能量代谢指标与失代偿期乙型肝炎肝硬化患者短期内自发性细菌性腹膜炎发生风险的相关性
DOI: 10.3969/j.issn.1001-5256.2022.06.018
Association of energy metabolic markers with the short-term risk of spontaneous bacterial peritonitis in patients with decompensated hepatitis B virus-related liver cirrhosis
-
摘要:
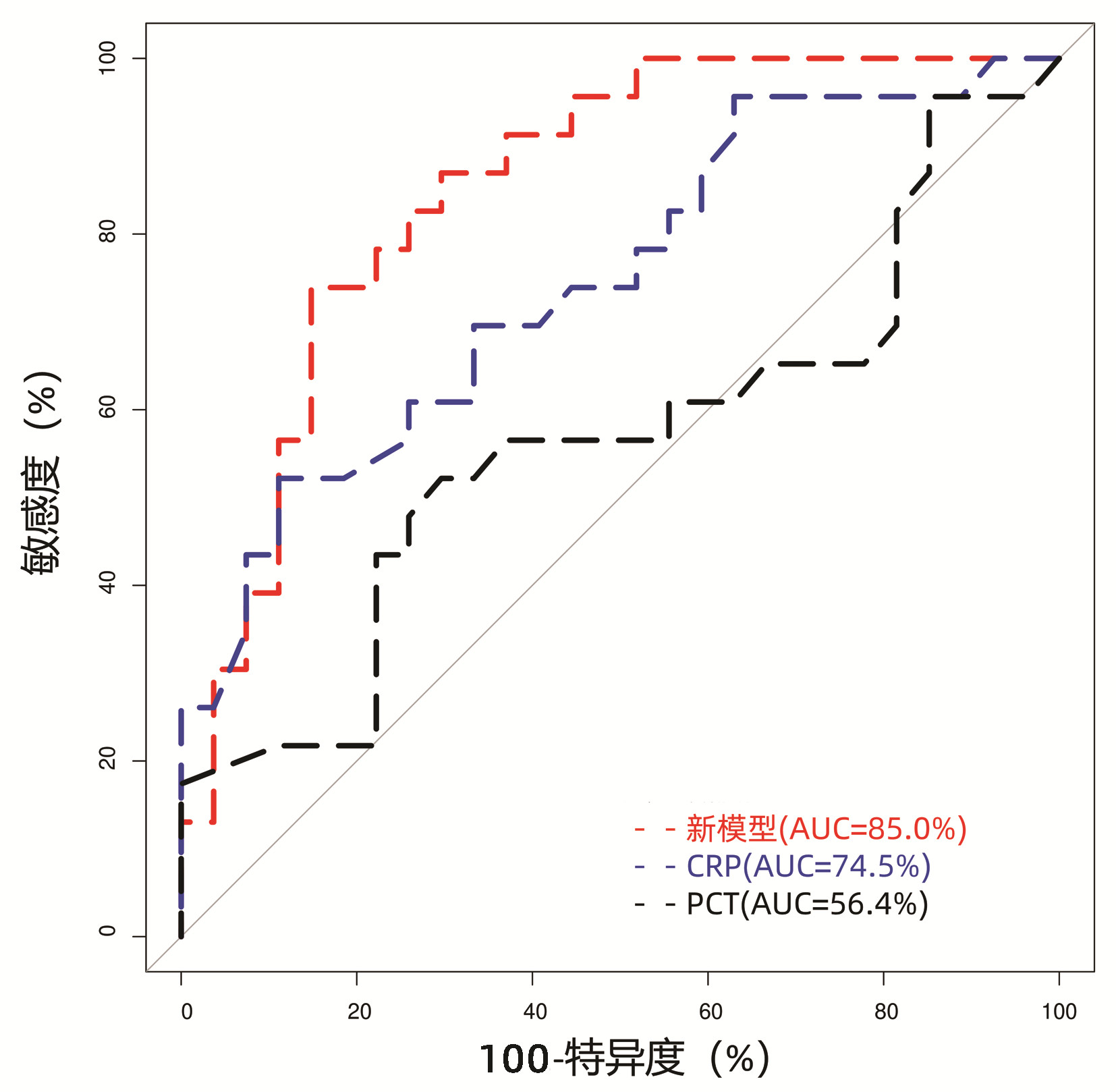
目的 探讨失代偿期乙型肝炎肝硬化(HBV-LC)患者的间接能量代谢指标与自发性细菌性腹膜炎(SBP)发生风险的相关性。 方法 回顾性分析2017年11月—2019年11月福建医科大学孟超肝胆医院收治住院的失代偿期HBV-LC患者的临床资料,比较住院后2周内发生SBP和无SBP患者的基线临床参数和能量代谢指标的差异,采用logistic多因素回归分析患者发生SBP的相关风险因素。符合正态分布的计量资料两组间比较采用t检验;非正态分布的计量资料两组间比较采用Kruskal-Wallis H秩和检验。计数资料两组间比较采用χ2检验或Fisher精确检验。绘制受试者工作特征曲线(ROC曲线)分析新建立logistic回归模型的诊断效应,以最大Youden指数对应的点为模型的截断值,采用DeLong检验比较ROC曲线下面积(AUC)。 结果 纳入失代偿期HBV-LC患者50例,住院后2周内发生SBP患者23例(46%),无SBP患者27例(54%)。SBP患者的甘油三酯、前白蛋白以及凝血酶原活动度(PTA)均显著低于无SBP患者(P值均<0.05);而SBP患者的国际标准化比值、C反应蛋白(CRP)和终末期肝病模型评分则显著高于无SBP患者(P值均<0.05)。比较两组患者的基线能量代谢指标:SBP患者的呼吸熵(RQ)和碳水化合物氧化率(CHO)均较无SBP患者低[RQ: 0.79(0.76~0.86) vs 0.85(0.79~0.91), P=0.041; CHO: 20.50%(15.25%~41.05%) vs 41.6%(22.25%~68.05%), P=0.041]。logistic多因素回归分析提示PTA为失代偿期HBV-LC患者住院期间发生SBP的独立危险因素(比值比=0.004,P=0.008),并以PTA、CRP、RQ和CHO等变量构建回归模型,模型AUC为85.0%,当曲线的Youden指数为最大值时,模型截断值为0.60,特异度为85.19%,敏感度为73.91%,模型的区分度优于CRP(AUC=74.5%, P=0.049)和PCT(AUC=56.4%, P<0.01)。 结论 失代偿期HBV-LC患者短期内发生SBP的患者能量代谢指标RQ和CHO明显降低,结合PTA、CRP和CHO/RQ比值等指标,有助于临床医师早期判断SBP的高风险患者,并加强对高风险患者的营养支持。 Abstract:Objective To investigate the association of energy metabolic markers with the risk of spontaneous bacterial peritonitis (SBP) in patients with decompensated hepatitis B virus-related liver cirrhosis (HBV-LC). Methods A retrospective analysis was performed for the clinical data of the patients with decompensated HBV-LC who were admitted to Mengchao Hepatobiliary Hospital of Fujian Medical University from November 2017 to November 2019, and baseline clinical parameters and energy metabolic markers were compared between the patients with SBP and those without SBP within 2 weeks after admission. A multivariate logistic regression analysis was performed to investigate the risk factors for SBP. The t-test was used for comparison of normally distributed continuous data between two groups, and the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between two groups; the Fisher's exact test was used for comparison of categorical data between two groups. The receiver operating characteristic (ROC) curve was plotted to evaluate the diagnostic efficiency of the newly established logistic regression model, and with the corresponding point of Youden index as the cut-off value, the DeLong test was used to compare the area under the ROC curve (AUC). Results A total of 50 patients with decompensated HBV-LC were included, among whom 23 (46%) developed SBP within 2 weeks after admission and 27 (54%) had no SBP during hospitalization. Compared with the non-SBP patients, the SBP patients had significantly lower triglyceride, prealbumin, and prothrombin time activity (PTA) and significantly higher international normalization ratio, C-reactive protein (CRP), and Model for End-Stage Liver Disease score (all P < 0.05). Comparison of baseline energy metabolic markers showed that compared with the non-SBP patients, the SBP patients had significantly lower respiratory quotient (RQ) [0.79(0.76-0.86) vs 0.85(0.79-0.91), P=0.041] and carbohydrate oxidation (CHO) rate [20.50%(15.25%-41.05%) vs 41.6%(22.25%-68.05%), P=0.041]. The multivariate logistic regression analysis showed that PTA was an independent risk factor for SBP in the patients with decompensated HBV-LC during hospitalization (odd ratio=0.004, P=0.008), and the regression model established based on the variables including PTA, CRP, RQ, and CHO had an AUC of 85.0% and a cut-off value of 0.60 at the maximum Youden index, with a specificity of 85.19% and a sensitivity of 73.91%, suggesting that this model had a better discriminatory ability than CRP (AUC=74.5%, P=0.049) and procalcitonin (AUC=56.4%, P < 0.01). Conclusion There are significant reductions in the energy metabolic markers RQ and CHO in the patients with decompensated HBV-LC who develop SBP within a short term, and their combination with PTA, CRP, and CHO/RQ ratio can help clinicians identify the patients at a high risk of SBP in the early stage and enhance nutrition support for such patients. -
Key words:
- Hepatitis B virus /
- Liver Cirrhosis /
- Peritonitis /
- Energy Metabolism
-
表 1 基线特征比较
Table 1. Comparison of baseline characteristics
指标 所有患者(n=50) SBP组(n=23) 无SBP组(n=27) 统计值 P值 性别[例(%)] 0.689 女 7(14) 4(17) 3(11) 男 43(86) 19(83) 24(89) 肝衰竭[例(%)] χ2=2.7 0.103 是 21(42) 13(57) 8(30) 否 29(58) 10(43) 19(70) 年龄(岁) 49.98±11.61 53.13±11.62 47.30±11.11 t=1.8 0.078 身高(cm) 168(165~170) 165(163~170) 168(165~170) H=230.5 0.117 体质量(kg) 62.1(55.2~72.0) 63.0(53.7~71.0) 61.0(55.9~71.9) H=304.5 0.915 体表面积(m2) 1.73±0.17 1.71±0.16 1.74±0.18 H=-0.7 0.517 BMI[例(%)] 0.793 下降 5(10) 2(9) 3(11) 正常 32(64) 14(61) 18(67) 超重 10(20) 6(26) 4(15) 肥胖 3(6) 1(4) 2(7) 表 2 实验室指标比较
Table 2. Comparison of laboratory parameters
指标 所有患者(n=50) SBP组(n=23) 无SBP组(n=27) 统计值 P值 总胆红素(μmol/L) 124.95(43.75~274.40) 185.80(83.40~280.60) 77.70(29.30~245.45) H=403.0 0.073 白蛋白(g/L) 33.2±5.8 31.6±5.4 34.6±5.9 t=-1.8 0.071 前白蛋白(g/L) 55(45~88) 50(38~65) 78(51~126) H=177.5 0.010 胆碱酯酶(U/L) 3401(2230~5029) 3120(2094~4299) 4111(2367~5396) H=256.5 0.298 血糖(mmol/L) 5.27(4.74~7.18) 5.50(4.68~6.58) 5.22(4.84~7.28) H=311.0 0.992 甘油三酯(mmol/L) 0.88(0.71~1.20) 0.81(0.69~0.94) 1.09(0.76~1.27) H=208.5 0.048 总胆固醇(mmol/L) 2.94±1.19 2.69±1.15 3.15±1.20 t=-1.4 0.179 低密度脂蛋白(mmol/L) 1.57±0.70 1.52±0.73 1.62±0.68 t=-0.5 0.600 高密度脂蛋白(mmol/L) 0.56(0.21~0.90) 0.46(0.17~0.65) 0.74(0.29~1.04) H=216.5 0.069 血肌酐(μmol/L) 73(63~87) 70(65~85) 73(61~88) H=306.5 0.946 PTA(%) 0.56±0.22 0.45±0.16 0.66±0.21 t= -4.0 <0.001 INR 1.55(1.20~2.04) 1.99(1.50~2.46) 1.25(1.12~1.67) H= 482.0 <0.001 血红蛋白(g/L) 124.12±21.03 118.78±17.31 128.67±23.10 t=-1.7 0.091 血小板(×109/L) 81.0(53.5~110.3) 74.0(51.5~95.0) 92.0(57.5~124.5) H=234.5 0.142 MELD评分 16.81±6.84 19.83±6.22 14.25±6.37 t=3.1 0.003 CRP(mg/L) 8.37(3.32~14.79) 13.42(6.82~21.44) 6.79(2.05~9.42) H=462.5 0.003 PCT(ng/mL) 0.36(0.11~0.71) 0.55(0.08~0.76) 0.35(0.15~0.60) H=350.5 0.442 注:PCT,降钙素原。 表 3 能量代谢指标比较
Table 3. Comparison of energy metabolism indices
指标 所有患者(n=50) SBP组(n=23) 无SBP组(n=27) 统计值 P值 REE(kcal) 1 513.30±323.65 1 493.78±266.95 1 529.93±369.44 t=-0.4 0.691 pREE((kcal) 1396(1312~1565) 1383(1259~1504) 1401(1327~1594) H=266.0 0.392 RQ 0.82(0.78~0.89) 0.79(0.76~0.86) 0.85(0.79~0.91) H=205.0 0.041 npRQ 0.82(0.78~0.90) 0.79(0.76~0.86) 0.85(0.79~0.91) H=210.0 0.051 FAT(%) 49.70(28.15~65.33) 55.80(40.85~69.30) 43.80(15.35~61.40) H=385.0 0.149 CHO(%) 31.55(18.15~52.83) 20.50(15.25~41.05) 41.60(22.25~68.05) H=205.0 0.041 PRO(%) 15.2(8.0~22.1) 15.1(5.1~24.4) 15.5(8.4~19.3) H=302.5 0.884 CHO/RQ 0.38(0.23~0.59) 0.26(0.20~0.48) 0.49(0.28~0.62) H=202.5 0.036 注:pREE,预测静息能量消耗;FAT,脂肪氧化率。 表 4 logistic回归模型参数
Table 4. The parameters of logistic regression model
参数 系数 比值比 95%置信区间 Z值 P值 截距 2.18 0.611~163.911 1.559 0.119 PTA -5.43 0.004 0.000~0.179 -2.634 0.008 CHO/RQ -0.93 0.396 0.011~13.843 -0.517 0.605 CRP 0.08 1.086 1.012~1.204 1.899 0.058 -
[1] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of ascites and complications in cirrhosis[J]. J Clin Hepatol, 2017, 33(10): 1847-1863. DOI: 10.3969/j.issn.1001-5256.2017.10.003.中华医学会肝病学分会. 肝硬化腹水及相关并发症的诊疗指南[J]. 临床肝胆病杂志, 2017, 33(10): 1847-1863. DOI: 10.3969/j.issn.1001-5256.2017.10.003. [2] European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis[J]. J Hepatol, 2018, 69(2): 406-460. DOI: 10.1016/j.jhep.2018.03.024. [3] MARCIANO S, DÍAZ JM, DIRCHWOLF M, et al. Spontaneous bacterial peritonitis in patients with cirrhosis: incidence, outcomes, and treatment strategies[J]. Hepat Med, 2019, 11: 13-22. DOI: 10.2147/HMER.S164250. [4] CHINNOCK B, AFARIAN H, MINNIGAN H, et al. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis[J]. Ann Emerg Med, 2008, 52(3): 268-273. DOI: 10.1016/j.annemergmed.2008.02.016. [5] TANDON P, RAMAN M, MOURTZAKIS M, et al. A practical approach to nutritional screening and assessment in cirrhosis[J]. Hepatology, 2017, 65(3): 1044-1057. DOI: 10.1002/hep.29003. [6] LI YT, HUANG JR, PENG ML. Current status and prospects of spontaneous peritonitis in patients with cirrhosis[J]. Biomed Res Int, 2020, 2020: 3743962. DOI: 10.1155/2020/3743962. [7] MONTANO-LOZA AJ, MEZA-JUNCO J, PRADO CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis[J]. Clin Gastroenterol Hepatol, 2012, 10(2): 166-173, 173. e1. DOI: 10.1016/j.cgh.2011.08.028. [8] RODRIGUES SG, BRABANDT B, STIRNIMANN G, et al. Adipopenia correlates with higher portal pressure in patients with cirrhosis[J]. Liver Int, 2019, 39(9): 1672-1681. DOI: 10.1111/liv.14175. [9] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Gastroenterology, Chinese Medical Association. Clinical guidelines on nutrition in end-stage liver disease[J]. J Clin Hepatol, 2019, 35(6): 1222-1230. DOI: 10.3969/j.issn.1001-5256.2019.06.010.中华医学会肝病学分会, 中华医学会消化病学分会. 终末期肝病临床营养指南[J]. 临床肝胆病杂志, 2019, 35(6): 1222-1230. DOI: 10.3969/j.issn.1001-5256.2019.06.010. [10] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [11] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [12] TERAKURA Y, SHIRAKI M, NISHIMURA K, et al. Indirect calorimetry and anthropometry to estimate energy metabolism in patients with liver cirrhosis[J]. J Nutr Sci Vitaminol (Tokyo), 2010, 56(6): 372-379. DOI: 10.3177/jnsv.56.372. [13] PENG S, PLANK LD, MCCALL JL, et al. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study[J]. Am J Clin Nutr, 2007, 85(5): 1257-1266. DOI: 10.1093/ajcn/85.5.1257. [14] PRIETO-FRÍAS C, CONCHILLO M, PAYERAS M, et al. Factors related to increased resting energy expenditure in men with liver cirrhosis[J]. Eur J Gastroenterol Hepatol, 2016, 28(2): 139-145. DOI: 10.1097/MEG.0000000000000516. [15] BELARMINO G, SINGER P, GONZALEZ MC, et al. Prognostic value of energy expenditure and respiratory quotient measuring in patients with liver cirrhosis[J]. Clin Nutr, 2019, 38(4): 1899-1904. DOI: 10.1016/j.clnu.2018.07.001. [16] de WAELE E, MALBRAIN M, SPAPEN H. Nutrition in Sepsis: A bench-to-bedside review[J]. Nutrients, 2020, 12(2): 395. DOI: 10.3390/nu12020395. [17] KAO CC, GUNTUPALLI KK, BANDI V, et al. Whole-body CO2 production as an index of the metabolic response to sepsis[J]. Shock, 2009, 32(1): 23-28. DOI: 10.1097/SHK.0b013e3181970f32. [18] GLASS C, HIPSKIND P, TSIEN C, et al. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study[J]. J Appl Physiol (1985), 2013, 114(5): 559-565. DOI: 10.1152/japplphysiol.01042.2012. [19] TAJIKA M, KATO M, MOHRI H, et al. Prognostic value of energy metabolism in patients with viral liver cirrhosis[J]. Nutrition, 2002, 18(3): 229-234. DOI: 10.1016/s0899-9007(01)00754-7. [20] NISHIKAWA H, ENOMOTO H, IWATA Y, et al. Prognostic significance of nonprotein respiratory quotient in patients with liver cirrhosis[J]. Medicine (Baltimore), 2017, 96(3): e5800. DOI: 10.1097/MD.0000000000005800. [21] LI A, MUKHOPADHYAY A. Correction to: Substrate utilization and energy expenditure pattern in sepsis by indirect calorimetry[J]. Crit Care, 2020, 24(1): 660. DOI: 10.1186/s13054-020-03391-7. [22] TANG L, YIN X. Diagnostic value of serum procalcitonin, C-reactive protein and neutrophil CD64 index in early diagnosis of neonatal infection with umbilical vein catheterization[J/CD]. Chin J Exp Clin Infect Dis(Electronic Edition), 2020, 14(4): 336-339. DOI: 10.3877/cma.j.issn.1674-1358.2020.04.013.唐磊, 尹旭. 血清降钙素原、C反应蛋白和中性粒细胞CD64指数对脐静脉置管新生儿感染早期诊断价值[J/CD]. 中华实验和临床感染病杂志(电子版), 2020, 14(4): 336-339. DOI: 10.3877/cma.j.issn.1674-1358.2020.04.013. [23] LI T, CHEN YY, SUN HS, et al. Application of hematology-related indexes in early diagnosis of bacterial infection of tumor patients in ICU[J]. J Jilin Univ(Med Edit), 2020, 46(4): 816-821. DOI: 10.13481/j.1671-587x.20200424.李铤, 陈媛媛, 孙洪帅, 等. 血液学相关指标在ICU肿瘤患者细菌感染早期诊断中的应用[J]. 吉林大学学报(医学版), 2020, 46(4): 816-821. DOI: 10.13481/j.1671-587x.20200424. [24] METWALLY K, FOUAD T, ASSEM M, et al. Predictors of spontaneous bacterial peritonitis in patients with cirrhotic ascites[J]. J Clin Transl Hepatol, 2018, 6(4): 372-376. DOI: 10.14218/JCTH.2018.00001. [25] ABDEL-RAZIK A, MOUSA N, ELHAMMADY D, et al. Ascitic fluid calprotectin and serum procalcitonin as accurate diagnostic markers for spontaneous bacterial peritonitis[J]. Gut Liver, 2016, 10(4): 624-631. DOI: 10.5009/gnl15120. [26] MIKUŁA T, SAPUŁA M, JABŁOŃSKA J, et al. Significance of heparin-binding protein and D-dimers in the early diagnosis of spontaneous bacterial peritonitis[J]. Mediators Inflamm, 2018, 2018: 1969108. DOI: 10.1155/2018/1969108. [27] SHEN HX, LOU XP, CHANG XW, et al. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients: A meta analysis[J]. World Chin J Dig, 2016, 24(12): 1903-1909. DOI: 10.11569/wcjd.v24.i12.1903.申红霞, 娄小平, 苌新伟, 等. 肝硬化患者自发性细菌性腹膜炎危险因素的Meta分析[J]. 世界华人消化杂志, 2016, 24(12): 1903-1909. DOI: 10.11569/wcjd.v24.i12.1903. -



 PDF下载 ( 2128 KB)
PDF下载 ( 2128 KB)

 下载:
下载:


