to the interval between phosphate buffer or HLSC-exo injection and ConA injection. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), tumor necrosis factor-α (TNF-α), and interleukin-10 (IL-10) were measured, and the gross morphology and histopathology of the liver were compared between groups. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results HLSC-exo was a membranous vesicle with a diameter of 90-110 nm, with a clear saucer-like structure under an electron microscope and marked expression of its specific marker proteins CD9 and CD63. In the blank control group, the levels of ALT and AST were 31.81±6.74 U/L and 69.75±8.30 U/L, respectively. Compared with the blank control group, the 3-, 6-, and 12-hour ConA model groups had significant increases in the levels of ALT and AST (all P < 0.001); compared with the 3-and 6-hour ConA model groups, the 3-and 6-hour HLSC-exo treatment groups had significant reductions in the levels of ALT and AST (225.58±115.59 U/L vs 1989.32±347.67 U/L, 1174.71±203.30 U/L vs 2208.33±349.96 U/L, 303.53±126.68 U/L vs 2534.27±644.72 U/L, 1340.70±262.56 U/L vs 2437.13±288.13 U/L, all P < 0.001); compared with the 6-hour HLSC-exo treatment group, the 3-hour HLSC-exo treatment group had significantly greater reductions (P < 0.001). In the blank group, the levels of IL-10 and TNF-α were 313.51±10.97 pg/ml and 476.05±7.31 pg/ml, respectively. Compared with the blank control group, the 3-, 6-, and 12-hour ConA model groups had a significant reduction in the level of IL-10 (all P < 0.001); compared with the 3-and 6-hour ConA model groups, the 3-and 6-hour HLSC-exo treatment groups had a significant increase in the level of IL-10(331.61±10.46 pg/ml vs 266.20±8.15 pg/ml, 288.13±10.74 pg/ml vs 264.41±9.12 pg/ml, both P < 0.001); compared with the 6-hour HLSC-exo treatment group, the 3-hour HLSC-exo treatment group had a significantly greater increase (P < 0.001). Compared with the blank control group, the 3-, 6-, and 12-hour ConA model groups had a significant increase in the level of TNF-α (all P < 0.001); compared with the 3-and 6-hour ConA model groups, the 3-and 6-hour HLSC-exo treatment groups had a significant reduction in the level of TNF-α (478.26±12.99 pg/ml vs 551.31±17.70 pg/ml, 515.58±7.18 pg/ml vs 556.21±11.15 pg/ml, both P < 0.001); compared with the 6-hour HLSC-exo treatment group, the 3-hour HLSC-exo treatment group had a significantly greater reduction (P < 0.001). Compared with the 3-and 6-hour ConA model groups in terms of the gross morphology and histopathology of the liver, the 3-and 6-hour HLSC-exo treatment groups had a significant reduction in the degree of hepatocyte necrosis, and the 3-hour HLSC-exo treatment group had a basically complete lobular structure, with sporadic spotty necrosis; the 12-hour HLSC-exo treatment group had no significant improvement in hepatocyte necrosis compared with the 12-hour ConA model group. Conclusion Intravenous injection of adult HLSC-exo can alleviate acute liver injury induced by ConA in mice, and injection at 3 hours in advance has the most significant protective effect. Regulation of cytokines is one of the important mechanisms for HLSC-exo to alleviate liver injury. -
Key words:
- Liver Failure, Acute /
- Stem Cells /
- Exosomes /
- Concanavalin A
-
短期内严重而持续的肝损伤可导致肝脏合成、代谢和生物转化等功能迅速衰竭,出现以凝血障碍、进行性黄疸、顽固性腹水、肝性脑病等为主要表现的临床综合征,一般药物治疗效果差,病死率极高[1]。刀豆蛋白A(concanavalin A,ConA)是从巴西刀豆中分离纯化的一种凝集素,可激活CD4+T淋巴细胞,进而导致大量炎症因子释放,24 h内即可出现大面积肝细胞坏死,目前该模型被广泛用于研究免疫介导的急性肝损伤[2]。肝移植是目前内科综合治疗难以救治的急性肝衰竭(acute liver failure,ALF)最有效的方法。然而,供体肝的缺乏、术后并发症的高发生率和高昂的经济成本限制了其临床应用。近年来,越来越多的研究表明,脐带间充质干细胞及其衍生的外泌体可有效治疗ALF[3]。本课题组前期研究[4-5]显示,成体人肝源性干细胞腹腔移植后可分泌大量对肝脏有保护作用的细胞因子。本实验将进一步探讨成体人肝源性干细胞外泌体(human liver stem cells-exosome,HLSC-exo)不同时间静脉注射对ConA诱导的小鼠急性肝损伤的保护作用。
1. 材料与方法
1.1 实验材料
1.1.1 实验动物及细胞系
C57BL/6雄性小鼠56只(济南朋悦实验动物繁育有限公司),SPF级,6~8周龄,体质量19~21 g,使用许可证号:SYXK(鲁)20180002,生产许可证号:SCXK(鲁)20190003,饲养于济宁医学院实验动物中心。成体人肝源性干细胞(human liver stem cells,HLSC)由济宁医学院附属医院洪丰教授建系,在37 ℃、5%CO2培养箱,DMEM高糖培养液(含10%胎牛血清和100 U/mL青链霉素)中培养,0.25%胰酶消化传代。
1.1.2 主要试剂及仪器
ConA(Sigma);DMEM高糖培养基、0.25%胰酶、10%胎牛血清、100 U/mL青链霉素(Gibco);异氟烷(瑞沃德);超净无菌工作台、超速离心机(Themo Fisher);单克隆抗体CD9、CD63(润基生物);TNFα、IL-10试剂盒(酶免);纳米粒径示踪分析仪(Particle Metrix);透射电镜(Hitachi)。
1.2 实验方法
1.2.1 HLSC-exo的提取及鉴定
差速离心法提取HLSC细胞培养上清的外泌体,Western blot检测HLSC-exo特异性标志蛋白CD9、CD63的表达,纳米颗粒跟踪分析粒径分布,透射电镜拍摄电镜图片。
1.2.2 实验动物及分组
采用随机数字表法将56只C57BL/6小鼠分为7组,每组8只。空白对照组:每只小鼠经尾静脉给予200 μL PBS。ConA模型组:每只小鼠经尾静脉注射PBS 200 μL,分别间隔3、6、12 h后经尾静脉注射ConA 12 mg/kg,分为ConA 3 h、6 h、12 h模型组;HLSC-exo治疗组:每只小鼠经尾静脉注射含3.2×109个HLSC-exo的PBS混悬液200 μL,分别间隔3、6、12 h后经尾静脉注射ConA 12 mg/kg,HLSC-exo 3 h、6 h、12 h治疗组。各组在给予ConA 24 h后,处死小鼠,收集外周血,取标本。
1.3 肝生化指标检测
注射ConA 24 h后,眼球取血于促凝管中,4000 r/min,10 min离心收集血清,送本院检验科检测ALT、AST。
1.4 血清TNFα、IL-10水平测定
按照ELISA试剂盒说明书步骤测定血清中TNFα、IL-10水平。
1.5 小鼠肝组织病理检测
处死小鼠后,暴露肝脏并留取照片,后取同一部位肝组织,福尔马林固定,梯度乙醇脱水,石蜡包埋切片,二甲苯脱蜡,苏木素和伊红染色后光镜下观察。
1.6 统计学方法
采用SPSS 25.0统计软件进行分析,计量资料以x±s表示,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。P<0.05为差异有统计学意义。
2. 实验结果
2.1 外泌体鉴定
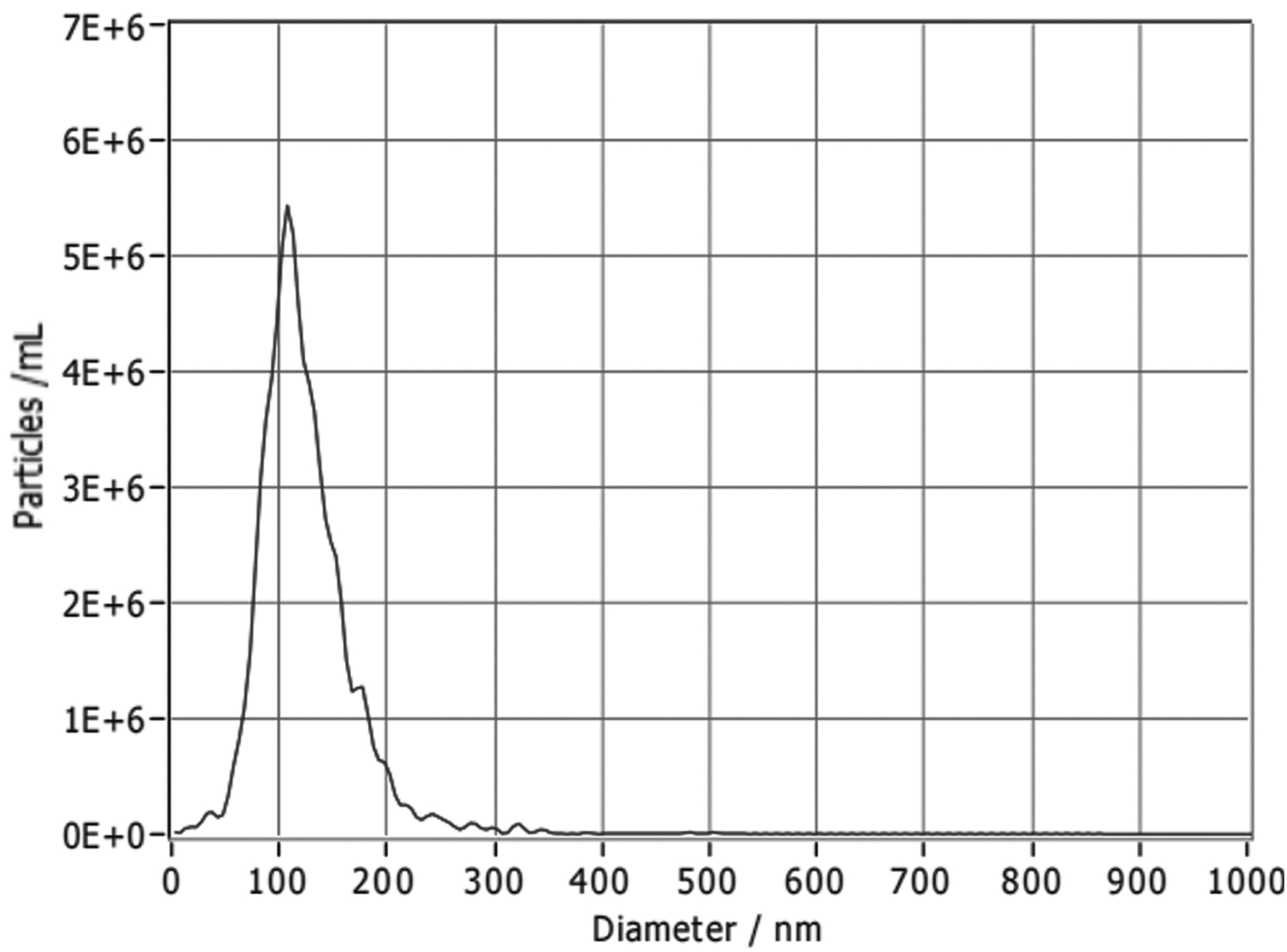
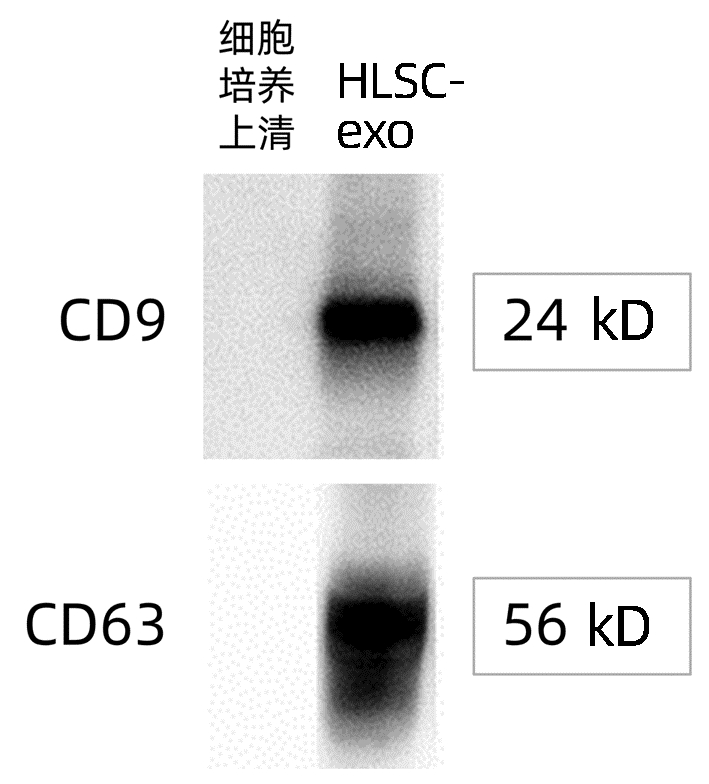
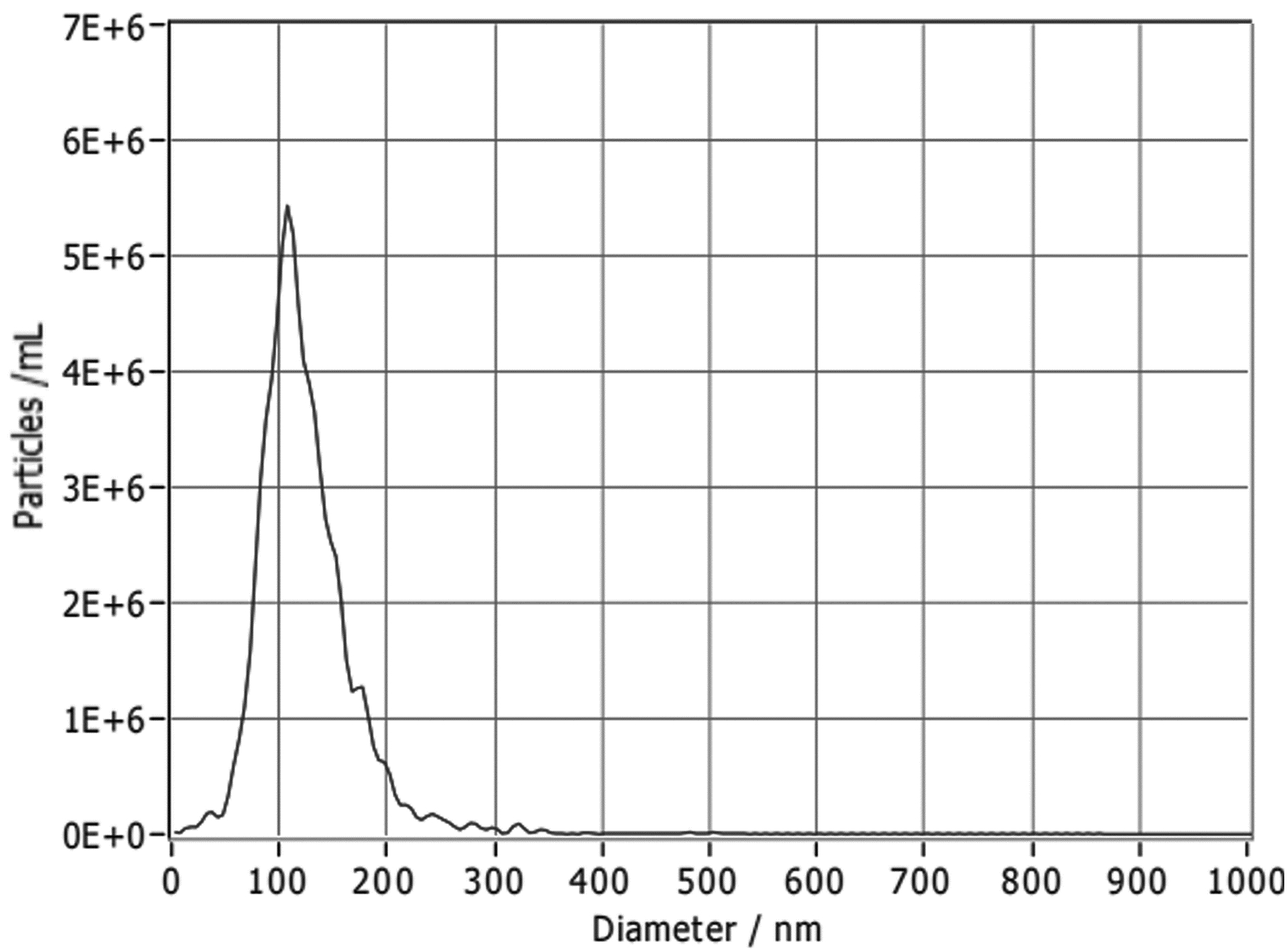
纳米颗粒跟踪分析结果显示,约98.8%的HLSC-exo囊泡粒径在90~110 nm(图 1)。透射电镜下可见HLSC-exo清晰的“茶托样”结构(图 2)。Western blot结果显示,CD9和CD63蛋白均有明显的表达(图 3)。
2.2 肝功能指标变化
空白对照组小鼠ALT、AST水平分别为(31.81±6.74)U/L、(69.75±8.30)U/L。相较于空白对照组,ConA 3 h、6 h、12 h模型组小鼠ALT、AST水平明显升高(P值均<0.001);HLSC-exo 3 h、6 h治疗组ALT、AST水平较ConA 3 h、6 h模型组明显下降(P值均<0.001);与HLSC-exo 6 h治疗组相比,HLSC-exo 3 h治疗组下降更明显(P<0.001);而HLSC-exo 12 h治疗组与ConA 12 h模型组相比,ALT、AST变化不明显(P值均>0.05)(表 1)。
表 1 各组小鼠肝功能ALT、AST水平Table 1. ALT and AST levels of mice in each group组别 ALT(U/L) AST(U/L) 空白组 31.81±6.74 69.75±8.30 ConA模型组 3 h 1989.32±347.671) 2534.27±644.721) 6 h 2208.33±349.961) 2437.13±288.131) 12 h 2223.12±318.481) 2289.88±274.591) HLSC-exo治疗组 3 h 225.58±115.592)3) 303.53±126.682)3) 6 h 1174.71±203.304) 1340.70±262.564) 12 h 1990.00±302.59 2293.36±341.96 F值 101.26 79.60 P值 <0.001 <0.001 注:与空白组比较,1)P<0.001;与ConA 3h组比较,2)P<0.001;与HLSC-exo 6 h组比较,3)P<0.001;与ConA 6 h组比较,4)P<0.001。 2.3 血清TNFα、IL-10水平变化
空白对照组小鼠IL-10、TNFα水平分别为(313.51±10.97)pg/mL,(476.05±7.31)pg/mL。相较于空白对照组,ConA 3 h、6 h、12 h模型组IL-10水平均明显下降(P值均<0.001);HLSC-exo 3 h、6 h治疗组IL-10水平相较于ConA 3 h、6 h模型组明显升高(P值均<0.001);且与HLSC-exo 6 h治疗组相比,HLSC-exo 3 h治疗组升高更明显(P<0.001)。
相较于空白对照组,ConA 3 h、6 h、12 h模型组TNFα水平明显升高(P值均<0.001);HLSC-exo 3 h、6 h治疗组TNFα水平相较于ConA 3 h、6 h模型组明显下降(P值均<0.001);且与HLSC-exo 6 h治疗组相比,HLSC-exo 3 h治疗组下降更明显(P<0.001);而ConA 12 h模型组与HLSC-exo 12 h治疗组比较,IL-10、TNFα水平均无统计学意义(P>0.05)(表 2)。
表 2 各组小鼠血清IL-10、TNFα水平比较Table 2. IL-10 and TNFα levels of mice in each group组别 IL-10(pg/mL) TNFα(pg/mL) 空白组 313.51±10.97 476.05±7.31 ConA模型组 3 h 266.20±8.151) 551.31±17.701) 6 h 264.41±9.121) 556.21±11.151) 12 h 260.23±4.561) 564.42±4.081) HLSC-exo治疗组 3 h 331.61±10.462)3) 478.26±12.992)3) 6 h 288.13±10.744) 515.58±7.184) 12 h 260.10±7.25 559.85±12.02 F值 81.89 96.24 P值 <0.001 <0.001 注:与空白组比较,1)P<0.001;与ConA 3h组比较,2)P<0.001;与HLSC-exo 6 h组比较,3)P<0.001;与ConA 6 h组比较,4)P<0.001。 2.4 肝脏大体和病理结果
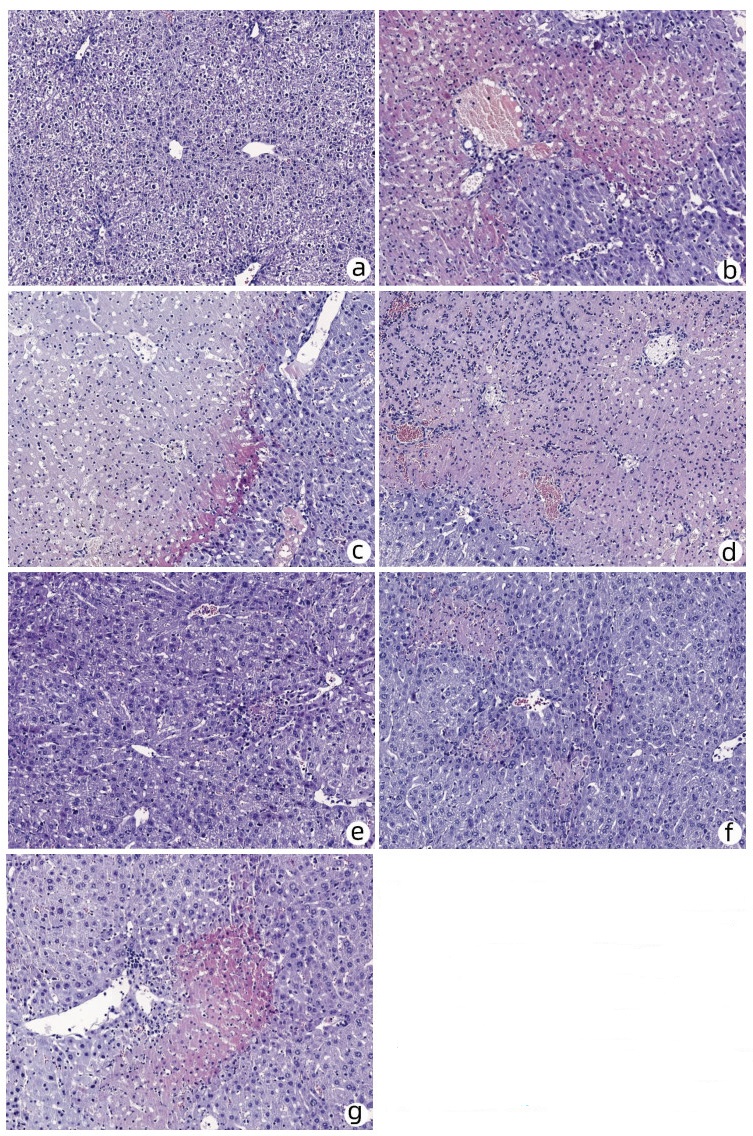
正常小鼠肝脏大体表面光滑,质地柔软,呈鲜红色,HE染色可见肝小叶结构完整清晰,无肝细胞变性、坏死或炎性浸润的迹象。ConA 3 h、6 h、12 h模型组和HLSC-exo 12 h治疗组小鼠肝脏表面粗糙,质地变硬,有大面积灰白色点状及片状坏死,HE染色见肝细胞充血水肿明显,肝实质及胆管内有炎性细胞浸润,肝小叶受损,可见大面积坏死,界版不清。HLSC-exo 3 h、6 h治疗组小鼠肝脏坏死程度较ConA组明显减轻,HE染色可见炎症细胞浸润,肝细胞坏死较ConA模型组得到改善。与HLSC-exo 6 h治疗组组相比,HLSC-exo 3 h治疗组肝小叶基本完整,可见散在点状坏死(图 4),炎性浸润和坏死程度明显降低,而这些变化与肝功能变化一致, 均提示HLSC-exo可逆转ConA诱导的急性肝损伤,且以提前3 h注射保护作用最强。
3. 讨论
ALF进展迅速,病死率高达80%,常伴有多器官功能障碍,临床上内科综合治疗难以纠正的肝衰竭可选择肝移植、血浆置换和人工肝治疗。然而,肝源不足、人工肝治疗的短期疗效及高花费使其临床应用受限[6]。近年来,干细胞疗法受到更多关注,但需要大量时间来培养细胞以达到治疗所需数量,而ALF患者往往需要立即救治。外泌体是由细胞释放到细胞外基质中的膜囊泡,直径30~100 nm,其成分包括DNA片段、mRNA、功能蛋白及转录因子等,可以用标准化技术提纯冷存,且无需使用冷冻保存剂,可承受多次冻融循环,还可制备成冻干粉剂以备临床应用[7]。静脉注射时,可通过肺循环到达靶器官,避免了干细胞疗法肺栓塞的风险,且无致瘤副作用。
差速离心法仍是提取外泌体最广泛的应用方法,首先低速离心去除细胞和凋亡碎片,更高速离心以消除更大的囊泡,最后高速离心沉淀外泌体,该方法操作简单、成本低、耗时较长、不需要分离试剂、外泌体获量大,适合大量初始样品的提取。商用外泌体提取试剂盒主要由2个孔径分别为200 nm和20 nm的膜过滤器组成,样品通过低速离心进行预处理,以分离较大颗粒和细胞碎片,高速离心使样品通过膜过滤器,最终外泌体被截留在两层膜中间,该方法简单、快速、耗时短、获量大,但存在分离试剂污染可能,而且通过膜孔的过程中由于剪切应力和离心作用,外泌体可能会损伤,形态被改变,进而影响后续分析[8]。本实验使用差速离心法提取HLSC-exo,获量大,浓度可达1.6×1010个/mL,电镜下结构完整,可见清晰的“茶托样”结构。
本实验结果表明,HLSC-exo可显著降低转氨酶水平,改善肝脏病理损伤,对ConA诱导的急性肝损伤有显著的保护作用,尤其以提前3 h给予HLSC-exo的保护作用最好,提前6 h效果次之,提前12 h无明显保护作用。HLSC-exo 3 h、6 h治疗组转氨酶水平较ConA 3 h、6 h组均明显下降,且HLSC-exo 3 h治疗组较HLSC-exo 6 h治疗组下降更明显,差异有统计学意义。同样,病理结果也表明HLSC-exo 3 h、6 h治疗组肝损伤程度较ConA组减轻,尤其HLSC-exo 3 h治疗组肝小叶结构基本正常,仅有轻度充血水肿和炎性细胞浸润,未见明显肝细胞坏死情况。
CD4+T淋巴细胞被过度激活,TNFα、IL-1、IFNγ等炎症因子的大量释放以及肝组织严重坏死是ConA诱导的急性肝损伤模型的主要特点。IL-10主要通过抑制活化的T淋巴细胞分泌炎症介质,尤其是抑制辅助性T淋巴细胞(Th)1、Th2、Th17介导的免疫应答,从而减轻肝损伤[9-10]。本实验结果证实,HLSC-exo 3 h、6 h治疗组小鼠血清TNFα水平较ConA组显著降低,而IL-10水平较ConA组升高,表明HLSC-exo与人肝源性干细胞作用相似[5],通过抑制促炎因子释放,促进抑炎因子表达是减轻肝损伤的其中主要机制之一。
综上所述,HLSC-exo静脉注射可显著减轻ConA诱导的小鼠急性肝损伤,调控细胞因子是其发挥治疗作用的重要机制之一。此外,HLSC-exo静脉注射时机不同可影响治疗效果。
-
表 1 各组小鼠肝功能ALT、AST水平
Table 1. ALT and AST levels of mice in each group
组别 ALT(U/L) AST(U/L) 空白组 31.81±6.74 69.75±8.30 ConA模型组 3 h 1989.32±347.671) 2534.27±644.721) 6 h 2208.33±349.961) 2437.13±288.131) 12 h 2223.12±318.481) 2289.88±274.591) HLSC-exo治疗组 3 h 225.58±115.592)3) 303.53±126.682)3) 6 h 1174.71±203.304) 1340.70±262.564) 12 h 1990.00±302.59 2293.36±341.96 F值 101.26 79.60 P值 <0.001 <0.001 注:与空白组比较,1)P<0.001;与ConA 3h组比较,2)P<0.001;与HLSC-exo 6 h组比较,3)P<0.001;与ConA 6 h组比较,4)P<0.001。 表 2 各组小鼠血清IL-10、TNFα水平比较
Table 2. IL-10 and TNFα levels of mice in each group
组别 IL-10(pg/mL) TNFα(pg/mL) 空白组 313.51±10.97 476.05±7.31 ConA模型组 3 h 266.20±8.151) 551.31±17.701) 6 h 264.41±9.121) 556.21±11.151) 12 h 260.23±4.561) 564.42±4.081) HLSC-exo治疗组 3 h 331.61±10.462)3) 478.26±12.992)3) 6 h 288.13±10.744) 515.58±7.184) 12 h 260.10±7.25 559.85±12.02 F值 81.89 96.24 P值 <0.001 <0.001 注:与空白组比较,1)P<0.001;与ConA 3h组比较,2)P<0.001;与HLSC-exo 6 h组比较,3)P<0.001;与ConA 6 h组比较,4)P<0.001。 -
[1] ESCORSELL À, CASTELLOTE J, SÁNCHEZ-DELGADO J, et al. Management of acute liver failure. Clinical guideline from the Catalan Society of Digestology[J]. Gastroenterol Hepatol, 2019, 42(1): 51-64. DOI: 10.1016/j.gastrohep.2018.07.013. [2] YE T, WANG T, YANG X, et al. Comparison of concanavalin a-induced murine autoimmune hepatitis models[J]. Cell Physiol Biochem, 2018, 46(3): 1241-1251. DOI: 10.1159/000489074. [3] JIANG W, TAN Y, CAI M, et al. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect[J]. Stem Cells Int, 2018, 2018: 6079642. DOI: 10.1155/2018/6079642. [4] BI Y, LI J, YANG Y, et al. Human liver stem cells attenuate concanavalin A-induced acute liver injury by modulating myeloid-derived suppressor cells and CD4+ T cells in mice[J]. Stem Cell Res Ther, 2019, 10(1): 22. DOI: 10.1186/s13287-018-1128-2. [5] FAN Z, ZHANG K, HONG F, et al. The mechanisms of intraperitoneal transplantation of human liver-derived stem cells against concanavalin A-induced acute liver injury in mice[J]. Chin J Lab Diag, 2018, 22(6): 1070-1073. DOI: 10.3969/j.issn.1007-4287.2018.06.043.樊增, 张凯, 洪丰, 等. 人肝源性干细胞腹腔移植抗刀豆蛋白A诱导小鼠急性肝损伤的机制研究[J]. 中国实验诊断学, 2018, 22(6): 1070-1073. DOI: 10.3969/j.issn.1007-4287.2018.06.043. [6] HU C, ZHAO L, ZHANG L, et al. Mesenchymal stem cell-based cell-free strategies: Safe and effective treatments for liver injury[J]. Stem Cell Res Ther, 2020, 11(1): 377. DOI: 10.1186/s13287-020-01895-1. [7] LOU G, CHEN Z, ZHENG M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases[J]. Exp Mol Med, 2017, 49(6): e346. DOI: 10.1038/emm.2017.63. [8] SHIREJINI SZ, INCI F. The Yin and Yang of exosome isolation methods: conventional practice, microfluidics, and commercial kits[J]. Biotechnol Adv, 2022, 54: 107814. DOI: 10.1016/j.biotechadv.2021.107814. [9] WANG L, YANG RN. Study on role and clinical significance of IL-9, IL-10 in immune liver damage[J]. Lab Med Clin, 2017, 14(20): 3011-3014. DOI: 10.3969/j.issn.1672-9455.2017.20.014.王兰, 杨瑞宁. IL-9、IL-10在免疫性肝损伤中的作用及临床意义[J]. 检验医学与临床, 2017, 14(20): 3011-3014. DOI: 10.3969/j.issn.1672-9455.2017.20.014. [10] GAO YD, TIAN Y, CHEN Y, et al. Effect of magnesium isoglycyrrhizinate on concanavalin A-induced acute liver failure in mice[J]. J Clin Hepatol, 2020, 36(7): 1571-1576. DOI: 10.3969/j.issn.1001-5256.2020.07.024.高钰迪, 田原, 陈煜, 等. 异甘草酸镁对刀豆蛋白A诱导的急性肝衰竭小鼠模型的影响[J]. 临床肝胆病杂志, 2020, 36(7): 1571-1576. DOI: 10.3969/j.issn.1001-5256.2020.07.024. -




 PDF下载 ( 4326 KB)
PDF下载 ( 4326 KB)


 下载:
下载:



 下载:
下载: