255例肝穿刺证实的慢性药物性肝损伤患者预后的影响因素分析
DOI: 10.3969/j.issn.1001-5256.2022.06.022
Influencing factors for the prognosis of biopsy proven patients with chronic drug-induced liver injury: An analysis of 255 cases
-
摘要:
目的 探讨成人慢性药物性肝损伤(DILI)患者预后的影响因素。 方法 选取2014年1月—2018年12月于解放军总医院第五医学中心经肝穿刺明确诊断为慢性DILI患者255例,根据2年后患者的肝功能水平分为未恢复组和恢复组。统计两组患者的年龄、性别、BMI、用药种类、DILI损伤分型、DILI损伤严重程度、合并基础疾病、实验室指标、肝组织学结果及2年预后等临床资料。正态分布的计量资料两组间比较采用t检验; 非正态分布的计量资料两组间比较采用Mann -Whitney U检验。计数资料两组间比较采用χ2检验。采用logistic单因素及多因素回归分析慢性DILI预后的独立影响因素。 结果 经过两年的随访,195例患者肝功能恢复(76.5%),60例(23.5%)未恢复。两组患者在肝损伤类型(P=0.028)、合并糖尿病(P=0.048)比例以及肝组织纤维化程度(P<0.001)的差异具有统计学意义,且未恢复组患者基线WBC、PLT、ALT、AST、GGT和总胆汁酸水平明显高于恢复组患者,ChE水平低于恢复组患者(P值均<0.05)。将基线特征纳入单因素分析logistic回归,结果显示PLT、ALT、AST、ChE、肝纤维化程度分级对预后的影响具有统计学意义(P值均<0.05)。上述变量的多因素logistic回归分析结果显示,PLT<100×109/L(OR=3.592,95%CI: 1.128~11.438,P=0.003)、ALT>2×ULN(OR=3.080,95%CI: 1.331~7.127,P=0.009)是慢性DILI预后的独立危险因素。 结论 当患者达慢性DILI诊断标准时,基线PLT<100×109/L、ALT>2×ULN作为独立危险因素,可以用于筛选出更易出现不良预后的患者。 -
关键词:
- 药物性肝损伤, 慢性 /
- 预后 /
- 危险因素
Abstract:Objective To investigate the influencing factors for the prognosis of adult patients with chronic drug-induced liver injury (DILI). Methods A total of 255 patients who were diagnosed with chronic DILI by liver biopsy in The Fifth Medical Center of Chinese PLA General Hospital from January 2014 to December 2018 were enrolled, and according to the liver function after 2 years, they were divided into non-recovery group and recovery group. The two groups were analyzed in terms of the clinical data including age, sex, body mass index, types of drugs used, type of DILI injury, severity of DILI injury, underlying diseases, laboratory markers, liver histology, and 2-year prognosis. The t-test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. Univariate and multivariate logistic regression analyses were used to investigate the independent risk factors for the prognosis of chronic DILI. Results After 2 years of follow-up, 195 patients (76.5%) achieved the recovery of liver function, while 60 patients (23.5%) did not achieve such recovery. There were significant differences between the two groups in the type of DILI injury (P=0.028), the proportion of patients with diabetes (P=0.048), and the degree of liver fibrosis (P < 0.001), and compared with the recovery group, the non-recovery group had significantly higher levels of baseline white blood cell count, platelet count (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase, and total bile acid and a significantly lower level of cholinesterase (ChE) (all P < 0.05). The baseline characteristics were included in the univariate logistic regression analysis, and the results showed that PLT, ALT, AST, ChE, and fibrosis degree were significantly associated with the prognosis of chronic DILI (all P < 0.05). The multivariate logistic regression analysis of the above variables showed that PLT < 100×109/L (odds ratio [OR]=3.592, 95% confidence interval [CI]: 1.128-11.438, P=0.003) and ALT > 2×upper limit of normal (ULN) (OR=3.080, 95%CI: 1.331-7.127, P=0.009) were independent risk factors for the prognosis of chronic DILI. Conclusion When patients meet the diagnostic criteria for chronic DILI, the independent risk factors PLT < 100×109/L and ALT > 2×ULN may be used to screen out the patients who are more likely to have poor prognosis. -
Key words:
- Drug-Induced Liver Injury, Chronic /
- Prognosis /
- Risk Factors
-
药物性肝损伤(DILI)是指由各类处方或非处方的化学药物、生物制剂、传统中药、天然药物、保健品、膳食补充剂及其代谢产物乃至辅料等所诱发的肝损伤[1]。DILI发病率逐年增长,已引起医疗界的关注与重视[1-2]。虽然DILI通常呈现急性病程,但是约20%的患者会进展为慢性DILI[2]。目前,国内外的研究[2-5]集中于DILI的发生率、致病药物、临床特征、慢性化预测因素以及预测模型等方面,较少有研究关注慢性DILI的长期预后, 因此本文通过对肝穿刺证实的成人慢性DILI患者进行回顾性研究分析,探索慢性DILI的预后影响因素。
1. 资料与方法
1.1 研究对象
回顾分析解放军总医院第五医学中心2014年1月—2018年12月收治的经肝穿刺证实的成人慢性DILI患者临床资料。纳入标准:(1)慢性DILI的诊断标准[1]满足“DILI发生6个月后,血清ALT、AST、ALP及TBil仍持续异常,或存在门静脉高压或慢性肝损伤的影像学和组织学证据”; (2)年龄≥18岁; (3)行肝组织活检; (4)RUCAM评分≥6分。排除标准:(1)单独使用对乙酰氨基酚引起的肝损伤; (2)环境毒物引起的肝损伤; (3)合并其他肝脏疾病(甲型肝炎、乙型肝炎、丙型肝炎、戊型肝炎、巨细胞病毒感染、EB病毒感染、单纯疱疹病毒感染、自身免疫性肝病、酒精性肝病、非酒精性脂肪性肝病、遗传代谢性肝病、缺血缺氧性肝病、先天性肝病等); (4)与自身免疫性肝炎无法鉴别(自身免疫性肝炎综合诊断积分系统评分为10~15分); (5)既往行肝脏或骨髓移植。
1.2 临床分型
根据《药物性肝损伤诊治指南》[1]的临床分型标准制订。(1)肝细胞损伤型: ALT≥3倍正常值上限(ULN),且R≥5; (2)胆汁淤积型: ALP≥2×ULN,且R≤2; (3)混合型: ALT≥3×ULN,ALP≥2×ULN,且2<R<5。R=[ALT(实测值)/ALT(ULN)]/[ALP(实测值)/ALP(ULN)]。根据Scheuer评分系统[6]进行肝脏炎症程度分级及纤维化分期。
1.3 研究方法
随访慢性DILI患者2年,根据2年后的随访结局分为2组:(1)恢复组; (2)未恢常组。“未恢复”定义为随访2年后患者的实验室指标满足以下任何1个标准:(1)ALT>1.5×ULN[7]; (2)AST>1.5×ULN[7]; (3)ALP>1.1×ULN[8]。收集患者性别、年龄、BMI等人口学特征,以及临床表现、血常规、肝生化、肾功能、血脂、凝血功能、免疫球蛋白、抗核抗体等指标。
1.4 统计学方法
采用SPSS 26.0统计软件进行数据分析。正态分布的计量资料以x±s表示,两组间比较采用t检验; 非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。采用单因素及多因素logistic回归对预后的影响因素进行分析。P<0.05为差异具有统计学意义。
2. 结果
2.1 一般资料
共纳入255例成人慢性DILI患者,其中男74例(29.0%),女181例(71.0%),中位年龄48(41~55)岁,中位BMI为23.67(21.48~25.74) kg/m2。
2.2 致肝损伤药物种类
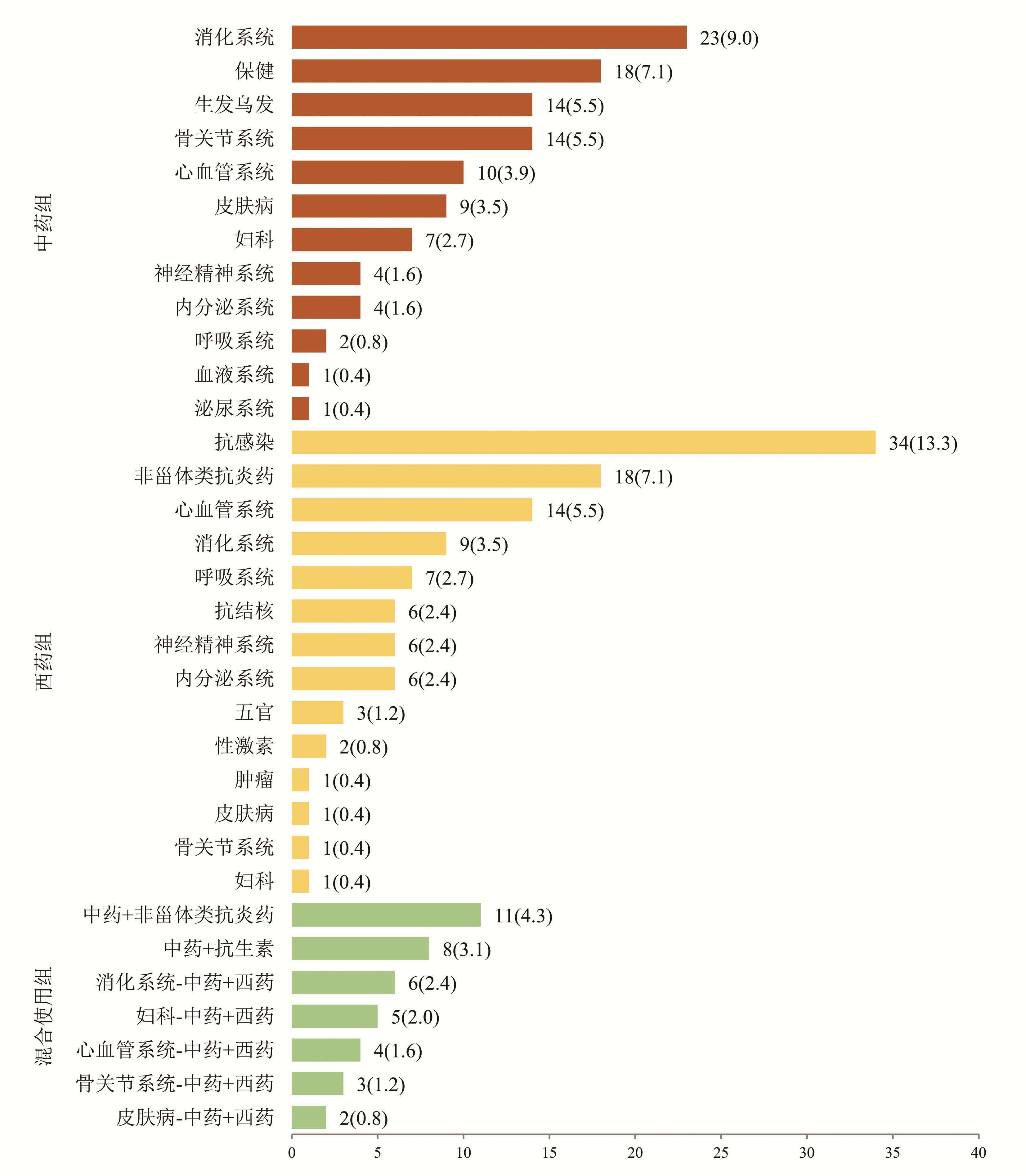
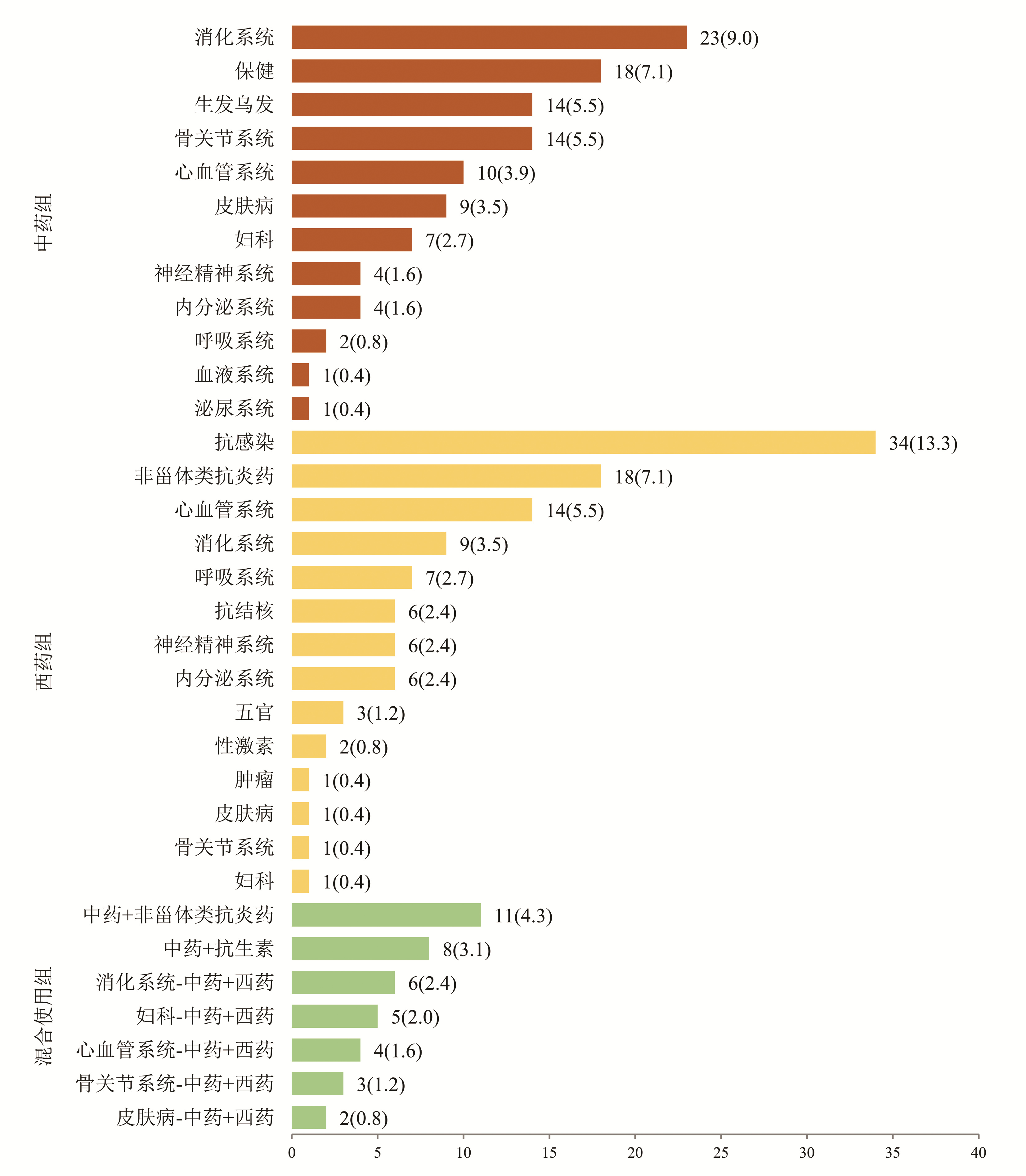
根据患者使用的致病药物种类分为3组:中药组、西药组和混合使用组,各组占比依次为42.0%、42.7%、15.3%(表 1)。中药组排名前4位的致病药物分别为消化系统、保健、生发乌发、骨关节系统用药; 西药组位于前4位的致病药物分别为抗感染药物、非甾体类抗炎药、心血管系统、消化系统用药; 混合使用组前3位致病药物分别为中药联合非甾体类抗炎药、中药联合抗生素、消化系统中药联合西药(图 1)。
表 1 255例慢性DILI患者的人口统计学和临床特点Table 1. Demographics and clinical features of 255 patients with chronic DILI项目 总患者
(n=255)恢复组
(n=195)未恢复组
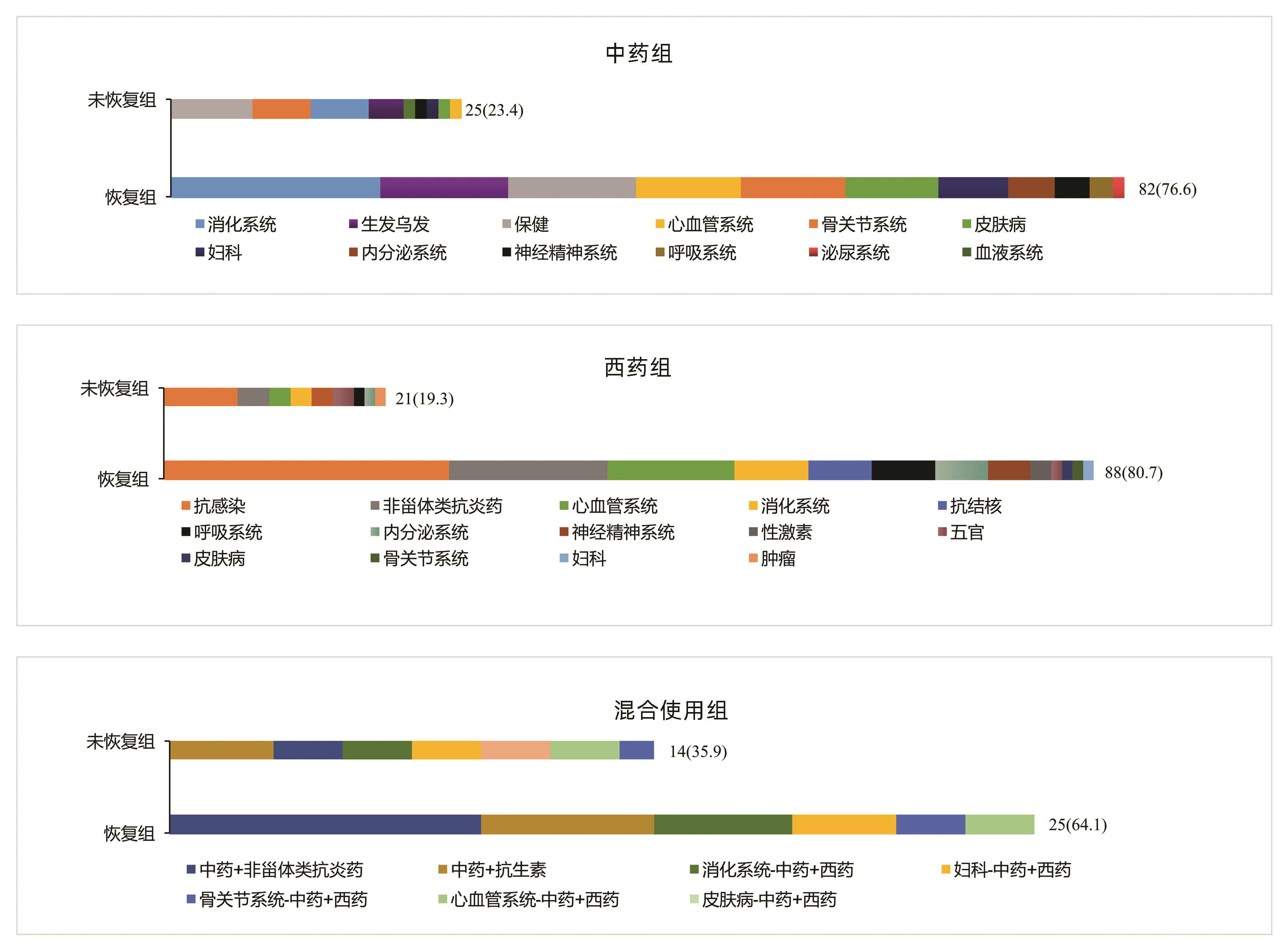
(n=60)统计值 P值 女性[例(%)] 181(71.0) 141(72.3) 40(66.7) χ2=0.709 0.400 年龄(岁) 48(41~55) 48(40~55) 49(42~55) U=5 585.5 0.596 BMI(kg/m2) 23.67(21.48~25.74) 23.72(21.40~25.80) 23.60(21.70~25.40) U=5 709.0 0.966 用药史[例(%)] χ2=4.418 0.110 中药1) 107(42.0) 82(42.1) 25(41.7) 西药 109(42.7) 88(45.1) 21(35.0) 混合使用 39(15.3) 25(12.8) 14(23.3) 临床分型[例(%)] χ2=7.152 0.028 肝细胞型 71(27.8) 57(29.2) 14(23.3) 胆汁淤积型 95(37.3) 64(32.8) 31(51.7) 混合型 89(34.9) 74(37.9) 15(25.0) DILI严重程度分级[例(%)] χ2=5.172 0.160 1级 201(78.8) 155(79.5) 46(76.7) 2级 24(9.4) 21(10.7) 3(5.0) 3级 8(3.1) 6(3.1) 2(3.3) 4级 22(8.6) 13(6.7) 9(15.0) 合并基础疾病[例(%)] 高血压 43(16.9) 35(17.9) 8(13.3) χ2=0.697 0.404 糖尿病 13(5.1) 7(3.6) 6(10.0) χ2=3.897 0.048 血脂异常 91(35.7) 65(33.3) 26(43.3) χ2=1.999 0.157 实验室指标 WBC(×109/L) 4.75(4.04~5.84) 4.87(4.16~5.91) 4.45(3.77~5.34) U=4 669.5 0.018 嗜酸性粒细胞(×109/L) 0.11(0.06~0.18) 0.10(0.06~0.18) 0.11(0.05~0.18) U=5 695.5 0.757 Hb(g/L) 131(122~142) 131(122~142) 130(119~143) U=5 682.5 0.737 PLT(×109/L) 190.0(151.0~243.0) 198.5(154.5~246.5) 165.5(136.0~213.5) U=4 549.5 0.009 INR 0.96(0.90~1.03) 0.95(0.90~1.01) 0.96(0.90~1.05) U=5 421.0 0.390 ALT(U/L) 121.0(73.0~254.0) 112.0(67.0~222.5) 160.0(93.5~303.7) U=4 654.0 0.017 AST(U/L) 111.0(58.0~216.0) 106.0(56.0~186.5) 139.5(66.0~281.5) U=4 840.5 0.043 ALP(U/L) 150.0(120.0~194.0) 148.0(117.5~191.0) 155.0(135.0~222.0) U=5 085.0 0.126 TBil(μmol/L) 16.7(11.6~36.5) 16.2(11.1~33.4) 17.3(13.5~49.7) U=5 095.5 0.131 GGT(U/L) 140.0(91.0~222.0) 133.0(77.5~193.5) 164.3(122.0~291.0) U=4 311.0 0.002 前白蛋白(mg/L) 160.0(114.0~208.0) 158.5(115.5~207.5) 168.5(112.5~211.5) U=5 810.5 0.937 ChE(U/L) 6136(5048~7294) 6466(5261~7472) 6435(5289~7346) U=3 800.0 <0.001 TBA(μmol/L) 10.0(6.0~33.0) 9.2(5.0~26.5) 12.9(7.0~44.0) U=4 729.0 0.025 IgG(g/L) 13.0(11.0~15.7) 13.0(10.9~15.5) 12.7(11.2~16.0) U=5 527.5 0.519 IgM(g/L) 1.02(0.65~1.45) 1.00(0.70~1.40) 1.20(0.70~1.60) U=5 470.5 0.447 抗核抗体[例(%)] 51(20.0) 39(20.0) 12(20.0) χ2=0.000 1.000 注:1)包括保健品。 在中药组中,未恢复组排名前3位的致病药物为保健、骨关节、消化系统用药; 恢复组为消化系统、生发乌发、保健用药。在西药组中,未恢复组与恢复组一样,排名前2位的致病药物均为抗感染药物、非甾体类抗炎药。在混合组中,未恢复组排名第1位的致病药物组合为中药联合抗生素; 恢复组为中药联合非甾体类抗炎药(图 2)。
2.3 恢复组与未恢复组患者的临床特点
根据随访2年患者肝功能水平进行分组,恢复组195例(76.5%),未恢复组60例(23.5%)。恢复组以混合型肝损伤为主(37.9%),未恢复组以胆汁淤积型肝损伤为主(51.7%),差异具有统计学意义(P=0.028)。在DILI损伤严重程度方面,2组均以1级肝损伤为主。伴随基础疾病方面,未恢复组有更多的患者伴有糖尿病(P=0.048)。未恢复组患者基线WBC、PLT、ALT、AST、GGT和总胆汁酸(TBA)水平明显高于恢复组患者,ChE低于恢复组患者(P值均<0.05)(表 1)。
2.4 恢复组与未恢复组患者的肝组织学特点
2组患者在发病6个月后均做了肝组织学活检,结果提示,在炎症程度分级方面,恢复组G2~G4总占比为60.5%,未恢复组G2~G4总占比为71.7%;在纤维化程度方面,未恢复组在S3和S4的占比均高于恢复组(13.3% vs 11.3%,13.3% vs 1.0%,P值均<0.001)(表 2)。
表 2 255例慢性DILI患者的肝组织病理学结果Table 2. Pathological features of 255 patients with chronic DILI项目 总患者
(n=255)恢复组
(n=195)未恢复组
(n=60)χ2值 P值 炎症程度分级[例(%)] 3.883 0.384 G1 94(36.9) 77(39.5) 17(28.3) G2 98(38.4) 74(37.9) 24(40.0) G3 59(23.1) 42(21.5) 17(28.3) G4 4(1.6) 2(1.0) 2(3.3) 纤维化程度分级[例(%)] 21.573 <0.001 S1 128(50.2) 109(55.9) 19(31.7) S2 87(34.1) 62(31.8) 25(41.7) S3 30(11.8) 22(11.3) 8(13.3) S4 10(3.9) 2(1.0) 8(13.3) 2.5 慢性DILI预后的影响因素分析
logistic回归单因素结果显示,PLT<100×109/L、ALT>2×ULN、AST>5×ULN、ChE<5000 U/L和纤维化程度S3~S4对慢性DILI预后的影响具有统计学意义(P值均<0.05)(表 3)。将上述变量纳入多因素分析,结果显示,PLT<100×109/L、ALT>2×ULN是慢性DILI预后的独立危险因素(P值均<0.05)(表 4)。
表 3 预后影响因素的logistic单因素回归分析Table 3. Univariate logistic regression analysis of influencing factors of prognosis变量 OR 95%CI P值 变量 OR 95%CI P值 女性 0.766 0.411~1.426 0.401 ALT 年龄 ≤2×ULN ≤60岁 >2×ULN 2.899 1.345~6.250 0.007 >60岁 1.029 0.391~2.707 0.953 AST BMI ≤5×ULN ≤24 kg/m2 >5×ULN 1.930 1.036~3.594 0.038 >24 kg/m2 1.003 0.561~1.794 0.992 ALP 用药史 ≤2×ULN 中药1) >2×ULN 1.859 0.657~5.258 0.243 西药 0.812 0.418~1.575 0.537 TBil 混合使用 1.375 0.644~2.937 0.411 ≤2×ULN 临床分型 >2×ULN 1.532 0.791~2.965 0.206 肝细胞型 GGT 非肝细胞型 1.357 0.692~2.661 0.374 ≤2.5×ULN DILI严重程度分级 >2.5×ULN 1.954 0.897~4.255 0.092 1~2级 ChE 3~4级 2.079 0.928~4.662 0.075 <5000 U/L 2.154 1.140~4.069 0.018 合并症 ≥5000 U/L 高血压 0.703 0.307~1.612 0.406 TBA 糖尿病 2.984 0.962~9.253 0.058 ≤4×ULN 血脂异常 1.529 0.847~2.762 0.159 >4×ULN 1.502 0.766~2.945 0.236 PLT 纤维化程度分级 <100×109/L 3.087 1.070~8.904 0.037 S1~S2 ≥100×109/L S3~S4 2.591 1.269~5.291 0.009 注:1)包括保健品。 表 4 预后影响因素的logistic多因素回归分析Table 4. Multivariate logistic regression analysis of influencing factors of prognosis变量 OR 95%CI P值 PLT <100×109/L 3.592 1.128~11.438 0.003 ≥100×109/L ALT ≤2×ULN >2×ULN 3.080 1.331~7.127 0.009 AST ≤5×ULN >5×ULN 1.195 0.599~2.384 0.614 ChE <5000 U/L 1.647 0.816~3.325 0.164 ≥5000 U/L 纤维化程度分级 S1~S2 S3~S4 2.084 0.970~4.479 0.060 3. 讨论
本研究中女性患者约占70.1%,略高于既往文献[9-10]报道的50%~60%,这可能与本研究的患者群体有关,既往文献[2, 4-5, 9]也报道过女性是DILI慢性化的危险因素。在致病药物方面,未恢复组以服用中药为主(41.7%),恢复组以服用西药为主(42.7%),但2组差异无统计学意义,多因素分析也未发现与患者预后有关。既往研究[11]发现,服用中药引发的肝损伤比其他类别药物引起的肝损伤严重,但也有学者[3-4]认为服用药物的类别与预后无关。
在肝损伤类型方面,未恢复组以胆汁淤积型为主(51.7%),既往有学者[12-13]报道DILI损伤分型与患者的预后密切相关,且既往国外研究[14]报道过胆汁淤积型患者的肝功能需要较长时间才能恢复正常,但也有国外学者[8]持不同意见:“DILI损伤类型与DILI患者的恢复时长无关”。本研究的多因素分析发现,肝损伤类型与慢性DILI患者的预后无关。
既往有学者[12-13]报道DILI损伤严重程度与患者的预后密切相关,但是本研究的多因素分析未发现DILI损伤严重程度与预后有关。未恢复组的基线WBC、PLT和ChE水平均低于恢复组,提示未恢复组肝脏功能储备功能较差。既往中国学者[4]发现PLT可能是DILI慢性化的独立预测指标美国学者[15]也报道过低水平的PLT是预测DILI预后的一个重要指标,在本研究的多因素分析中也发现PLT与慢性DILI的预后有关,PLT<100×109/L是慢性DILI预后的独立危险因素。
本研究的患者均在DILI发病6个月后做了肝组织学活检,未恢复组G3和G4的总占比是恢复组的1.4倍(31.6% vs 22.5%),提示未恢复组患者的肝脏炎症程度较重。未恢复组的基线ALT和AST值较恢复组高,与组织学结果相呼应,也提示未恢复组患者的肝脏炎症程度较恢复组重。此外,本研究的多因素分析发现ALT>2×ULN是慢性DILI预后的独立危险因素。
在纤维化程度方面,未恢复组的S3和S4的总占比约为恢复组的2.2倍(26.6% vs 12.3%),这提示纤维化程度较高的患者可能预后较差,纤维化程度较高的患者意味着存在较严重的肝损伤,同时肝脏自身修复能力也较弱。此外,未恢复组的基线GGT和TBA水平均较恢复组高,提示慢性组患者肝功能较差且有恶化趋势。
综上所述,与恢复组相比,未恢复组中胆汁淤积型比例、合并糖尿病比例及肝纤维化程度较高,预后较差。当患者达慢性DILI诊断标准时,基线PLT<100×109/L、ALT>2×ULN作为独立危险因素,可以用于筛选出更易出现不良预后的患者。
-
表 1 255例慢性DILI患者的人口统计学和临床特点
Table 1. Demographics and clinical features of 255 patients with chronic DILI
项目 总患者
(n=255)恢复组
(n=195)未恢复组
(n=60)统计值 P值 女性[例(%)] 181(71.0) 141(72.3) 40(66.7) χ2=0.709 0.400 年龄(岁) 48(41~55) 48(40~55) 49(42~55) U=5 585.5 0.596 BMI(kg/m2) 23.67(21.48~25.74) 23.72(21.40~25.80) 23.60(21.70~25.40) U=5 709.0 0.966 用药史[例(%)] χ2=4.418 0.110 中药1) 107(42.0) 82(42.1) 25(41.7) 西药 109(42.7) 88(45.1) 21(35.0) 混合使用 39(15.3) 25(12.8) 14(23.3) 临床分型[例(%)] χ2=7.152 0.028 肝细胞型 71(27.8) 57(29.2) 14(23.3) 胆汁淤积型 95(37.3) 64(32.8) 31(51.7) 混合型 89(34.9) 74(37.9) 15(25.0) DILI严重程度分级[例(%)] χ2=5.172 0.160 1级 201(78.8) 155(79.5) 46(76.7) 2级 24(9.4) 21(10.7) 3(5.0) 3级 8(3.1) 6(3.1) 2(3.3) 4级 22(8.6) 13(6.7) 9(15.0) 合并基础疾病[例(%)] 高血压 43(16.9) 35(17.9) 8(13.3) χ2=0.697 0.404 糖尿病 13(5.1) 7(3.6) 6(10.0) χ2=3.897 0.048 血脂异常 91(35.7) 65(33.3) 26(43.3) χ2=1.999 0.157 实验室指标 WBC(×109/L) 4.75(4.04~5.84) 4.87(4.16~5.91) 4.45(3.77~5.34) U=4 669.5 0.018 嗜酸性粒细胞(×109/L) 0.11(0.06~0.18) 0.10(0.06~0.18) 0.11(0.05~0.18) U=5 695.5 0.757 Hb(g/L) 131(122~142) 131(122~142) 130(119~143) U=5 682.5 0.737 PLT(×109/L) 190.0(151.0~243.0) 198.5(154.5~246.5) 165.5(136.0~213.5) U=4 549.5 0.009 INR 0.96(0.90~1.03) 0.95(0.90~1.01) 0.96(0.90~1.05) U=5 421.0 0.390 ALT(U/L) 121.0(73.0~254.0) 112.0(67.0~222.5) 160.0(93.5~303.7) U=4 654.0 0.017 AST(U/L) 111.0(58.0~216.0) 106.0(56.0~186.5) 139.5(66.0~281.5) U=4 840.5 0.043 ALP(U/L) 150.0(120.0~194.0) 148.0(117.5~191.0) 155.0(135.0~222.0) U=5 085.0 0.126 TBil(μmol/L) 16.7(11.6~36.5) 16.2(11.1~33.4) 17.3(13.5~49.7) U=5 095.5 0.131 GGT(U/L) 140.0(91.0~222.0) 133.0(77.5~193.5) 164.3(122.0~291.0) U=4 311.0 0.002 前白蛋白(mg/L) 160.0(114.0~208.0) 158.5(115.5~207.5) 168.5(112.5~211.5) U=5 810.5 0.937 ChE(U/L) 6136(5048~7294) 6466(5261~7472) 6435(5289~7346) U=3 800.0 <0.001 TBA(μmol/L) 10.0(6.0~33.0) 9.2(5.0~26.5) 12.9(7.0~44.0) U=4 729.0 0.025 IgG(g/L) 13.0(11.0~15.7) 13.0(10.9~15.5) 12.7(11.2~16.0) U=5 527.5 0.519 IgM(g/L) 1.02(0.65~1.45) 1.00(0.70~1.40) 1.20(0.70~1.60) U=5 470.5 0.447 抗核抗体[例(%)] 51(20.0) 39(20.0) 12(20.0) χ2=0.000 1.000 注:1)包括保健品。 表 2 255例慢性DILI患者的肝组织病理学结果
Table 2. Pathological features of 255 patients with chronic DILI
项目 总患者
(n=255)恢复组
(n=195)未恢复组
(n=60)χ2值 P值 炎症程度分级[例(%)] 3.883 0.384 G1 94(36.9) 77(39.5) 17(28.3) G2 98(38.4) 74(37.9) 24(40.0) G3 59(23.1) 42(21.5) 17(28.3) G4 4(1.6) 2(1.0) 2(3.3) 纤维化程度分级[例(%)] 21.573 <0.001 S1 128(50.2) 109(55.9) 19(31.7) S2 87(34.1) 62(31.8) 25(41.7) S3 30(11.8) 22(11.3) 8(13.3) S4 10(3.9) 2(1.0) 8(13.3) 表 3 预后影响因素的logistic单因素回归分析
Table 3. Univariate logistic regression analysis of influencing factors of prognosis
变量 OR 95%CI P值 变量 OR 95%CI P值 女性 0.766 0.411~1.426 0.401 ALT 年龄 ≤2×ULN ≤60岁 >2×ULN 2.899 1.345~6.250 0.007 >60岁 1.029 0.391~2.707 0.953 AST BMI ≤5×ULN ≤24 kg/m2 >5×ULN 1.930 1.036~3.594 0.038 >24 kg/m2 1.003 0.561~1.794 0.992 ALP 用药史 ≤2×ULN 中药1) >2×ULN 1.859 0.657~5.258 0.243 西药 0.812 0.418~1.575 0.537 TBil 混合使用 1.375 0.644~2.937 0.411 ≤2×ULN 临床分型 >2×ULN 1.532 0.791~2.965 0.206 肝细胞型 GGT 非肝细胞型 1.357 0.692~2.661 0.374 ≤2.5×ULN DILI严重程度分级 >2.5×ULN 1.954 0.897~4.255 0.092 1~2级 ChE 3~4级 2.079 0.928~4.662 0.075 <5000 U/L 2.154 1.140~4.069 0.018 合并症 ≥5000 U/L 高血压 0.703 0.307~1.612 0.406 TBA 糖尿病 2.984 0.962~9.253 0.058 ≤4×ULN 血脂异常 1.529 0.847~2.762 0.159 >4×ULN 1.502 0.766~2.945 0.236 PLT 纤维化程度分级 <100×109/L 3.087 1.070~8.904 0.037 S1~S2 ≥100×109/L S3~S4 2.591 1.269~5.291 0.009 注:1)包括保健品。 表 4 预后影响因素的logistic多因素回归分析
Table 4. Multivariate logistic regression analysis of influencing factors of prognosis
变量 OR 95%CI P值 PLT <100×109/L 3.592 1.128~11.438 0.003 ≥100×109/L ALT ≤2×ULN >2×ULN 3.080 1.331~7.127 0.009 AST ≤5×ULN >5×ULN 1.195 0.599~2.384 0.614 ChE <5000 U/L 1.647 0.816~3.325 0.164 ≥5000 U/L 纤维化程度分级 S1~S2 S3~S4 2.084 0.970~4.479 0.060 -
[1] Drug-induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury[J]. J Clin Hepatol, 2015, 31(11): 1752-1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002.中华医学会肝病学分会药物性肝病学组. 药物性肝损伤诊治指南[J]. 临床肝胆病杂志, 2015, 31(11): 1752-1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002. [2] WANG Q, HUANG A, WANG JB, et al. Chronic drug-induced liver injury: Updates and future challenges[J]. Front Pharmacol, 2021, 12: 627133. DOI: 10.3389/fphar.2021.627133. [3] WANG Y, WANG Y, WANG L, et al. A comparison study of clinical features and prognosis between herbal and dietary supplements and Wester medicine induced liver injuries[J]. Chin Hepatol, 2021, 26(4): 364-369. DOI: 10.14000/j.cnki.issn.1008-1704.2021.04.007.王艳, 王昱, 王岚, 等. 中草药与西药致药物性肝损伤的临床特征及其预后的对比研究[J]. 肝脏, 2021, 26(4): 364-369. DOI: 10.14000/j.cnki.issn.1008-1704.2021.04.007. [4] JI TT, LU HY, TAN N, et al. A comparative analysis of the clinical features of acute and chronic drug-induced liver injury[J]. J Clin Hepatol, 2020, 36(7): 1556-1561. DOI: 10.3969/j.issn.1001-5256.2020.07.021.纪童童, 陆海英, 谭宁, 等. 急、慢性药物性肝损伤临床特征的对比分析[J]. 临床肝胆病杂志, 2020, 36(7): 1556-1561. DOI: 10.3969/j.issn.1001-5256.2020.07.021. [5] HUANG A, SUN Y, ZOU ZS. Recent research ad-vances in chronicity of drug-induced liver injury[J]. J Clin Hepatol, 2020, 36(3): 501-504. DOI: 10.3969/j.issn.1001-5256.2020.03.004.黄昂, 孙颖, 邹正升. 药物性肝损伤慢性化的研究近况[J]. 临床肝胆病杂志, 2020, 36(3): 501-504. DOI: 10.3969/j.issn.1001-5256.2020.03.004. [6] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [7] FONTANA RJ, HAYASHI PH, BARNHART H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury[J]. Am J Gastroenterol, 2015, 110(10): 1450-1459. DOI: 10.1038/ajg.2015.283. [8] MEDINA-CALIZ I, ROBLES-DIAZ M, GARCIA-MUÑOZ B, et al. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury[J]. J Hepatol, 2016, 65(3): 532-542. DOI: 10.1016/j.jhep.2016.05.003. [9] CHALASANI N, BONKOVSKY HL, FONTANA R, et al. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN prospective study[J]. Gastroenterology, 2015, 148(7): 1340-1352. e7. DOI: 10.1053/j.gastro.2015.03.006. [10] SHEN T, LIU Y, SHANG J, et al. Incidence and etiology of drug-induced liver injury in Mainland China[J]. Gastroenterology, 2019, 156(8): 2230-2241. e11. DOI: 10.1053/j.gastro.2019.02.002. [11] JING J, TESCHKE R. Traditional Chinese Medicine and herb-induced liver injury: Comparison with drug-induced liver injury[J]. J Clin Transl Hepatol, 2018, 6(1): 57-68. DOI: 10.14218/JCTH.2017.00033. [12] LI FL, HUANG ZG, GE CL, et al. Logistic regression analysis of the clinical characteristics and prognostic factors of drug-induced liver injury[J]. J Hepatobiliary Surg, 2019, 27(4): 258-262. DOI: 10.3969/j.issn.1006-4761.2019.04.007.李飞龙, 黄赵刚, 葛朝亮, 等. 药物性肝损伤的临床特点及预后因素Logistic回归分析[J]. 肝胆外科杂志, 2019, 27(4): 258-262. DOI: 10.3969/j.issn.1006-4761.2019.04.007. [13] ZHANG XY, CHEN HQ, BAI C. Clinical characteristics and prognosis of drug-induced liver injury[J]. Contemp Med, 2019, 25 (7): 137-139. DOI: 10.3969/j.issn.1009-4393.2019.07.055.张旭艳, 陈慧群, 白成. 药物性肝损伤的临床特点及其预后评估[J]. 当代医学, 2019, 25(7): 137-139. DOI: 10.3969/j.issn.1009-4393.2019.07.055. [14] BJÖRNSSON E, KALAITZAKIS E, AV KLINTEBERG V, et al. Long- term follow-up of patients with mild to moderate drug-induced liver injury[J]. Aliment Pharmacol Ther, 2007, 26(1): 79-85. DOI: 10.1111/j.1365-2036.2007.03355.x. [15] LO RE V 3rd, HAYNES K, FORDE KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: Evaluation of Hy's law and a new prognostic model[J]. Clin Gastroenterol Hepatol, 2015, 13(13): 2360-2368. DOI: 10.1016/j.cgh.2015.06.020. 期刊类型引用(6)
1. 许姗姗,仇丽霞,柳雅立,张晶. 360例病理诊断的药物性肝损伤患者临床特征及预后分析. 肝脏. 2025(01): 16-20 .  百度学术
百度学术2. 贺艳,柯洪琴,李洪亮,朱建勇,赵利军,于慧斌. 美罗培南相关肝损伤的影响因素及其预测价值. 临床肝胆病杂志. 2025(03): 506-512 .  本站查看
本站查看3. 李明静,李贝贝,孟庆宇,钟新梅. 病情复发的慢性药物性肝损伤患者临床特征分析. 肝脏. 2024(09): 1128-1131 .  百度学术
百度学术4. 赖荣陶,娄玮蒨,陈成伟,于乐成. 药物性肝损伤. 肝脏. 2023(01): 11-13 .  百度学术
百度学术5. 关敬,哈森,袁颢,陈颖,刘鹏举,刘智,姜爽. 小陷胸汤加味方对2型糖尿病大鼠肝损伤的保护作用及其机制. 吉林大学学报(医学版). 2023(03): 608-616 .  百度学术
百度学术6. 杨红,谢鹏飞,姜曼. 慢性药物性肝损伤后肝功能恢复的风险预测模型构建. 肝脏. 2023(12): 1480-1483+1526 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 3674 KB)
PDF下载 ( 3674 KB)

 下载:
下载:


 下载:
下载:
 百度学术
百度学术