基于高通量测序分析凉血解毒方对HBV相关慢加急性肝衰竭患者肠道菌群的影响
DOI: 10.3969/j.issn.1001-5256.2022.06.013
Effect of Liangxue Jiedu decoction on intestinal flora in patients with hepatitis B virus-related acute-on-chronic liver failure: An analysis based on high-throughput sequencing
-
摘要:
目的 观察凉血解毒方对HBV相关慢加急性肝衰竭(HBV-ACLF)患者肠道菌群的影响。 方法 选取2018年10月—2019年10月于北京地坛医院住院诊断为HBV-ACLF的患者,招募同期正常健康人群作为对照(HP组),通过高通量测序的方法筛选出HBV-ACLF患者与正常健康人群的菌群多样性和菌种的差异,从门、属水平筛选出两组的差异菌;利用体外模拟发酵实验,HBV-ACLF患者给予不同浓度(0、10%、50%、100%)凉血解毒方培养基培养24 h,从属分类水平比较分析HBV-ACLF用药组、HBV-ACLF未用药组及HP组菌群变化。正态分布的计量资料两组间比较采用t检验;非正态分布的计量资料两组间比较采用Mann-Whitney U检验。 结果 共纳入HBV-ACLF患者10例,其中HBV-ACLF用药组5例,未用药组5例;HP组15例。HBV-ACLF未用药组较HP组肠道菌群多样性及丰度下降。在门分类水平,HP组每个样本均以拟杆菌门和厚壁菌门为主;HBV-ACLF未用药组拟杆菌门明显减少,梭杆菌门、变形菌门、纤维杆菌门增多。在属分类水平上,HBV-ACLF未用药组瘤胃菌、布劳特菌属、真杆菌属较HP组明显减少,而副杆菌属、乳酸杆菌、梭杆菌、链球菌较HP组增多。体外模拟发酵实验结果显示,HBV-ACLF用药组瘤胃菌、毛螺菌属、拟杆菌、氏菌属相对丰度值较HBV-ACLF未用药组显著升高,而梭杆菌、变形菌较未用药组显著减少(P值均<0.05)。 结论 凉血解毒方能够调节肠道菌群紊乱,恢复肠道菌群多样性,增加优势菌,减少致病菌,这可能是其治疗HBV-ACLF重要的作用机制之一。 Abstract:Objective To investigate the effect of Liangxue Jiedu decoction on intestinal flora in patients with hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF). Methods The patients who were hospitalized and diagnosed with HBV-ACLF in Beijing Ditan Hospital from October 2018 to October 2019 were enrolled, and healthy individuals were enrolled as HP group. High-throughput sequencing was used to screen for the differences in bacterial diversity and species between HBV-ACLF patients and healthy individuals, and differentially expressed bacteria between the two groups were screened out at the phylum and genus levels. With the help of in vitro simulated fermentation experiment, fecal samples were collected from the patients with HBV-ACLF and were then cultured in the medium containing different concentrations of Liangxue Jiedu decoction (0, 10%, 50%, and 100%) for 24 hours, and the changes in intestinal flora were analyzed and compared between the HBV-ACLF treatment group, the HBV-ACLF non-treatment group, and the HP group at the genus level. The t-test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups. Results A total of 10 HBV-ACLF patients were enrolled, with 5 in the HBV-ACLF treatment group and 5 in the HBV-ACLF non-treatment group, and there were 15 individuals in the HP group. Compared with the HP group, the HBV-ACLF non-treatment group had significant reductions in the diversity and abundance of intestinal flora. At the phylum level, Bacteroidetes and Firmicutes were mainly observed in the samples of the HP group, while the HBV-ACLF non-treatment group had a significant reduction in Bacteroidetes and significant increases in Fusobacteria, Proteobacteria, and Fibrobacteres. At the genus level, compared with the HP group, the HBV-ACLF non-treatment group had significant reductions in Ruminococcus, Blautia, and Eubacterium and significant increases in Parabacteroides, Lactobacillus, Fusobacterium, and Streptococcus. The in vitro fermentation experiment showed that compared with the HBV-ACLF non-treatment group, the HBV-ACLF treatment group had significant increases in Ruminococcus, Lachnospira, Bacteroides, and Genusgenus and significant reductions in Fusobacterium and Proteobacteria (all P < 0.05). Conclusion Liangxue Jiedu decoction can regulate intestinal flora disturbance, restore the diversity of intestinal flora, increase dominant bacteria, and reduce pathogenic bacteria, which may be one of its important mechanisms of action in the treatment of HBV-ACLF. -
慢加急性肝衰竭(ACLF)的临床表现以极度乏力、严重消化道症状、黄疸、腹水及凝血功能障碍为主,突出的病理表现为广泛肝细胞坏死[1],病死率极高(50%~90%)[2-4],目前,仍然是世界性难题。在我国,HBV感染所致ACLF最为多见,约占90%左右[5],一旦发生ACLF,目前尚缺乏特效药物。
近年来,人们对肠道菌群的认识逐渐深入。越来越多的研究通过16S rDNA和宏基因组等技术揭示出肠道菌群在慢性肝脏疾病中的改变[6]。北京地坛医院中西医结合中心以诊治肝病为特色,多年来在中医药治疗肝衰竭方面不断的探索和总结,尤其在治疗HBV相关ACLF(HBV-ACLF)方面积累了丰富的经验。基于ACLF“热、毒、瘀”的核心病机及病证症相结合的治疗理念,中西医结合中心创立了“凉血解毒重通腑,健脾化湿顾中焦”的治疗法则,并且在治疗用药方面独具匠心。本研究采用体外模拟发酵实验的方法分析凉血解毒方治疗ACLF的肠道菌群特征,为进一步研究凉血解毒方治疗HBV-ACLF的作用机制奠定基础。
1. 资料与方法
1.1 研究对象
选取2018年10月—2019年10月于本院中西医结合中心住院的HBV-ACLF患者,根据是否加凉血解毒方,分为HBV-ACLF用药组与HBV-ACLF未用药组。另选取同期健康志愿者作为对照(HP组)。
1.2 纳入标准
HBV-ACLF入组标准:(1)诊断标准参考《肝衰竭诊治指南(2012年版)》[7];(2)年龄18~65岁。HP组纳入标准:在本院住院HBV-ACLF患者家属中,招募与疾病对象年龄、性别、地域相匹配的健康志愿者,病史调查显示身体健康,无肝脏和胃肠道疾病史,既往无高血压、糖尿病等其他系统性疾病,6个月内无手术史,肝肾功能无异常。
1.3 排除标准
重叠HAV、HCV、HEV等感染;合并自身免疫性肝炎、药物性肝炎、酒精性肝病及各种遗传代谢性肝病;合并结核感染;HIV感染者;有严重心、肾等并发症或合并其他原发性疾病者;恶性肿瘤患者;上消化道出血;肝肾综合征;妊娠或哺乳期妇女;近1个月内应用过抗生素或益生菌;饮食习惯特殊者,例如素食者。
1.4 材料
1.4.1 主要仪器
移液器(Eppendorf N13462C Eppendorf);小型离心机[ABSON MiFly-6,合肥艾本森科学仪器有限公司(中国)];PCR仪[ABI GeneAmpⓇ9700型,ABI(美国)];测序仪[Illumina Miseq,Illumina(美国)]等。
1.4.2 试剂
凉血解毒方(茵陈30 g、栀子15 g、生地15 g、生黄芪15 g、赤芍30 g、丹参15 g、丹皮15 g、升麻15 g、白术15 g、茯苓15 g、黄芩15 g,批号:190631-1,北京康仁堂药业有限公司订购免煎颗粒剂);DNA抽提试剂盒[E.Z.N.A.Ⓡ Soil DNA Kit,Omega Bio-Tek(美国)];建库试剂盒[NEXTFLEXⓇ Rapid DNA-Seq Kit,Bioo Scientific(美国)];测序试剂盒[MiSeq Reagent Kit v3,Illumina(美国)]等。YCFA培养基(酪胨10 g/L,酵母提取物2.5 g/L,L-半胱氨酸盐酸盐0.8 g/L,血红素0.05 g/L,NaCl 4.5 g/L,CaCl2·6H2O 0.09 g/L,KH2PO4 0.45 g/L,K2HPO4 0.45 g/L,MgSO4·7H2O 0.09 g/L)。
1.5 研究方法和步骤
1.5.1 粪便样本的采集和处理
收集新鲜粪便,30 min内送到实验室,立即置于超净工作台进行处理,挑取中心部位粪便样本(防止外源细菌污染以及肠壁细胞干扰)2 mL短时间内进行体外模拟发酵实验。
1.5.2 凉血解毒方对HBV-ACLF患者肠道菌群干预的体外模拟发酵实验
1.5.2.1 取样、接种与厌氧培养
本研究严格按照上述纳排标准收集了HBV-ACLF患者的新鲜粪便样本,样本采集后短时间内置于超净台并分取4份,每份约0.5 g,然后加入到含0、10%、50%、100%中药的培养基(表 1)中混匀,立即放置在厌氧罐中厌氧培养24 h。
表 1 含有不同凉血解毒方浓度的培养基配方Table 1. Culture medium containing different concentrations of Liangxue Jiedu formula含药浓度 YCFA培养基 凉血解毒方溶液 0 10 mL 0 mL 10% 9 mL 1 mL 50% 5 mL 5 mL 100% 0 mL 10 mL 1.5.2.2 培养后样本处理
(1) 过滤:将无菌漏斗置于50 mL离心管上,用无菌纱布将培养好的粪便菌液进行过滤以滤除粪便沉渣。(2)保菌:取1 mL菌液加入含有500 μL甘油(50%)的离心管中,混匀,-80 ℃冻存。(3)离心:高速离心机离心(12 000 r/min,15 min),分装上清和沉菌,-80 ℃冻存。
1.5.3 粪便及发酵沉菌的DNA提取
采用QIAamp DNA Mini Kit,具体按照试剂说明书进行操作。
1.5.4 粪菌群16S rDNA测序分析
本研究所涉及的Illumina平台MiSeq高通量测序由海欧易生物医学科技有限公司完成。
1.5.5 生物信息学分析
(1) 测序数据处理;(2)操作分类单元(operational taxonomic units,OTU)聚类;(3)分类学分析;(4)多样性分析;(5)样本层次聚类分析;(6)微生物多元变量统计分析。
1.6 统计学方法
使用SPSS 20.0统计软件进行数据分析。符合正态分布的计量资料以x±s表示,两组间比较采用t检验;非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。运用t检验和Wilcoxon检验比较HBV-ACLF未用药组与HP组各个水平菌群差异。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入HBV-ACLF患者10例,其中HBV-ACLF用药组5例,未用药组5例;健康志愿者15例。HP组与HBV-ACLF组的基本信息见表 2。两组年龄、BMI比较差异均无统计学意义(t值分别为2.110、4.256,P值均>0.05)。
表 2 两组研究对象的一般资料Table 2. General information of the two groups of subjects指标 HP组
(n=15)HBV-ACLF组
(n=10)年龄(岁) 41.3±13.2 42.3±10.2 BMI(kg/m2) 20.3±6.2 21.0±7.8 Child-Pugh分级(例) A级 0 B级 6 C级 4 MELD评分 24.7±3.5 TBil(μmol/L) 293.5±103.5 Alb(g/L) 29.5±4.9 PTA(%) 25.0(20.6~30.7) INR 2.2(1.9~2.8) 2.2 测序数据
本研究共收集25个样本,质控之后的空白clean tags数据量分布在38 009~41 802,clean tags经过去除嵌合体得到有效数据valid tags(即最终用于分析的数据)数据量分布在32 184~37 043,valid tags平均长度分布在409.92~432.39 bp,各样本OTU个数分布在300~536。每个样本的tags信息见表 3。
表 3 每个样本信息表Table 3. Information sheet for each sampleSample_ID clean_tags valid_tags valid_percent valid mean
lengthOTU_counts LF.1 41 033 33 826 82.44% 420.39 424 LF.2 41 802 37 043 88.62% 410.31 436 LF.3 40 602 36 443 89.76% 412.07 477 LF.4 39 174 33 063 84.40% 415.78 529 LF.5 40 065 35 353 88.24% 411.78 395 LF.6 40 416 36 302 89.82% 424.90 393 LF.7 39 940 34 481 86.33% 423.41 389 LF.8 41 002 35 022 85.42% 415.13 411 LF.9 39 690 35 844 90.31% 410.98 356 LF.10 40 678 35 815 88.05% 416.40 446 H.1 38 761 34 957 90.19% 425.93 315 H.2 40 329 34 492 85.53% 427.77 385 H.3 38 753 33 801 87.22% 429.53 357 H.4 38 848 34 017 87.56% 426.16 300 H.5 38 462 35 138 91.36% 432.39 405 H.6 38 194 32 350 84.70% 431.75 355 H.7 39 211 34 487 87.95% 410.10 439 H.8 41 783 35 965 86.08% 409.92 442 H.9 38 162 32 184 84.34% 413.61 449 H.10 40 125 36 203 90.23% 416.68 481 H.11 38 234 33 521 87.67% 414.28 524 H.12 38 990 34 198 87.71% 419.20 453 H.13 39 733 34 258 86.22% 416.84 451 H.14 40 022 33 981 84.91% 413.43 447 H.15 38 009 32 751 86.17% 413.66 536 注:Sample_ID,病例编码。 2.3 HBV-ACLF未用药组与HP组肠道菌群多样性分析
2.3.1 Alpha多样性
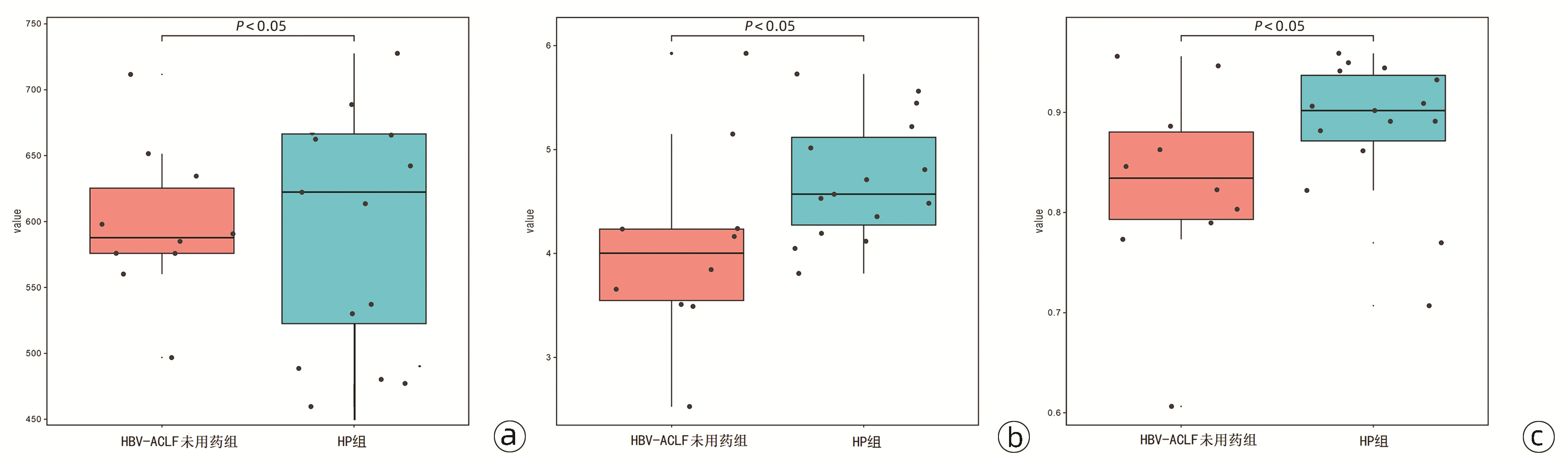
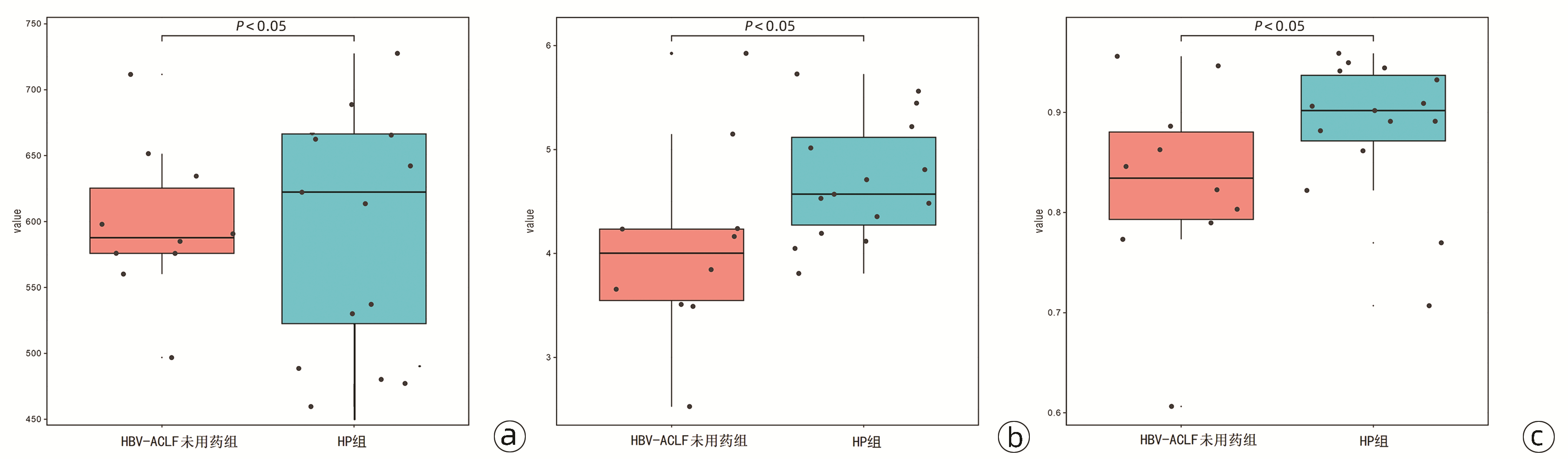
结果显示,HBV-ACLF患者肠道中微生物物种的丰富度降低,菌群多样性降低。(1)衡量菌群丰度的Chao指数:HBV-ACLF未用药组的Chao指数显著低于HP组[587.5(575.0~625.0) vs 623.5(524.0~673.5),Z=3.876,P=0.036](图 1a)。(2)衡量群落多样性的Shannon指数:HBV-ACLF未用药组的Shannon指数显著低于HP组[4.00(3.52~4.23) vs 4.62(4.24~5.13),Z=2.876,P=0.028](图 1b)。(3)衡量菌群多样性的Simpson指数:HBV-ACLF未用药组的Simpson指数显著低于HP组[0.84(0.78~0.88) vs 0.90(0.87~0.94),Z=3.563,P=0.049](图 1c)。
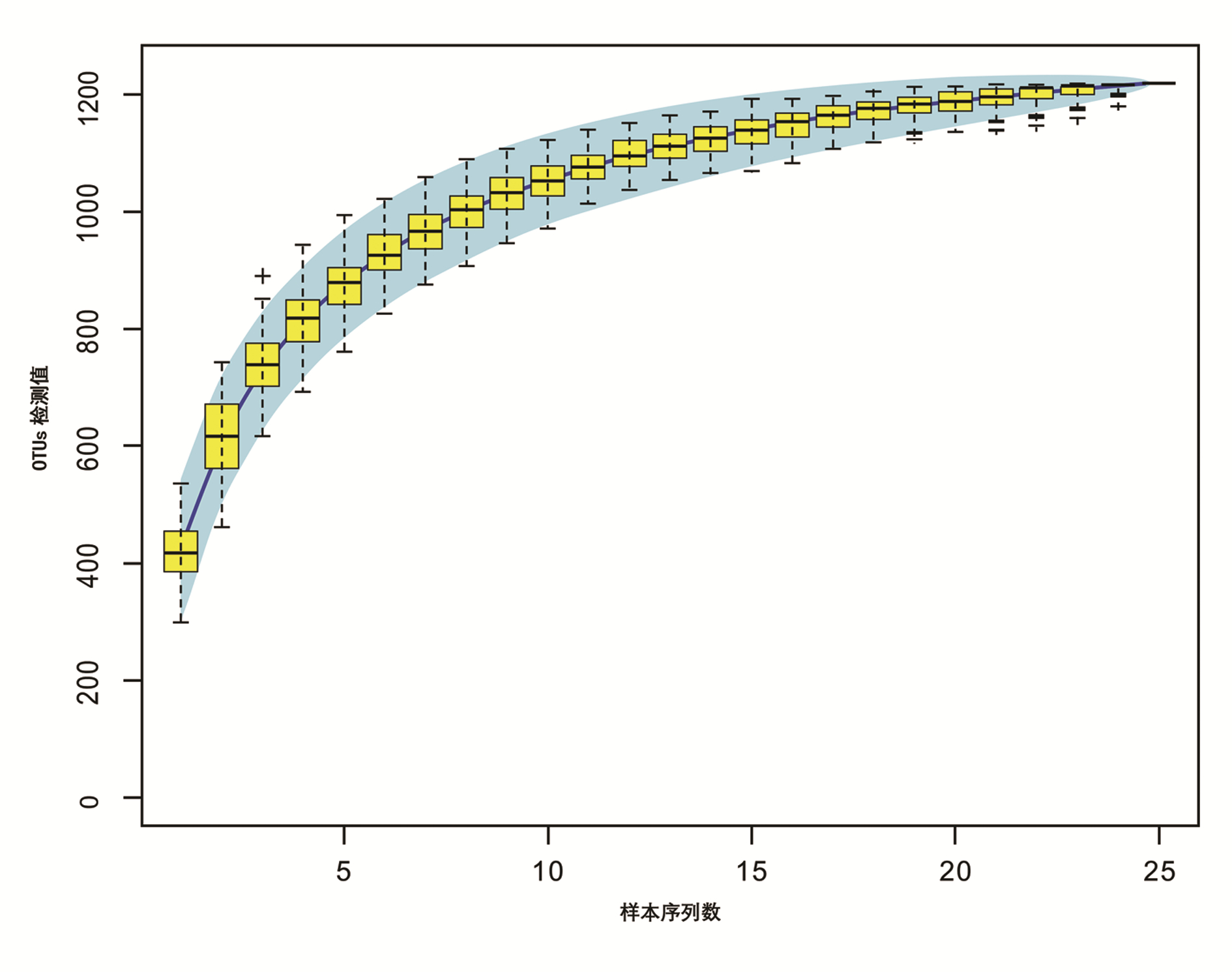
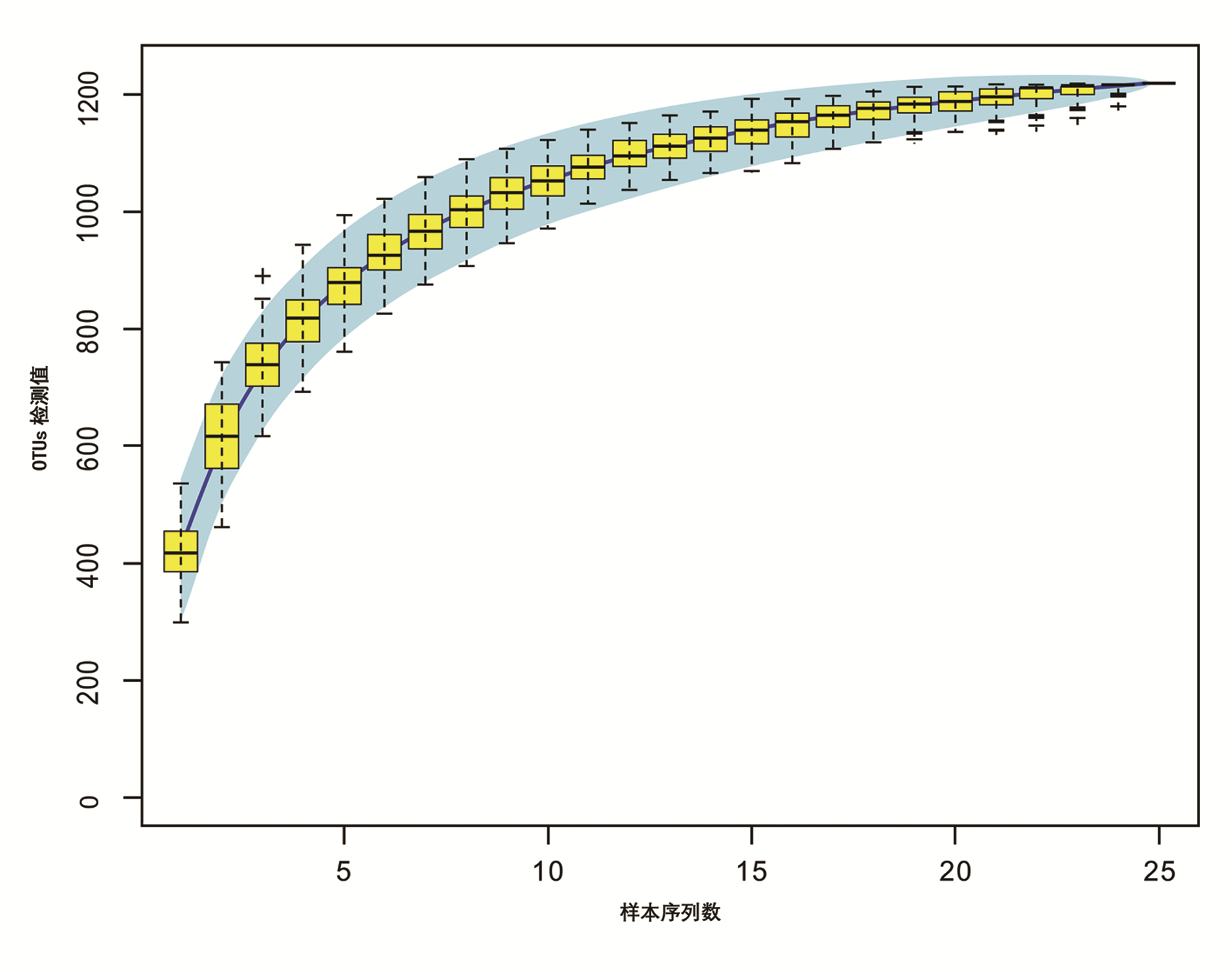
2.3.2 物种累积曲线
物种累积曲线显示,随着样本量的增加,曲线趋于平缓,表明该研究中的物种并不会随样本量的增加而显著增多,测序样本量足以反映样本中的绝大多数微生物信息(图 2)。
2.4 HBV-ACLF未用药组与HP组菌群比较
HBV-ACLF未用药组与HP组之间各个分类水平均存在一定的差异,运用OTU进行分析,可反映在门、纲、目、种、属等各分类水平上各样本的物种分类情况。在门水平,采用t检验,两组之间存在3种菌群差异[拟杆菌门(Bacteroidetes)、厚壁菌门(Firmicutes)和变形菌门(Proteobacteria)];采用Wilcoxon检验,两组之间存在2种菌群差异[拟杆菌门(Bacteroidetes)和厚壁菌门(Firmicutes)];尤其在属水平,采用Wilcoxon检验,两组之间存在77种菌群差异。具体差异物种统计汇总见表 4。
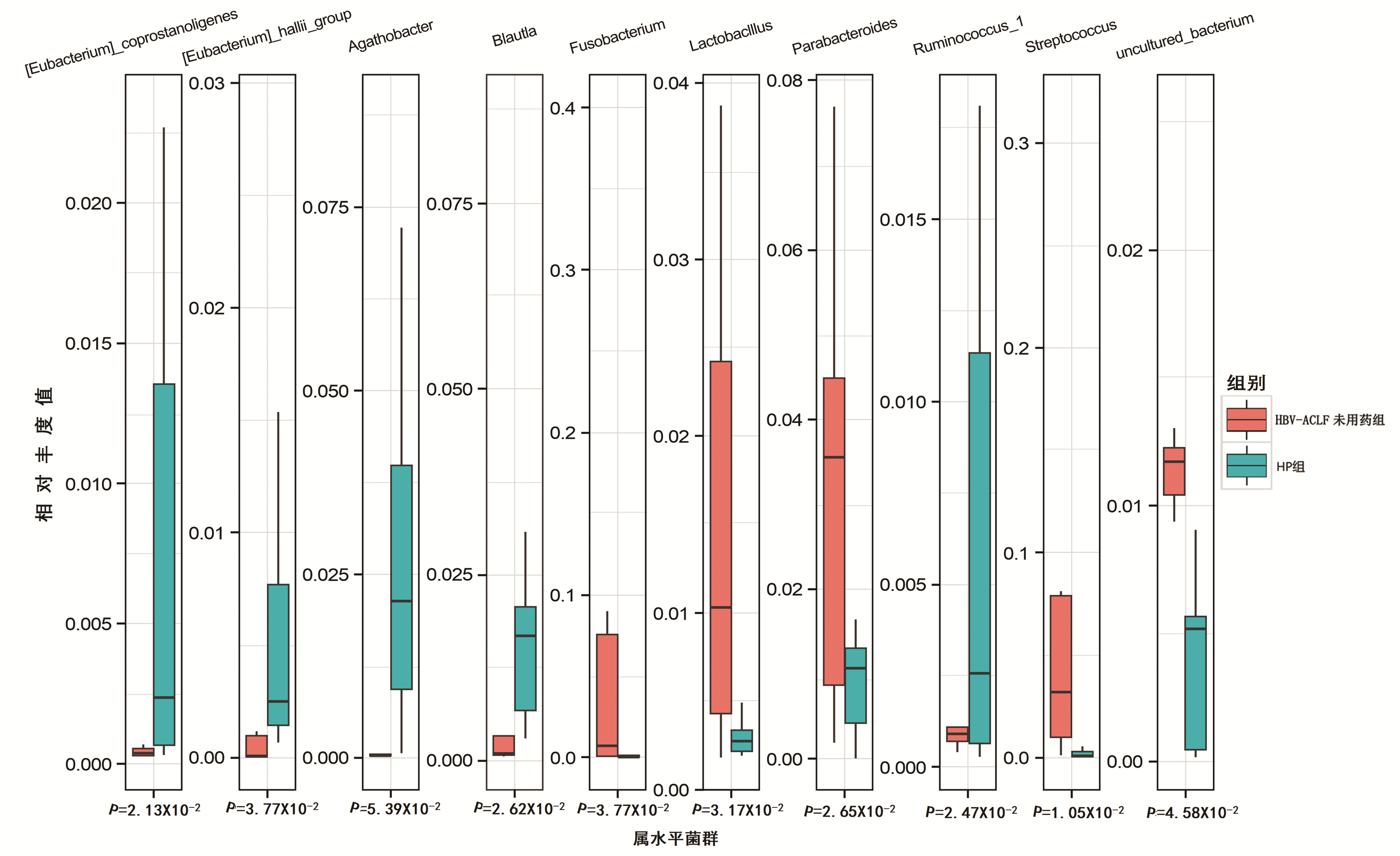
表 4 各个水平菌群比较Table 4. Comparison of microflora at different levels方法 OTU 门 纲 目 科 属 种 t检验 142 3 6 13 20 39 10 Wilcoxon检验 263 2 6 13 34 77 22 根据OTU分析,在门分类水平和属分类水平上通过柱状图反映肠道菌种情况,可以呈现不同物种在每例患者所占的比例以及两组不同的菌种。结果显示,HP组每个样本均以拟杆菌门(Bacteroidetes)和厚壁菌门(Firmicutes)为主,HBV-ACLF未用药组拟杆菌门(Bacteroidetes)明显减少,梭杆菌门(Fusobacterio)、变形菌门(Proteobacteria)、纤维杆菌门(Fibrobacteres)增多(图 3)。HBV-ACLF未用药组瘤胃菌(Ruminococus)、布劳特菌属(Blautia)、真杆菌属(Eubacterium)较HP组明显减少,而副杆菌属(Parabacteroides)、乳酸杆菌(Lactobacllus)、梭杆菌(Fusobacterio)、链球菌(Streptococcus)较HP组增多(图 4)。
 图 3 门水平菌群比较注:a,不同门分类在每个病例中所占的比例;b,HBV-ACLF未用药组和HP组3种门分类的比较。Actinobacteris,放线杆菌门;Epsilonbacteraeota,表皮菌门;Tenericutes,马鞭菌门;Gemmatimonadetes,芽单孢菌门;Cynobacteria,嗜肺杆菌门;Acidobacteria,酸杆菌门;Nitrospirae,硝基绣线菌门;Spirochaetes,螺旋体菌门;Deferribacteres,脱铁杆菌门;Laescibacteria,不动杆菌门;Fibrobacteres,纤维杆菌门;Fusobacterio,梭杆菌门;Proteobacteria,变形菌门。Figure 3. Phyla level flora ratio
图 3 门水平菌群比较注:a,不同门分类在每个病例中所占的比例;b,HBV-ACLF未用药组和HP组3种门分类的比较。Actinobacteris,放线杆菌门;Epsilonbacteraeota,表皮菌门;Tenericutes,马鞭菌门;Gemmatimonadetes,芽单孢菌门;Cynobacteria,嗜肺杆菌门;Acidobacteria,酸杆菌门;Nitrospirae,硝基绣线菌门;Spirochaetes,螺旋体菌门;Deferribacteres,脱铁杆菌门;Laescibacteria,不动杆菌门;Fibrobacteres,纤维杆菌门;Fusobacterio,梭杆菌门;Proteobacteria,变形菌门。Figure 3. Phyla level flora ratio 图 4 属水平菌群比较注:Eubacterium coprostanoligenes,粪甾烷内酯真杆菌属;Eubacterium hallii-group,真杆菌属;Agathobacter,阴沟杆菌属;Agathobacter Blautla,布劳特菌属;Fusobacterium,梭杆菌属;Lactobacllus,乳酸杆菌;Parabacteroides,副杆菌属;Ruminococcus,瘤胃菌;Streptococcus链球菌属;uncultured-bacterium, 未增养菌。Figure 4. Comparison of microflora at genus level
图 4 属水平菌群比较注:Eubacterium coprostanoligenes,粪甾烷内酯真杆菌属;Eubacterium hallii-group,真杆菌属;Agathobacter,阴沟杆菌属;Agathobacter Blautla,布劳特菌属;Fusobacterium,梭杆菌属;Lactobacllus,乳酸杆菌;Parabacteroides,副杆菌属;Ruminococcus,瘤胃菌;Streptococcus链球菌属;uncultured-bacterium, 未增养菌。Figure 4. Comparison of microflora at genus level2.5 肠道菌群聚类分析
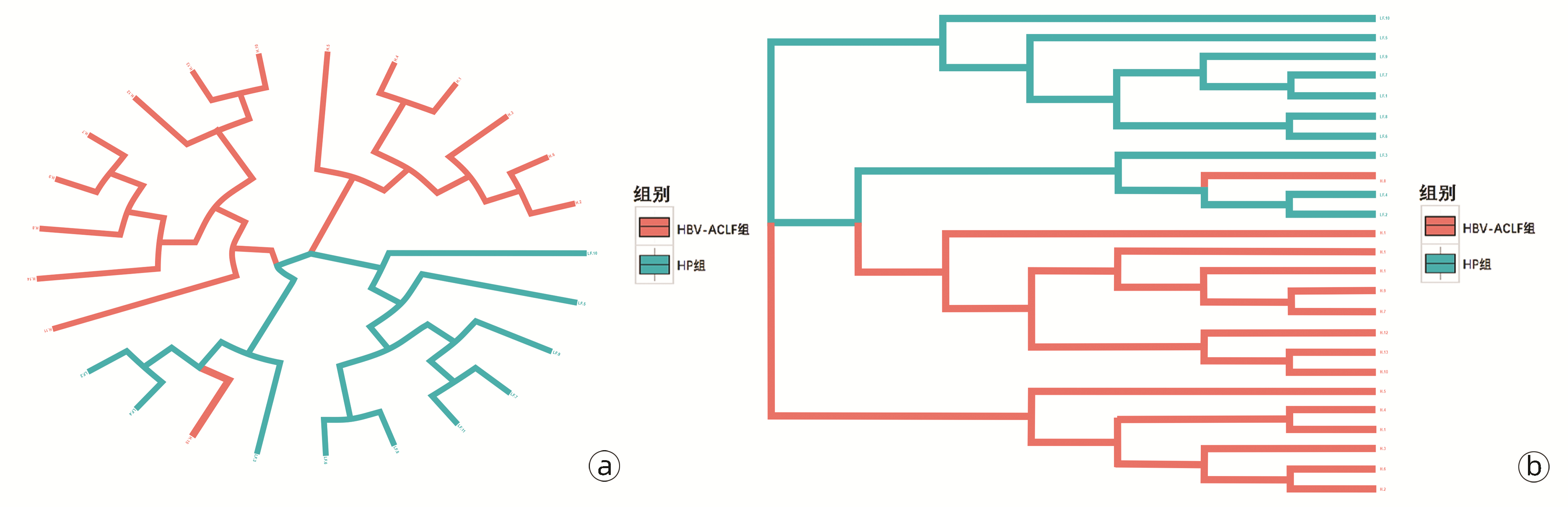
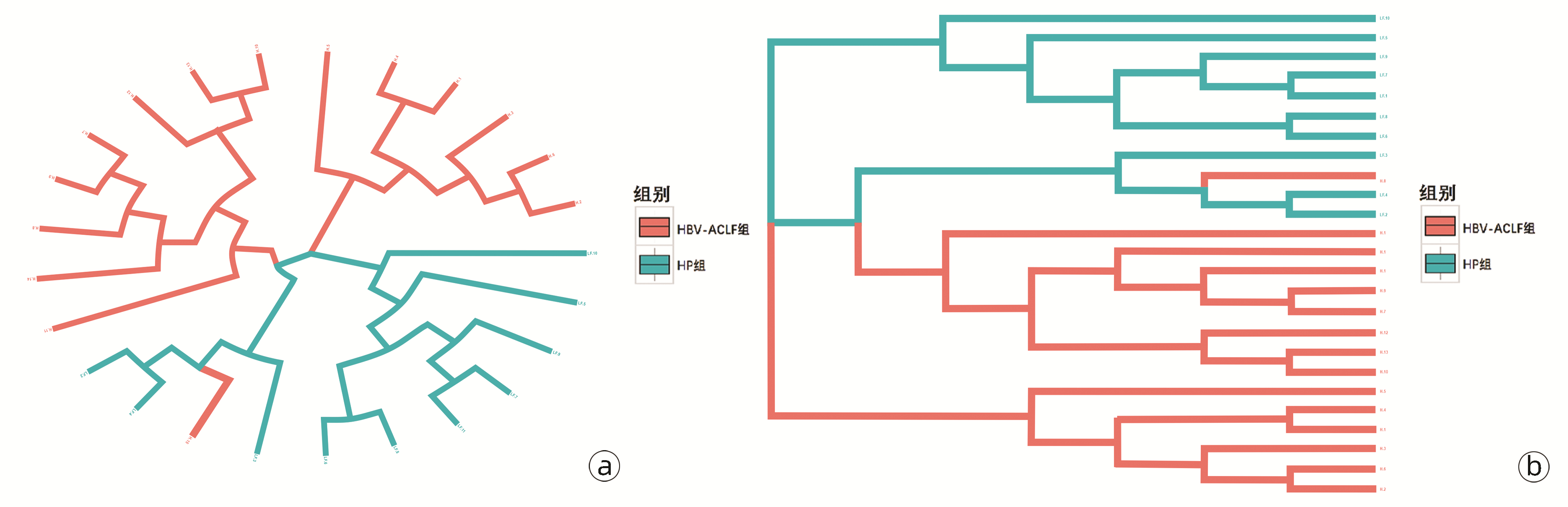
聚类分析结果整体趋势表现为病种相同的研究对象大致聚类到一起,提示病种相同者肠道菌群构成相似,即HBV-ACLF患者肠道菌群构成相似,HP组肠道菌群构成相似(图 5)。
2.6 凉血解毒方对HBV-ACLF患者肠道菌群多样性的影响
采用Shannon指数衡量HBV-ACLF患者肠道菌群多样性。结果显示,HBV-ACLF用药组(凉血解毒方含药浓度10%、50%、100%)Shannon指数[5.00(4.80~5.20)、5.30(4.78~6.12)、5.50(4.86~6.28)]较HBV-ACLF未用药组[3.80(3.00~4.12)]显著升高(Z值分别为2.443、3.374、3.235,P值分别为0.013、0.039、0.018),且随着含药浓度升高,Shannon指数逐渐升高,表明凉血解毒方能够恢复HBV-ACLF患者肠道菌群的多样性,且含药浓度越高,效果越明显(图 6)。
2.7 凉血解毒方对差异性菌属的影响
为了解凉血解毒方对HBV-ACLF患者肠道菌群构成的影响,从属分类水平进行了比较分析,结果显示:HBV-ACLF用药组瘤胃菌(Ruminococus)、毛螺菌属(Lachnospira)、拟杆菌(Bacteroidetes)、氏菌属(Genusgenus)相对丰度值较HBV-ACLF未用药组显著升高(图 7b、d、f、h),而梭杆菌(Fusobacterio)、变形菌(Proteobacteria)较未用药组显著降低(图 7j、l) (P值均<0.05)。
 图 7 凉血解毒方对菌属的影响注:a、c、e、g、i、k,HP组与HBV-ACLF未用药组瘤胃菌(Ruminococus)、毛螺菌属(Lachnospira)、拟杆菌(Bacteroidetes)、氏菌属(Genusgenus)、梭杆菌(Fusobacterio)、变形菌(Proteobacteria)菌群变化;b、d、f、h、j、l,HBV-ACLF不同凉血解毒方含药浓度瘤胃菌(Ruminococus)、毛螺菌属(Lachnospira)、拟杆菌(Bacteroidetes)、氏菌属(Genusgenus)、梭杆菌(Fusobacterio)、变形菌(Proteobacteria)菌群变化。Figure 7. Effect of Liangxue Jiedu Fang on fungi
图 7 凉血解毒方对菌属的影响注:a、c、e、g、i、k,HP组与HBV-ACLF未用药组瘤胃菌(Ruminococus)、毛螺菌属(Lachnospira)、拟杆菌(Bacteroidetes)、氏菌属(Genusgenus)、梭杆菌(Fusobacterio)、变形菌(Proteobacteria)菌群变化;b、d、f、h、j、l,HBV-ACLF不同凉血解毒方含药浓度瘤胃菌(Ruminococus)、毛螺菌属(Lachnospira)、拟杆菌(Bacteroidetes)、氏菌属(Genusgenus)、梭杆菌(Fusobacterio)、变形菌(Proteobacteria)菌群变化。Figure 7. Effect of Liangxue Jiedu Fang on fungi与HP组相比,HBV-ACLF未用药组瘤胃菌(Ruminococus)、毛螺菌属(Lachnospira)、拟杆菌(Bacteroidetes)、氏菌属(Genusgenus)相对丰度值明显降低(图 7a、c、e、g),梭杆菌(Fusobacterio)、变形菌(Proteobacteria)相对丰度值明显升高(图 7i、k)(P值均<0.05)。
3. 讨论
ACLF是在慢性肝病基础上,引起短期内急性或者亚急性肝功能失代偿的临床症候群,病情危重,短期病死率极高,预后差[8]。目前关于ACLF的病理机制尚不清楚,但近几年越来越多研究表明肠道细菌易位是引起ACLF很重要的原因[9]。然而目前缺乏ACLF患者肠道菌群相关的研究。
本研究运用16S rDNA通量测序法对HBV-ACLF患者和健康人群的肠道菌群进行测序分析,结果显示两组人群中肠道菌群具有显著差异。肠道菌群的多样性和丰度是衡量肠道菌群是否平衡的重要指标[10]。本研究发现,衡量患者肠道菌群丰度的Chao指数在HBV-ACLF患者中显著降低;衡量多样性的Shannon指数在HBV-ACLF患者中显著降低。进一步分析肠道菌群门、属水平的分类学特征性改变,发现HBV-ACLF患者与健康人群有显著差异。HBV-ACLF未用药组肠道菌群发生了广泛的群落改变。大量潜在致病菌如副杆菌属(Parabacteroides)、乳酸杆菌(Lactobacllus)、梭杆菌(Fusobacterio)、链球菌(Streptococcus)等在HBV-ACLF患者中显著升高。
本研究还观察到HBV-ACLF患者较健康人群中相对丰度降低的菌属,如瘤胃菌(Ruminococus)、真杆菌属(Eubacterium),其中,瘤胃球菌属是已知的产生短链脂肪酸的细菌[11]。短链脂肪酸能够发挥保护肠道的作用,其保护机制可能与促进肠道绒毛的生长修复,为肠道黏膜提供营养,增强肠道免疫以及促进微生物生长,抑制致病菌相关[12-13]。
本研究采用体外模拟发酵实验,进一步分析了凉血解毒方对HBV-ACLF患者肠道菌群构成的影响,结果发现:HBV-ACLF用药组瘤胃菌(Ruminococus)、毛螺菌属(Lachnospira)、拟杆菌(Bacteroidetes)、氏菌属(Genusgenus)较未用药组显著升高,而梭杆菌(Fusobacterio)、变形菌(Proteobacteria)较未用药组显著减少(P值均<0.05)。研究结果表明,凉血解毒方能有效增加肠道优势菌,减少致病菌,从而恢复肠道正常菌群生物多样性及丰度。毛螺菌、氏菌属亦属于短链脂肪酸细菌,同样具有修复肠道黏膜的作用[14]。本研究中HBV-ACLF组两种菌属丰度显著降低,凉血解毒方干预后明显增多。拟杆菌[15]是人体肠道内常见的健康菌群之一,具有分解碳水化合物的作用,其减少可引起肠道消化不良。本研究中发现HBV-ACLF组患者拟杆菌明显减少,应用凉血解毒方干预后,拟杆菌明显增加,由此推测,凉血解毒方改善临床症状的作用机制可能与调节肠道内拟杆菌水平相关。此外,本研究应用凉血解毒方干预后,HBV-ACLF用药组较未用药组梭杆菌属及变形菌属显著减少,提示减少致病菌,从而有效降低炎症反应,可能是凉血解毒方作用机制之一。
综上所述,本研究发现HBV-ACLF患者肠道菌群较正常健康人群肠道菌群结构丰度及多样性显著下降;HBV-ACLF患者肠道菌群区别于正常人群,表现为潜在致病菌异常升高,潜在有益菌异常降低,二者倒置导致肠道菌群紊乱;紊乱的肠道菌群可能通过细菌易位增加感染风险、炎症过度活化,故在HBV-ACLF发生发展过程中扮演重要角色。进一步体外模拟发酵实验结果表明,凉血解毒方能有效增加优势菌,减少致病菌,从而达到调节肠道菌群紊乱的作用,为进一步研究凉血解毒方治疗HBV-ACLF的作用机制奠定基础。
-
注:a,不同门分类在每个病例中所占的比例;b,HBV-ACLF未用药组和HP组3种门分类的比较。Actinobacteris,放线杆菌门;Epsilonbacteraeota,表皮菌门;Tenericutes,马鞭菌门;Gemmatimonadetes,芽单孢菌门;Cynobacteria,嗜肺杆菌门;Acidobacteria,酸杆菌门;Nitrospirae,硝基绣线菌门;Spirochaetes,螺旋体菌门;Deferribacteres,脱铁杆菌门;Laescibacteria,不动杆菌门;Fibrobacteres,纤维杆菌门;Fusobacterio,梭杆菌门;Proteobacteria,变形菌门。
图 3 门水平菌群比较
Figure 3. Phyla level flora ratio
注:Eubacterium coprostanoligenes,粪甾烷内酯真杆菌属;Eubacterium hallii-group,真杆菌属;Agathobacter,阴沟杆菌属;Agathobacter Blautla,布劳特菌属;Fusobacterium,梭杆菌属;Lactobacllus,乳酸杆菌;Parabacteroides,副杆菌属;Ruminococcus,瘤胃菌;Streptococcus链球菌属;uncultured-bacterium, 未增养菌。
图 4 属水平菌群比较
Figure 4. Comparison of microflora at genus level
注:a、c、e、g、i、k,HP组与HBV-ACLF未用药组瘤胃菌(Ruminococus)、毛螺菌属(Lachnospira)、拟杆菌(Bacteroidetes)、氏菌属(Genusgenus)、梭杆菌(Fusobacterio)、变形菌(Proteobacteria)菌群变化;b、d、f、h、j、l,HBV-ACLF不同凉血解毒方含药浓度瘤胃菌(Ruminococus)、毛螺菌属(Lachnospira)、拟杆菌(Bacteroidetes)、氏菌属(Genusgenus)、梭杆菌(Fusobacterio)、变形菌(Proteobacteria)菌群变化。
图 7 凉血解毒方对菌属的影响
Figure 7. Effect of Liangxue Jiedu Fang on fungi
表 1 含有不同凉血解毒方浓度的培养基配方
Table 1. Culture medium containing different concentrations of Liangxue Jiedu formula
含药浓度 YCFA培养基 凉血解毒方溶液 0 10 mL 0 mL 10% 9 mL 1 mL 50% 5 mL 5 mL 100% 0 mL 10 mL 表 2 两组研究对象的一般资料
Table 2. General information of the two groups of subjects
指标 HP组
(n=15)HBV-ACLF组
(n=10)年龄(岁) 41.3±13.2 42.3±10.2 BMI(kg/m2) 20.3±6.2 21.0±7.8 Child-Pugh分级(例) A级 0 B级 6 C级 4 MELD评分 24.7±3.5 TBil(μmol/L) 293.5±103.5 Alb(g/L) 29.5±4.9 PTA(%) 25.0(20.6~30.7) INR 2.2(1.9~2.8) 表 3 每个样本信息表
Table 3. Information sheet for each sample
Sample_ID clean_tags valid_tags valid_percent valid mean
lengthOTU_counts LF.1 41 033 33 826 82.44% 420.39 424 LF.2 41 802 37 043 88.62% 410.31 436 LF.3 40 602 36 443 89.76% 412.07 477 LF.4 39 174 33 063 84.40% 415.78 529 LF.5 40 065 35 353 88.24% 411.78 395 LF.6 40 416 36 302 89.82% 424.90 393 LF.7 39 940 34 481 86.33% 423.41 389 LF.8 41 002 35 022 85.42% 415.13 411 LF.9 39 690 35 844 90.31% 410.98 356 LF.10 40 678 35 815 88.05% 416.40 446 H.1 38 761 34 957 90.19% 425.93 315 H.2 40 329 34 492 85.53% 427.77 385 H.3 38 753 33 801 87.22% 429.53 357 H.4 38 848 34 017 87.56% 426.16 300 H.5 38 462 35 138 91.36% 432.39 405 H.6 38 194 32 350 84.70% 431.75 355 H.7 39 211 34 487 87.95% 410.10 439 H.8 41 783 35 965 86.08% 409.92 442 H.9 38 162 32 184 84.34% 413.61 449 H.10 40 125 36 203 90.23% 416.68 481 H.11 38 234 33 521 87.67% 414.28 524 H.12 38 990 34 198 87.71% 419.20 453 H.13 39 733 34 258 86.22% 416.84 451 H.14 40 022 33 981 84.91% 413.43 447 H.15 38 009 32 751 86.17% 413.66 536 注:Sample_ID,病例编码。 表 4 各个水平菌群比较
Table 4. Comparison of microflora at different levels
方法 OTU 门 纲 目 科 属 种 t检验 142 3 6 13 20 39 10 Wilcoxon检验 263 2 6 13 34 77 22 -
[1] SCHWEITZER A, HORN J, MIKOLAJCZYK RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013[J]. Lancet, 2015, 386(10003): 1546-1555. DOI: 10.1016/S0140-6736(15)61412-X. [2] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: A 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. [3] ALAM A, CHUN SUEN K, MA D. Acute-on-chronic liver failure: Recent update[J]. J Biomed Res, 2017, 31(3): 1-18. DOI: 10.7555/JBR.31.20160060. [4] ZHANG HL, YU LX, YANG W, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats[J]. J Hepatol, 2012, 57(4): 803-812. DOI: 10.1016/j.jhep.2012.06.011. [5] ARULRAJ R, NEUBERGER J. Liver transplantation: Filling the gap between supply and demand[J]. Clin Med (Lond), 2011, 11(2): 194-198. DOI: 10.7861/clinmedicine.11-2-194. [6] SOL C, GUILLY S, DA SILVA K, et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative met- agenomics: Relationship with acute - on - chronic liver failure and prognosis[J]. Gastroenterology, 2021, 160(1): 206-218. e13. DOI: 10.1053/j.gastro.2020.08.054. [7] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure[J]. Inter J Epidemiol Infect Dis, 2013, 40(1): 1-7. DOI: 10.3760/cma.j.issn.1673-4149.2013.01.001.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2012年版)[J]. 国际流行病学传染病学杂志, 2013, 40(1): 1-7. DOI: 10.3760/cma.j.issn.1673-4149.2013.01.001. [8] LIAO W, TANG Y, FAN WH, et al. Analysis of intestinal flora imbalance and its influencing factors in patients with acute-on-chronic liver failure[J]. Traum Crit Med, 2021, 9(5): 394-396. DOI: 10.16048/j.issn.2095-5561.2021.05.17.廖威, 唐艳, 范文瀚, 等. 慢加急性肝衰竭患者肠道菌群失调情况及影响因素分析[J]. 创伤与急危重病医学, 2021, 9(5): 394-396. DOI: 10.16048/j.issn.2095-5561.2021.05.17. [9] DALAL R, MCGEE RG, RIORDAN SM, et al. Probiotics for people with hepatic encephalopathy[J]. Cochrane Database Syst Rev, 2017, 2(2): CD008716. DOI: 10.1002/14651858.CD008716.pub3. [10] ALDAD A, MORENO RG, SHIBAYAYANA M, et al. Protective effects of allopurinol against acute liver damageand cirrhosis induced by carbon tetrachloride: Modulation of NF-κB, cytokine production and oxidative stress[J]. Biochim Biophys Acta, 2012, 1820(2): 65-75. [11] LOUIS P, YOUNG P, HOLTROP G, et al. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA: Acetate CoA-transferase gene[J]. Environ Microbiol, 2010, 12(2): 304-314. DOI: 10.1111/j.1462-2920.2009.02066.x. [12] MOOKERJEE RP, PAVESI M, THOMSEN KL, et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure[J]. J Hepatol, 2016, 64(3): 574-582. DOI: 10.1016/j.jhep.2015.10.018. [13] WOODHOUSE CA, PATEL VC, SINGANAYAGAM A, et al. Review article: The gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease[J]. Aliment Pharmacol Ther, 2018, 47(2): 192-202. DOI: 10.1111/apt.14397. [14] XIE G, ZHONG W, LI H, et al. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption[J]. FASEB J, 2013, 27(9): 3583-3593. DOI: 10.1096/fj.13-231860. [15] TELTSCHIK Z, WIEST R, BEISNER J, et al. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense[J]. Hepatology, 2012, 55(4): 1154-1163. DOI: 10.1002/hep.24789. -




 PDF下载 ( 5458 KB)
PDF下载 ( 5458 KB)

 下载:
下载:




 下载:
下载: