仑伐替尼治疗酪氨酸激酶抑制剂经治不可切除肝细胞癌患者的效果及安全性观察
DOI: 10.3969/j.issn.1001-5256.2022.06.020
Efficacy and safety of lenvatinib in patients with unresectable hepatocellular carcinoma previously treated with tyrosine kinase inhibitor
-
摘要:
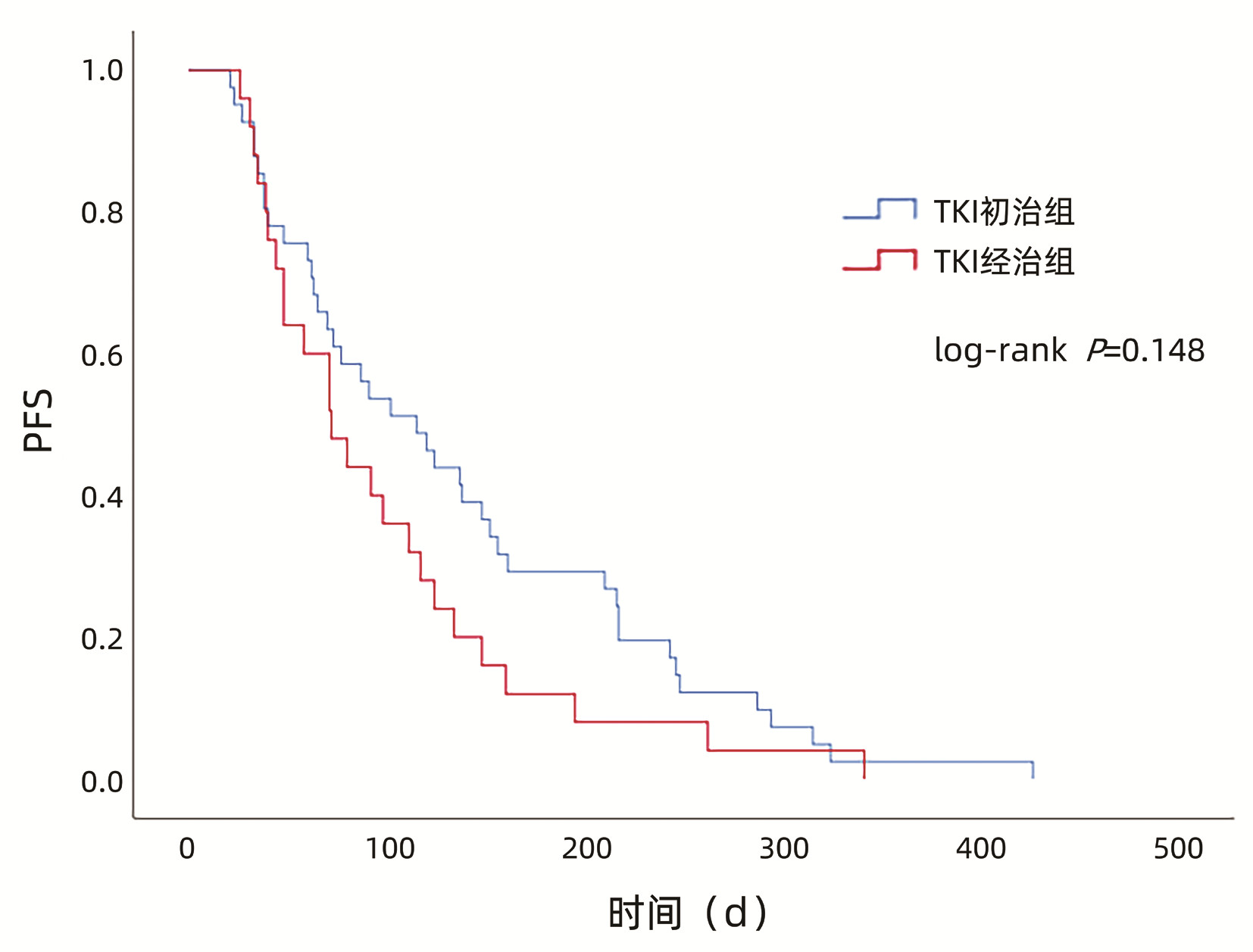
目的 探索仑伐替尼在酪氨酸激酶抑制剂(TKI)经治不可切除肝细胞癌(HCC)患者中的疗效和安全性。 方法 收集2019年1月—2020年1月就诊于首都医科大学附属北京地坛医院接受仑伐替尼治疗的76例不可切除HCC患者临床资料,根据治疗方式分为TKI初治组(n=49)和TKI经治组(n=27)。研究观察至入组后1年或调整治疗方案或肿瘤进展或死亡。比较两组无进展生存期(PFS)、客观缓解率(ORR)、疾病控制率(DCR)、不良事件发生率等。计量资料两组间比较采用t检验或Wilcoxon秩和检验。计数资料组间比较用χ2检验或Wilcoxon秩和检验。应用Kaplan-Meier法进行生存分析,组间比较采用log-rank检验。 结果 TKI初治组和TKI经治组中位PFS(115 d vs 72 d,P=0.148)、ORR(36.7% vs 18.5%,P=0.098)、DCR(65.3% vs 55.6%,P=0.402)、3级及以上不良事件发生率(24.5% vs 18.5%,P=0.550)比较,差异均无统计学意义。 结论 TKI经治不可切除HCC患者可以从仑伐替尼治疗中获益且安全性良好,与TKI初治患者相似。 Abstract:Objective To investigate the efficacy and safety of lenvatinib in patients with unresectable hepatocellular carcinoma (HCC) previously treated with tyrosine kinase inhibitor (TKI). Methods A retrospective analysis was performed for the clinical data of 76 patients with unresectable HCC who were treated with lenvatinib in Beijing Ditan Hospital, Capital Medical University, from January 2019 to January 2020, and according to the treatment method, they were divided into TKI previously untreated group with 49 patients and TKI treatment-experienced group with 27 patients. The patients were observed till one year after enrollment, adjustment of treatment regimen, tumor progression, or death. The two groups were compared in terms of progression-free survival (PFS) time, objective response rate (ORR), disease control rate (DCR), and incidence rate of adverse events. The t-test or the Wilcoxon rank-sum test was used for comparison of continuous data between two groups, and the chi-square test or the Wilcoxon rank-sum test was used for comparison of categorical data between two groups; the Kaplan-Meier method was used for survival analysis, and the log-rank test was used for comparison between groups. Results There were no significant differences between the TKI previously untreated group and the TKI treatment-experienced group in median PFS time (115 days vs 72 days, P=0.148), ORR (36.7% vs 18.5%, P=0.098), DCR (65.3% vs 55.6%, P=0.402), and incidence rates of grade ≥3 adverse events (24.5% vs 18.5%, P=0.550). Conclusion Patients with unresectable HCC previously treated with TKI can benefit from lenvatinib and have good safety, with similar results to those treated with TKI for the first time. -
Key words:
- Carcinoma, Hepatocellular /
- Lenvatinib /
- Treatment Outcome
-
表 1 两组患者基线资料
Table 1. Baseline patient characteristics
指标 TKI初治组(n=49) TKI经治组(n=27) 统计值 P值 性别[例(%)] χ2=0.000 0.987 男 40(81.6) 22(81.5) 女 9(18.4) 5(18.5) 年龄(岁) 57.88±10.62 55.67±10.78 t=0.864 0.390 糖尿病[例(%)] χ2=0.403 0.526 无 41(83.7) 21(77.8) 有 8(16.3) 6(22.2) HCC家族史[例(%)] χ2=2.298 0.130 无 43(87.8) 20(74.1) 有 6(12.2) 7(25.9) HBV感染[例(%)] χ2=0.362 0.547 有 39(79.6) 23(85.2) 无 10(20.4) 4(14.8) 肝硬化(影像)[例(%)] χ2=0.034 0.853 无 10(20.4) 6(22.2) 有 39(79.6) 21(77.8) 肝外转移[例(%)] χ2=1.040 0.308 无 33(67.3) 15(55.6) 有 16(32.7) 12(44.4) 门静脉癌栓[例(%)] χ2=1.983 0.159 无 19(38.8) 15(55.6) 有 30(61.2) 12(44.4) AST(U/L) 41.30(28.05~63.05) 41.20(29.50~61.00) Z=-0.168 0.866 ALT(U/L) 29.10(21.20~53.90) 24.10(22.30~37.10) Z=-1.020 0.308 TBil(μmol/L) 21.56±12.27 19.85±11.41 t=0.597 0.553 Alb(g/L) 37.27±5.09 36.86±5.12 t=0.329 0.743 PT(s) 12.80(12.00~14.45) 12.20(11.40~14.40) Z=-1.097 0.273 Child-Pugh分级[例(%)] χ2=0.009 0.922 A级 35(71.4) 19(70.4) B级 14(28.6) 8(29.6) mALBI分级[例(%)] χ2=0.244 0.621 1+2a 28(57.1) 17(63.0) 2b+3 21(42.9) 10(37.0) AFP(ng/mL) 240.10(22.79~2 703.93) 236.24(11.62~5 004.65) Z=-0.051 0.959 AFP [例(%)] χ2=0.044 0.835 <400 ng/mL 26(53.1) 15(55.6) ≥400 ng/mL 23(46.9) 12(44.4) CNLC分期[例(%)] χ2=-0.052 0.958 1+2 10(20.4) 9(33.3) 3a 23(46.9) 6(22.2) 3b 16(32.7) 12(44.4) BCLC分期[例(%)] χ2=1.551 0.213 A+B期 10(20.4) 9(33.3) C期 39(79.6) 18(66.7) WBC(×109/L) 4.79(3.57~5.78) 4.64(3.61~5.44) Z=-0.537 0.591 Hb(g/L) 132.35±20.07 129.65±25.08 t=0.607 0.546 PLT(×109/L) 122.00(88.70~164.00) 108.00(81.00~169.00) Z=-0.347 0.728 注:CNLC分期,中国肝癌分期。 -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. [2] SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2019[J]. CA Cancer J Clin, 2019, 69(1): 7-34. DOI: 10.3322/caac.21551. [3] LLOVET JM, RICCI S, MAZZAFERRO V, et al. Sorafenib in advanced hepatocellular carcinoma[J]. N Engl J Med, 2008, 359(4): 378-390. DOI: 10.1056/NEJMoa0708857. [4] CHENG AL, KANG YK, CHEN Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase Ⅲ randomised, double-blind, placebo-controlled trial[J]. Lancet Oncol, 2009, 10(1): 25-34. DOI: 10.1016/S1470-2045(08)70285-7. [5] KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391(10126): 1163-1173. DOI: 10.1016/S0140-6736(18)30207-1. [6] VOGEL A, CERVANTES A, CHAU I, et al. Correction to: "Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up"[J]. Ann Oncol, 2019, 30(5): 871-873. DOI: 10.1093/annonc/mdy510. [7] Chinese Society of Clinical Oncology. Guidelines for the diagnosis and treatment of primary liver cancer (2020)[DB/OL]. http://meeting.csco.org.cn/MUser/CscoPeriodical/1/2?keyword=&leibie=&pyear=2020&loc=54C24F8AFC08ECE3, 2020/11/9.中国临床肿瘤学会. 原发性肝癌诊疗指南(2020)[DB/OL]. http://meeting.csco.org.cn/MUser/CscoPeriodical/1/2?keyword=&leibie=&pyear=2020&loc=54C24F8AFC08ECE3, 2020/11/9. [8] LENCIONI R, LLOVET JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30(1): 52-60. DOI: 10.1055/s-0030-1247132. [9] U.S. Department of Health & Human Services. CTCAE 5.0[DB/OL]. https://www.hhs.gov/2017-11-27. [10] HIRAOKA A, KUMADA T, KARIYAMA K, et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: Multicenter analysis[J]. Hepatol Res, 2019, 49(1): 111-117. DOI: 10.1111/hepr.13243. [11] TOMONARI T, SATO Y, TANAKA H, et al. Potential use of lenvatinib for patients with unresectable hepatocellular carcinoma including after treatment with sorafenib: Real-world evidence and in vitro assessment via protein phosphorylation array[J]. Oncotarget, 2020, 11(26): 2531-2542. DOI: 10.18632/oncotarget.27640. [12] TAI WT, CHENG AL, SHIAU CW, et al. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma[J]. J Hepatol, 2011, 55(5): 1041-1048. DOI: 10.1016/j.jhep.2011.01.047. [13] WILHELM SM, CARTER C, TANG L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis[J]. Cancer Res, 2004, 64(19): 7099-7109. DOI: 10.1158/0008-5472.CAN-04-1443. [14] WAN PT, GARNETT MJ, ROE SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF[J]. Cell, 2004, 116(6): 855-867. DOI: 10.1016/s0092-8674(04)00215-6. [15] CHENG YR, YAN D, YANG JD, et al. Effect of transcatheter arterial chemoembolization combined with sorafenib in the treatment of primary liver cancer and its effect on immune function[J]. Clin J Med Offic, 2021, 49(3): 290-291. DOI: 10.16680/j.1671-3826.2021.03.16.程瑜蓉, 严冬, 杨建东, 等. 肝动脉栓塞化疗联合索拉非尼在原发性肝癌治疗中应用效果及对患者免疫功能影响[J]. 临床军医杂志, 2021, 49(3): 290-291. DOI: 10.16680/j.1671-3826.2021.03.16. [16] MATSUI J, YAMAMOTO Y, FUNAHASHI Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition[J]. Int J Cancer, 2008, 122(3): 664-671. DOI: 10.1002/ijc.23131. [17] TOHYAMA O, MATSUI J, KODAMA K, et al. Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models[J]. J Thyroid Res, 2014, 2014: 638747. DOI: 10.1155/2014/638747. [18] IKUTA K, YANO S, TRUNG VT, et al. E7080, a multi-tyrosine kinase inhibitor, suppresses the progression of malignant pleural mesothelioma with different proangiogenic cytokine production profiles[J]. Clin Cancer Res, 2009, 15(23): 7229-7237. DOI: 10.1158/1078-0432.CCR-09-1980. [19] XU B, ZHU XD, HUANG C, et al. Clinical efficacy of combination therapy with lenvatinib and programmed death-1 antibodies in unresectable or advanced hepatocellular carcinoma[J]. Chin J Dig Surg, 2021, 20(2): 197-204. DOI: 10.3760/cma.j.cn115610-20201217-00791.徐彬, 朱小东, 黄成, 等. 仑伐替尼联合程序性死亡受体-1抗体治疗不可切除或进展期肝细胞癌的临床疗效[J]. 中华消化外科杂志, 2021, 20(2): 197-204. DOI: 10.3760/cma.j.cn115610-20201217-00791. [20] GAO L, WANG X, TANG Y, et al. FGF19/FGFR4 signaling contributes to the resistance of hepatocellular carcinoma to sorafenib[J]. J Exp Clin Cancer Res, 2017, 36(1): 8. DOI: 10.1186/s13046-016-0478-9. [21] TOVAR V, CORNELLA H, MOEINI A, et al. Tumour initiating cells and IGF/FGF signalling contribute to sorafenib resistance in hepatocellular carcinoma[J]. Gut, 2017, 66(3): 530-540. DOI: 10.1136/gutjnl-2015-309501. [22] LI GX, ZHANG Y, YANG YM, et al. Efficacy and safety of lenvatinib for unresectable, advanced hepatocellular carcinoma in a real -world setting[J]. J Clin Hepatol, 2020, 36(10): 2266-2269. DOI: 10.3969/j.issn.1001-5256.2020.10.021.李广欣, 张钰, 杨艳美, 等. 真实世界中仑伐替尼治疗不可切除晚期肝细胞癌的效果及安全性观察[J]. 临床肝胆病杂志, 2020, 36(10): 2266-2269. DOI: 10.3969/j.issn.1001-5256.2020.10.021. [23] LEE J, SUNG PS, YANG H, et al. A real-world comparative analysis of lenvatinib and sorafenib as a salvage therapy for transarterial treatments in unresectable HCC[J]. J Clin Med, 2020, 9(12): 4121. DOI: 10.3390/jcm9124121. [24] CHUMA M, UOJIMA H, HIRAOKA A, et al. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: A multicenter analysis[J]. Hepatol Res, 2021, 51(2): 201-215. DOI: 10.1111/hepr.13592. [25] HIRAOKA A, KUMADA T, ATSUKAWA M, et al. Early relative change in hepatic function with lenvatinib for unresectable hepatocellular carcinoma[J]. Oncology, 2019, 97(6): 334-340. DOI: 10.1159/000502095. [26] UESHIMA K, NISHIDA N, HAGIWARA S, et al. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: A multicenter study[J]. Cancers (Basel), 2019, 11(7): 952. DOI: 10.3390/cancers11070952. [27] SHO T, SUDA G, OGAWA K, et al. Early response and safety of lenvatinib for patients with advanced hepatocellular carcinoma in a real-world setting[J]. JGH Open, 2020, 4(1): 54-60. DOI: 10.1002/jgh3.12209. 期刊类型引用(6)
1. 黄成,崔海坡,谭蔚锋,芦映希,姜赫宣. 肝门阻断夹持器齿型对夹持性能的影响. 生物医学工程学进展. 2022(03): 139-143 .  百度学术
百度学术2. 吴均政. 腹腔镜Pringle法联合左肝静脉阻断对左半肝切除术患者术中出血量及凝血指标的影响. 中外医学研究. 2021(26): 45-48 .  百度学术
百度学术3. 石川,常华,豆松萌,李恒. Pringle联合IVC法在巨大肝血管瘤切除术中的应用效果分析. 河南外科学杂志. 2021(06): 30-33 .  百度学术
百度学术4. 汪长青,吴珍宝. 不同肝血流阻断对腹腔镜肝切除术患者的影响. 西南国防医药. 2019(02): 116-118 .  百度学术
百度学术5. 刘雄友,高德山,郭忠涛. 不同肝血流阻断方式对肝切除术安全性及预后的影响. 肝胆外科杂志. 2018(01): 42-46 .  百度学术
百度学术6. 殷杰,徐新宝,朱日祥. 腹腔镜肝切除术治疗肝血管瘤58例临床分析. 中华肝脏外科手术学电子杂志. 2018(06): 469-472 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2021 KB)
PDF下载 ( 2021 KB)

 下载:
下载:
 百度学术
百度学术

