抗病毒治疗适应证变化对提高慢性乙型肝炎治疗率的影响
DOI: 10.3969/j.issn.1001-5256.2022.06.011
Effect of the change in antiviral therapy indication in increasing the treatment rate of chronic hepatitis B
-
摘要:
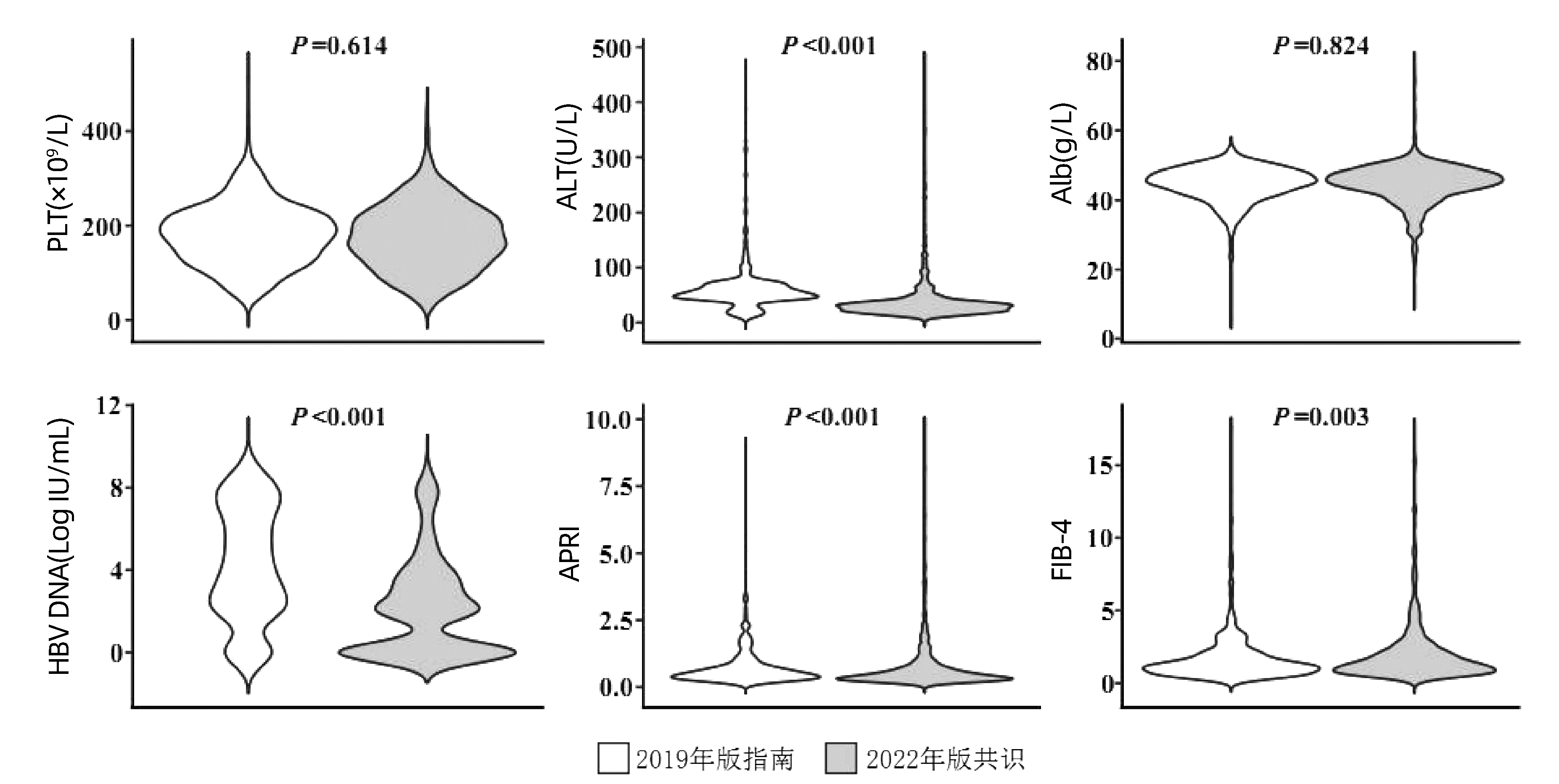
目的 探讨抗HBV治疗适应证变化对治疗率和需要治疗人群特征的影响。 方法 研究对象来自于中国消除乙肝临床研究平台(CR-HepB)数据库中未经治慢性乙型肝炎患者,收集其人口学、病毒学、血液学及血生化信息。不符合正态分布的定量资料在两组间比较采用Mann-Whitney U检验,多组间比较采用Kruskal-Wallis H检验,定性资料组间比较采用χ2检验或Fisher确切概率检验。 结果 本研究共纳入3640例未经治慢性乙型肝炎患者,64.4%为男性,68.7%年龄为30~59岁,46.8%临床分期不确定。根据2015年版、2019年版慢性乙型肝炎防治指南和2022年版专家共识,分别有625例(17.2%)、1333例(36.6%)、和2890例(79.4%)符合抗病毒治疗适应证。2022年版共识新增需治疗患者1557例,其中1424例(91.5%)符合男性ALT>30 U/L、女性ALT>19 U/L的治疗阈值。2022年版专家共识新增需治疗患者的ALT、HBV DNA水平、APRI和FIB-4评分显著低于2019年版指南新增需治疗患者(P值均<0.05)。 结论 随着慢性乙型肝炎抗病毒治疗的适应证扩展,将显著提高患者的治疗比例,有利于更多有疾病进展风险的轻症患者得到及时治疗并改善长期预后。 Abstract:Objective To investigate the impact of the change in anti-hepatitis B virus (HBV) therapy indication on treatment rate and the features of the population requiring treatment. Methods The treatment-naïve patients with chronic hepatitis B (CHB) in the China Registry of Hepatitis B (CR-HepB) database were selected as subjects, and related demographic, virological, hematological, and biochemical data were collected. The Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups, and the Kruskal-Wallis H test was used for comparison between multiple groups; the chi-square test or the Fisher's exact test was used for comparison of categorical data between groups. Results A total of 3640 treatment-naïve CHB patients were included in this study, among whom 64.4% were male, 68.7% had an age of 30-59 years, and 46.8% had an indeterminate clinical stage. According to the 2015 and 2019 editions of Guidelines for the prevention and treatment of chronic hepatitis B and the 2022 edition of expert consensus, the number of patients who had the indication for antiviral therapy was 625(17.2%), 1333(36.6%), and 2890(79.4%), respectively. The number of patients requiring treatment was increased by 1557 according to the 2022 edition of expert consensus, among whom 1424(91.5%) met the treatment threshold of alanine aminotransferase (ALT) > 30 U/L for male patients or ALT > 19 U/L for female patients. The additional patients requiring treatment according to the 2022 edition of expert consensus had significantly higher levels of ALT and HBV DNA and significantly lower scores of APRI and FIB-4 than the additional patients requiring treatment according to the 2019 edition of Guidelines (all P < 0.05). Conclusion The expansion of antiviral therapy indications for CHB may significantly increase the proportion of CHB patients receiving antiviral treatment and help mild CHB patients at the risk of disease progression to receive timely treatment and achieve the improvement in long-term prognosis. -
Key words:
- Hepatitis B, Chronic /
- Antiviral Agents /
- Indication
-
表 1 未经治CHB患者人口学和临床特征
Table 1. Demographic and clinical characteristics of treatment-naive CHB patients
指标 总人群
(n=3640)免疫耐受期
(n=121)免疫清除期
(n=562)免疫控制期
(n=885)再活动期
(n=369)分期不确定
(n=1703)χ2值 P值 年龄[例(%)] 388.800 <0.001 <30岁 896(24.6) 77(63.6) 269(47.9) 104(11.8) 61(16.5) 385(22.6) 30~59岁 2502(68.7) 44(36.4) 289(51.4) 686(77.5) 286(77.5) 1197(70.3) ≥60岁 242(6.6) 0 4(0.7) 95(10.7) 22(6.0) 121(7.1) 性别[例(%)] 60.713 <0.001 男性 2345(64.4) 49(40.5) 392(69.8) 530(59.9) 274(74.3) 1100(64.6) 女性 1295(35.6) 72(59.5) 170(30.2) 355(40.1) 95(25.7) 603(35.4) PLT(×109/L) 179.0(134.0~223.0) 202.0(168.0~237.0) 191.0(157.5~234.0) 179.0(133.3~223.8) 168.0(130.0~202.5) 176.0(126.0~221.0) 40.013 <0.001 ALT(U/L) 34.5(21.0~74.0) 24.0(17.2~31.5) 116.5(67.0~264.8) 20.9(16.0~27.0) 94.3(59.0~205.5) 33.0(21.1~55.3) 1 756.882 <0.001 AST(U/L) 30.0(22.1~52.1) 23.0(19.9~26.5) 71.1(42.9~149.7) 23.0(19.0~27.0) 61.5(41.9~123.0) 29.7(22.3~43.3) 1 429.019 <0.001 Alb(g/L) 45.0(41.6~47.7) 45.0(42.4~46.9) 43.5(40.3~46.6) 45.9(43.0~48.0) 43.8(40.0~46.2) 45.3(41.7~48.1) 101.599 <0.001 TBil(μmol/L) 15.0(11.2~21.6) 12.2(9.1~15.7) 16.7(12.4~23.0) 14.2(10.7~19.1) 17.0(12.5~26.3) 15.2(11.2~22.2) 97.197 <0.001 HBV DNA[例(%)] 2 769.582 <0.001 <2000 IU/mL 2105(57.8) 0 0 885(100.0) 0 1220(71.6) 2000~19 999 IU/mL 306(8.4) 0 0 0 82(22.2) 224(13.2) ≥20 000 IU/mL 1229(33.8) 121(100.0) 562(100.0) 0 287(77.8) 259(15.2) APRI评分 0.5(0.3~1.1) 0.3(0.2~0.4) 1.1(0.6~2.6) 0.3(0.2~0.5) 1.2(0.6~2.4) 0.5(0.3~1.0) 623.757 <0.001 FIB-4评分 1.3(0.8~2.3) 0.7(0.6~1.0) 1.2(0.8~2.4) 1.3(0.9~2.0) 1.8(1.1~3.3) 1.3(0.8~2.4) 66.331 <0.001 表 2 需抗病毒治疗患者临床特征
Table 2. Clinical characteristics of CHB patients requiring antiviral treatment
指标 2015年版指南需治疗患者
(n=625)2019年版指南需治疗患者
(n=1333)2022年版共识需治疗患者
(n=2890)P值1) P值2) P值3) 年龄[例(%)] 0.001 <0.001 <0.001 <30岁 238(38.1) 410(30.8) 668(23.1) 30~59岁 374(59.8) 871(65.3) 2051(71.0) ≥60岁 13(2.1) 52(3.9) 171(5.9) 性别[例(%)] 0.905 <0.001 <0.001 男性 455(72.8) 967(72.5) 1855(64.2) 女性 170(27.2) 366(27.5) 1035(35.8) PLT(×109/L) 179.0(145.0~221.0) 180.0(139.0~222.0) 177.0(132.0~222.0) 0.856 0.182 0.160 ALT(U/L) 190.0(116.0~377.0) 86.0(52.0~194.0) 45.0(27.0~97.8) <0.001 <0.001 <0.001 AST(U/L) 108.0(65.7~209.5) 54.0(36.0~110.2) 35.2(25.0~64.0) <0.001 <0.001 <0.001 Alb(g/L) 43.0(39.0~45.9) 44.2(40.8~47.1) 44.9(41.6~47.6) <0.001 <0.001 <0.001 TBil(μmol/L) 18.3(12.9~29.5) 16.3(12.2~23.5) 15.5(11.5~22.1) <0.001 <0.001 0.001 HBV DNA[例(%)] <0.001 <0.001 <0.001 <2000 IU/mL 0 306(23.0) 1445(50.0) 2000~19 999 IU/mL 28(4.5) 100(7.5) 285(9.9) ≥20 000 IU/mL 597(95.5) 927(69.5) 1160(40.1) APRI评分 1.7(0.9~3.7) 0.9(0.5~2.1) 0.6(0.4~1.4) <0.001 <0.001 <0.001 FIB-4评分 1.7(1.0~3.0) 1.4(0.9~2.5) 1.4(0.9~2.4) 0.002 <0.001 0.970 注:1)2015年版指南需治疗患者与2019年版指南需治疗患者比较;2)2015年版指南需治疗患者与2022年版共识需治疗患者比较; 3)2019年版指南需治疗患者与2022年版共识需治疗患者比较。 表 3 指南新增需抗病毒治疗患者的人口学和病毒学特征
Table 3. Demographic and virological characteristics of additional CHB patients requiring antiviral treatment
指标 2019版指南新增需抗病毒治疗(与2015版指南相比)
(n=708,19.5%)2022版共识新增需抗病毒治疗(与2019版指南相比)
(n=1557,42.8%)HBV DNA阳性 ALT>40 U/L 有家族史且年龄>30岁 ALT>19 U/L/ALT>30 U/L 有家族史 年龄>30岁 患者例数(%) 611(86.3) 599(84.6) 165(23.3) 1424(91.5) 20(1.3) 1299(83.4) 年龄[例(%)] <30岁 172(28.2) 172(28.7) 0 125(8.8) 20(100.0) 0 30~59岁 413(67.6) 398(66.4) 149(90.3) 1180(82.9) 0 1180(90.8) ≥60岁 26(4.3) 29(4.8) 16(9.7) 119(8.4) 0 119(9.2) 性别[例(%)] 男性 454(74.3) 449(75.0) 106(64.2) 760(53.4) 10(50.0) 760(58.5) 女性 157(25.7) 150(25.0) 59(35.8) 664(46.6) 10(50.0) 539(41.5) PLT(×109/L) 178.0(130.0~223.0) 176.0(129.5~222.0) 191.0(143.5~224.5) 172.0(123.0~222.0) 222.0(210.3~259.8) 166.0(120.5~217.0) ALT(U/L) 56.7(46.0~70.0) 57.6(48.0~72.0) 28.0(18.0~50.7) 29.0(21.0~39.0) 50.0(26.8~284.5) 29.5(21.0~39.0) AST(U/L) 39.0(32.0~50.0) 39.4(33.0~51.0) 26.4(21.0~38.0) 27.0(21.1~36.0) 31.0(23.3~153.8) 27.1(22.1~36.3) Alb(g/L) 45.3(42.0~48.0) 45.3(42.0~48.0) 44.5(41.0~46.1) 45.3(42.1~48.0) 45.0(40.2~47.7) 45.4(42.1~48.0) TBil(μmol/L) 15.4(11.6~20.5) 15.5(11.6~20.6) 15.1(11.4~19.5) 14.7(11.0~21.0) 14.8(12.5~18.0) 14.9(11.2~21.5) HBV DNA[例(%)] <2000 IU/mL 209(34.2) 213(35.6) 129(78.2) 1040(73.0) 16(80.0) 978(75.3) 2000~19 999 IU/mL 72(11.8) 65(10.9) 12(7.3) 171(12.0) 1(5.0) 164(12.6) ≥20 000 IU/mL 330(54.0) 321(53.6) 24(14.5) 213(15.0) 3(15.0) 157(12.1) APRI评分 0.6(0.3~0.8) 0.6(0.4~1.0) 0.3(0.2~0.5) 0.4(0.3~0.8) 0.4(0.3~1.9) 0.4(0.3~0.8) FIB-4评分 1.4(0.9~2.4) 1.2(0.7~2.1) 1.3(1.0~2.1) 1.5(0.9~2.5) 0.7(0.5~0.8) 1.6(1.0~2.6) -
[1] YUEN MF, CHEN DS, DUSHEIKO GM, et al. Hepatitis B virus infection[J]. Nat Rev Dis Primers, 2018, 4: 18035. DOI: 10.1038/nrdp.2018.35. [2] LIU J, LIANG W, JING W, et al. Countdown to 2030: eliminating hepatitis B disease, China[J]. Bull World Health Organ, 2019, 97(3): 230-238. DOI: 10.2471/BLT.18.219469. [3] HOU JL, ZHAO W, LEE C, et al. Outcomes of long-term treatment of chronic HBV infection with entecavir or other agents from a randomized trial in 24 countries[J]. Clin Gastroenterol Hepatol, 2020, 18(2): 457-467. e21. DOI: 10.1016/j.cgh.2019.07.010. [4] SINN DH, KIM SE, KIM BK, et al. The risk of hepatocellular carcinoma among chronic hepatitis B virus-infected patients outside current treatment criteria[J]. J Viral Hepat, 2019, 26(12): 1465-1472. DOI: 10.1111/jvh.13185. [5] TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. [6] European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. [7] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. [8] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. [9] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [10] Chinese Society of Hepatology, Chinese Medical Association. Expert opinion on expanding anti-HBV treatment for chronic hepatitis B[J]. Chin J Hepatol, 2022, 30(2): 131-136. DOI: 10.3760/cma.j.cn50113-20220209-00060.中华医学会肝病学分会. 扩大慢性乙型肝炎抗病毒治疗的专家意见[J]. 中华肝脏病杂志, 2022, 30(2): 131-136. DOI: 10.3760/cma.j.cn50113-20220209-00060. [11] SHAN S, YOU H, NIU J, et al. Baseline characteristics and treatment patterns of the patients recruited to the china registry of hepatitis B[J]. J Clin Transl Hepatol, 2019, 7(4): 322-328. DOI: 10.14218/JCTH.2019.00052. [12] KONG YY, WEI W, SHAN S, et al. Clinical profiles and treatment patterns of patients with HBV-related liver cirrhosis[J]. Chin Hepatol, 2020, 25(2): 123-127. DOI: 10.14000/j.cnki.issn.1008-1704.2020.02.011.孔媛媛, 魏巍, 单姗, 等. 乙型肝炎肝硬化患者的临床特征与抗病毒治疗模式变化[J]. 肝脏, 2020, 25(2): 123-127. DOI: 10.14000/j.cnki.issn.1008-1704.2020.02.011. [13] SHAN S, WEI W, KONG Y, et al. China registry of hepatitis B (CR-HepB): Protocol and implementation of a nationwide hospital-based registry of hepatitis B[J]. Scand J Public Health, 2020, 48(2): 233-239. DOI: 10.1177/1403494818772188. [14] XIAO G, YANG J, YAN L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis[J]. Hepatology, 2015, 61(1): 292-302. DOI: 10.1002/hep.27382. [15] LEE MH, YANG HI, LIU J, et al. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles[J]. Hepatology, 2013, 58(2): 546-554. DOI: 10.1002/hep.26385. [16] DUAN M, CHI X, XIAO H, et al. High-normal alanine aminotransferase is an indicator for liver histopathology in HBeAg-negative chronic hepatitis B[J]. Hepatol Int, 2021, 15(2): 318-327. DOI: 10.1007/s12072-021-10153-2. [17] LI M, KONG YY, WU SS, et al. Impact of reimbursement program on liver-related mortality in patients with chronic hepatitis B in Beijing, China[J]. J Dig Dis, 2019, 20(9): 467-475. DOI: 10.1111/1751-2980.12794. [18] TORDRUP D, HUTIN Y, STENBERG K, et al. Additional resource needs for viral hepatitis elimination through universal health coverage: projections in 67 low-income and middle-income countries, 2016-30[J]. The Lancet Global Health, 2019, 7(9): e1180-e1188. DOI: 10.1016/S2214-109X(19)30272-4. [19] NAYAGAM S, CHAN P, ZHAO K, et al. Investment case for a comprehensive package of interventions against hepatitis B in China: Applied modeling to help national strategy planning[J]. Clin Infect Dis, 2021, 72(5): 743-752. DOI: 10.1093/cid/ciaa134. [20] JIA JD, HOU JL, WEI L, et al. Highlights of the guidelines of prevention and treatment for chronic hepatitis B (2019 version)[J]. Chin J Hepatol, 2020, 28 (1): 21-23. DOI: 10.376/cma.j.issn.1007-3418.2020.01.006.贾继东, 侯金林, 魏来, 等. 《慢性乙型肝炎防治指南(2019年版)》新亮点[J]. 中华肝脏病杂志, 2020, 28 (1):21-23. DOI:10.3760/cma.j.issn.1007-3418.2020.01.006. -



 PDF下载 ( 2465 KB)
PDF下载 ( 2465 KB)


 下载:
下载: