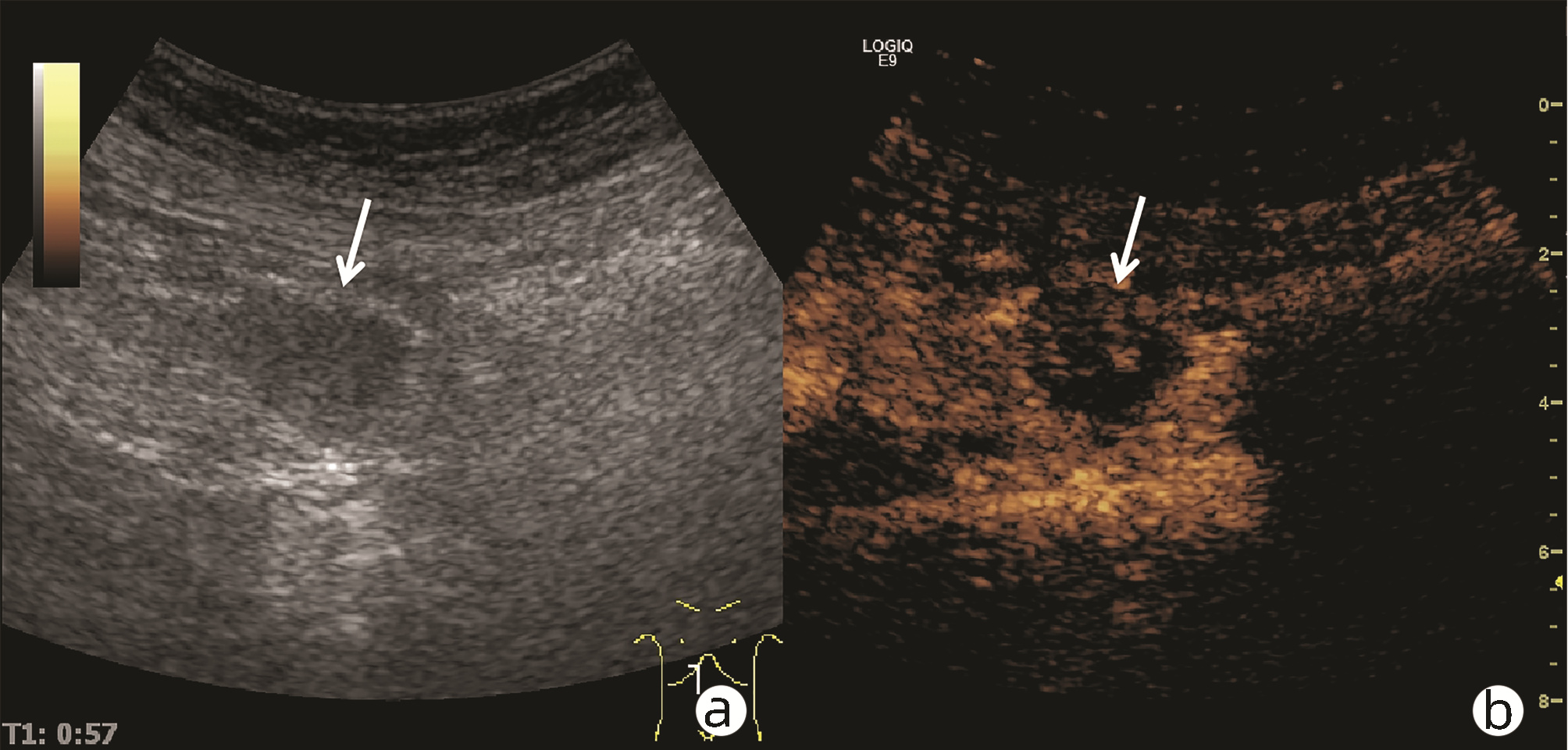
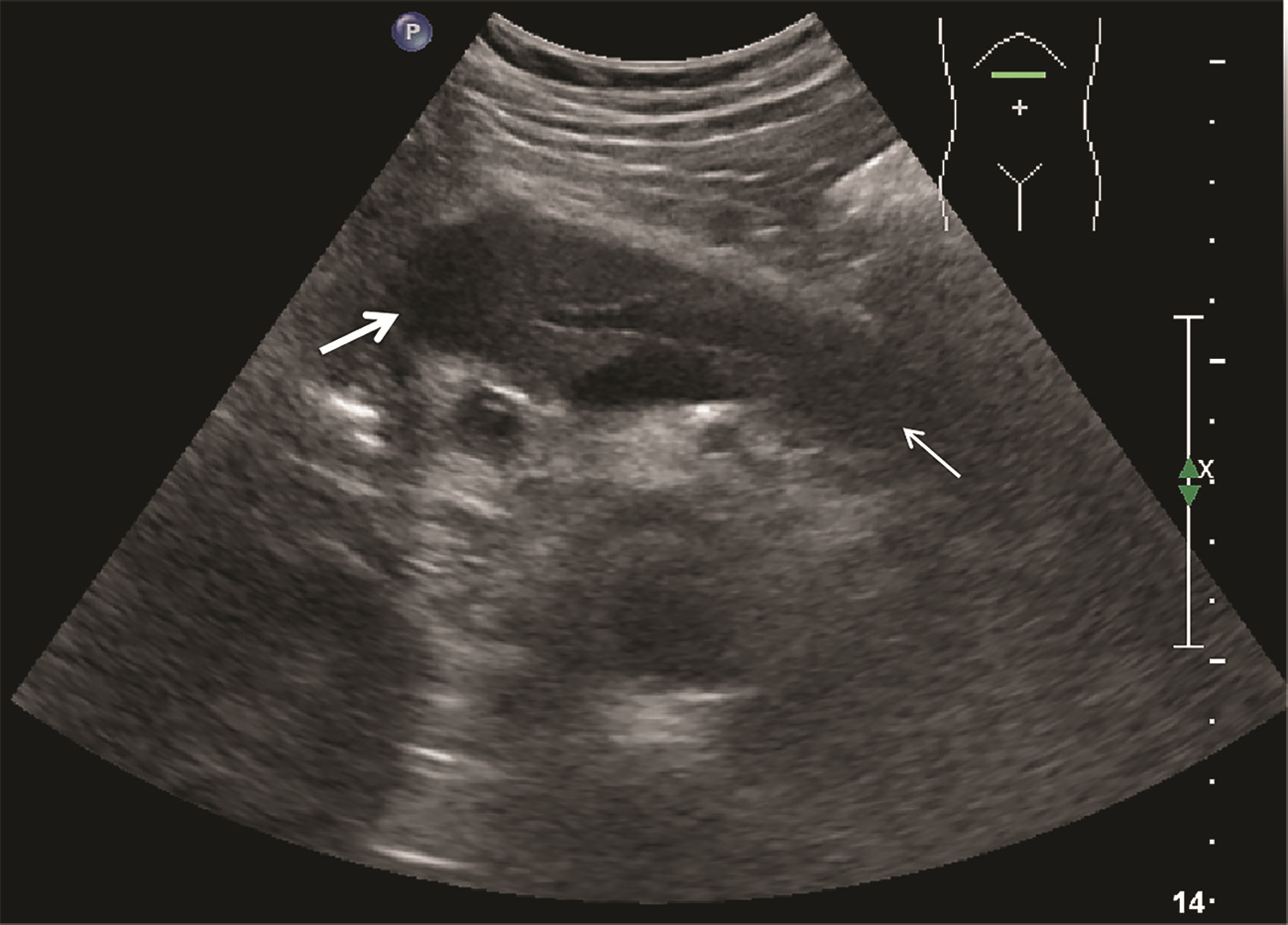
肿块型自身免疫性胰腺炎与胰腺导管腺癌的超声及超声造影表现分析
DOI: 10.3969/j.issn.1001-5256.2022.06.025
Ultrasound findings and contrast-enhanced ultrasound findings of mass-type autoimmune pancreatitis versus pancreatic ductal adenocarcinoma
-
摘要:
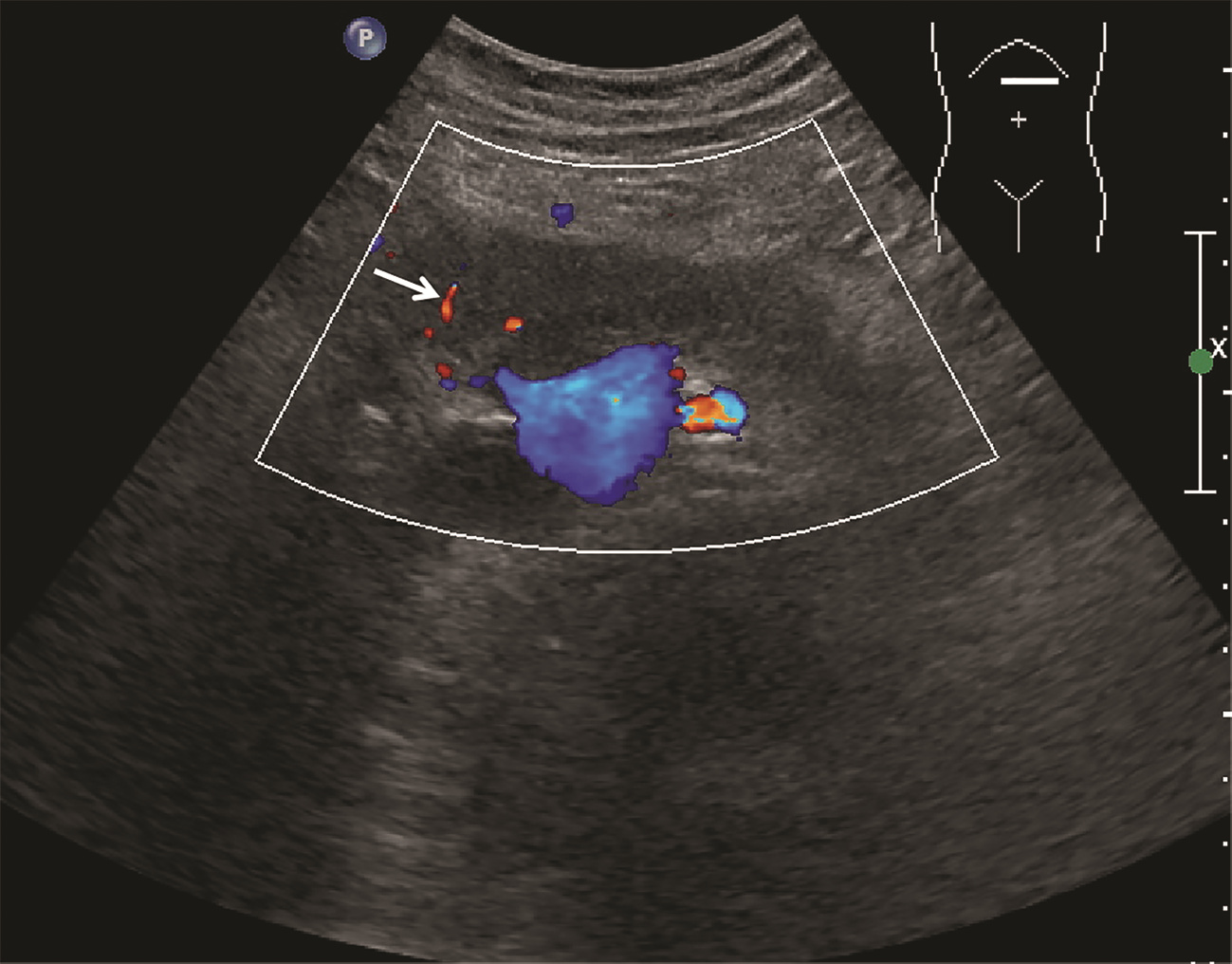
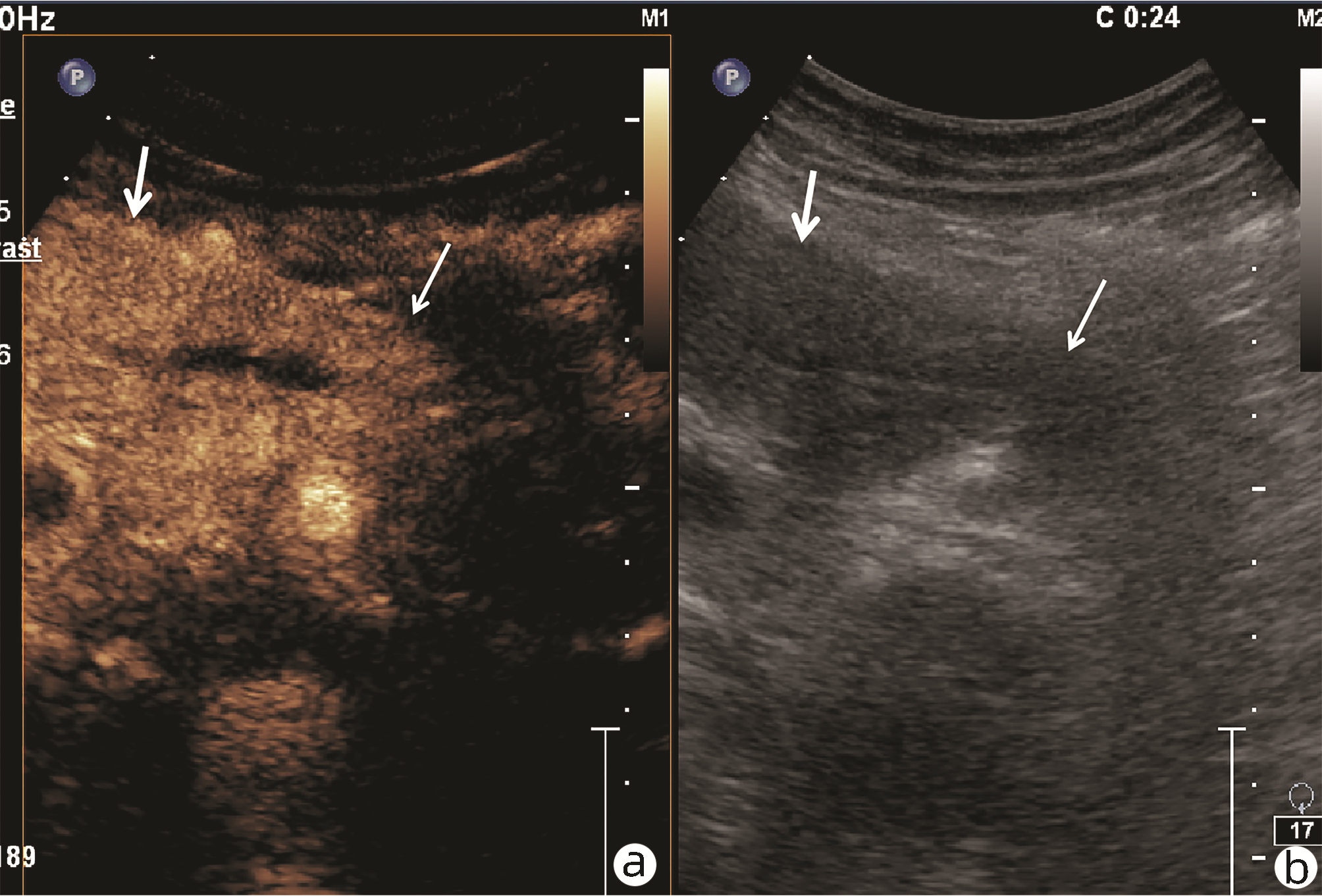
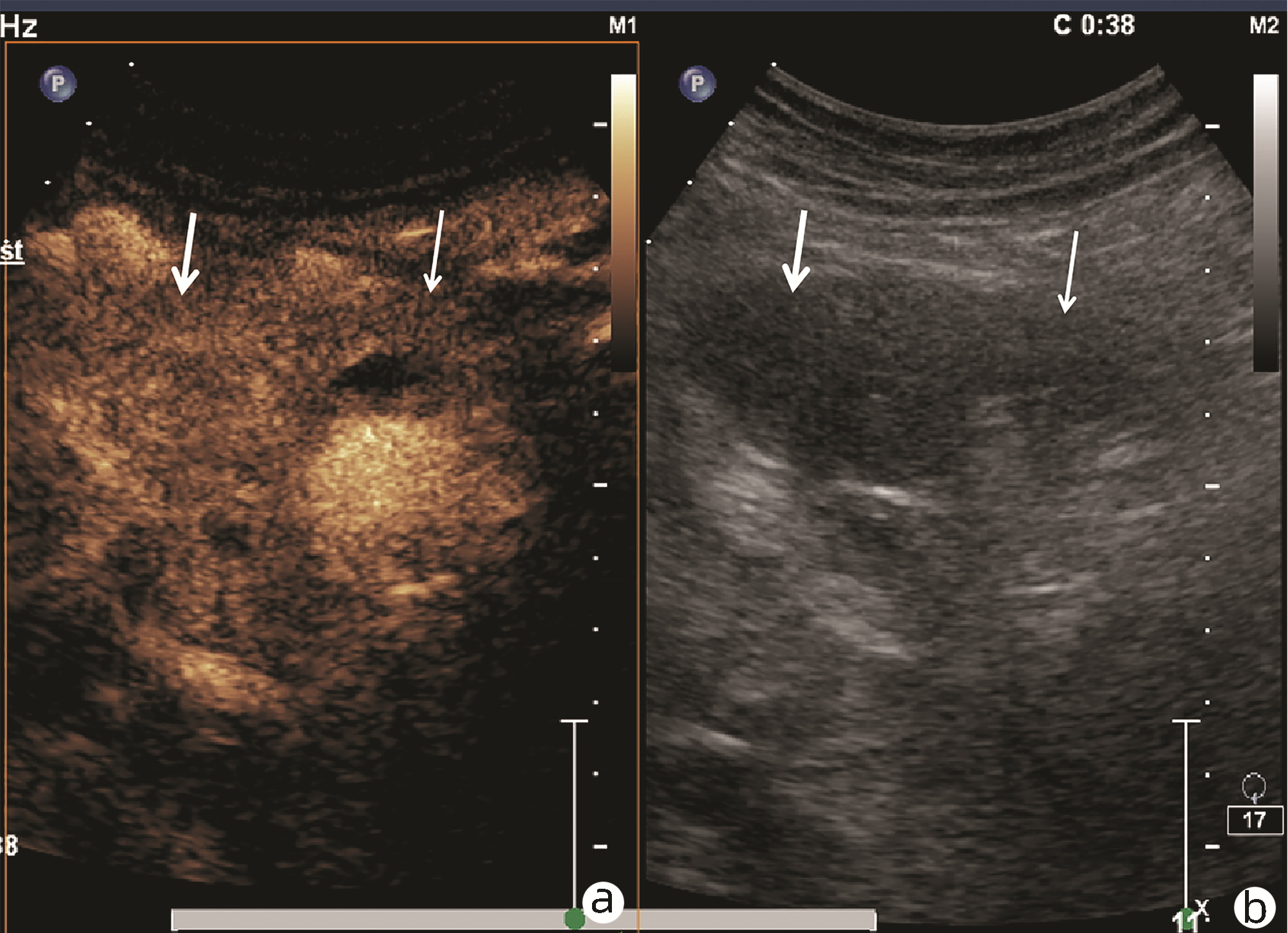
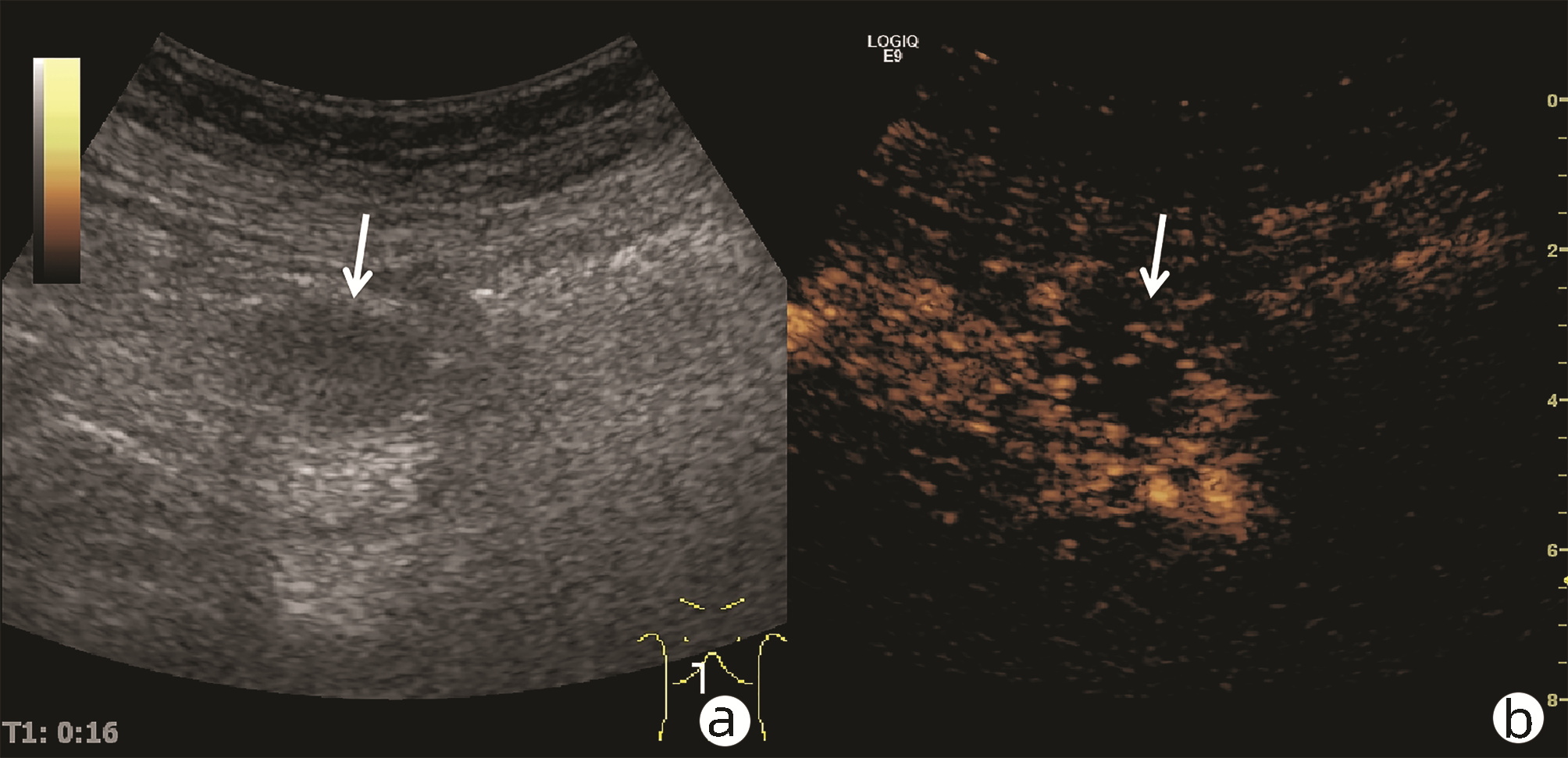
目的 探讨超声及超声造影对肿块型自身免疫性胰腺炎(AIP)与胰腺导管腺癌(PDAC)的鉴别诊断价值。 方法 回顾性分析2015年1月—2020年12月唐山市工人医院确诊的11例肿块型AIP患者的临床资料及常规超声、超声造影资料,分析其特征性表现,并与23例PDAC患者的资料进行对比,计数资料两组间比较采用χ2检验。 结果 11例肿块型AIP超声造影的诊断准确性为63.64%,均为单发病灶,且均低回声,在边界清晰、形态规则、胰管扩张或截断、血流信号方面所占比例分别为54.55%、63.64%、18.18%、36.36%,而PDCA组分别为30.43%、34.78%、78.26%、21.74%,两组间是否伴胰管扩张或截断方面存在统计学差异(χ2=11.089,P<0.05),其余指标均无明显统计学差异(P值均>0.05)。超声造影中7例(63.64%)肿块型AIP动脉期呈高增强,4例(36.36%)呈等增强,静脉期5例(45.45%)呈高增强、6例(54.55%)呈等增强;23例PDAC中22例(95.65%)病灶在动脉期及静脉期均呈低增强,两者动、静脉期强化方式差异均有统计学意义(χ2值分别为30.345、30.084,P值均<0.05)。 结论 超声造影检查增强模式及是否伴胰管扩张或截断方面在肿块型AIP与PDCA的鉴别诊断中具有较高的价值。 Abstract:Objective To investigate the value of ultrasound and contrast-enhanced ultrasound (CEUS) in the differential diagnosis of mass-type autoimmune pancreatitis (AIP) and pancreatic ductal adenocarcinoma (PDAC). Methods A retrospective analysis was performed for the clinical data, ultrasound findings, and CEUS findings of 11 patients with mass-type AIP who were diagnosed in Tangshan Workers' Hospital from January 2015 to December 2020, and their characteristic manifestations were analyzed and compared with the data of 23 patients with PDCA. The chi-square test was used for comparison of categorical data between two groups. Results For the 11 patients with mass-type AIP, CEUS had a diagnostic accuracy of 63.64%, and all of these patients had hypoechoic single lesions; the patients with clear boundaries, regular morphology, pancreatic duct dilatation or cutoff, and blood flow signal accounted for 54.55%, 63.64%, 18.18%, and 36.36%, respectively, while in the PDCA group, such patients accounted for 30.43%, 34.78%, 78.26%, and 21.74%, respectively, and there was a significant difference in the presence or absence of pancreatic duct dilatation or cutoff between the two groups(χ2=11.089, P < 0.05), with no significant differences in the other indices (all P > 0.05). For the 11 patients with mass-type AIP, CEUS showed that 7 patients (63.64%) had hyperenhancement and 4 (36.36%) had iso-enhancement in the arterial phase, and 5 patients (45.45%) had hyperenhancement in the arterial phase and 6 (54.55%) had iso-enhancement in the venous phase; for the 23 patients with PDCA, 22 (95.65%) had hypoenhancement of lesions in both arterial and venous phases, and there were significant differences in the enhancement pattern in arterial and venous phases between the two groups (χ2=30.345 and 30.084, both P < 0.05). Conclusion The enhancement pattern of CEUS and the presence or absence of pancreatic duct dilatation or cutoff have a relatively high value in the differential diagnosis of mass-type AIP and PDCA. -
表 1 肿块型AIP与PDCA常规超声表现比较
Table 1. Ultrasonographic characteristics between focal autoimmune pancreatitis and pancreatic ductal adenocarcinoma
超声表现 肿块型AIP
(n=11)PDCA
(n=23)χ2值 P值 病灶部位[例(%)] 2.543 0.280 胰头 9(81.82) 17(73.91) 胰体 2(18.18) 2(8.70) 胰尾 0 4(17.39) 病灶边界[例(%)] 1.832 0.176 清晰 6(54.55) 7(30.43) 不清晰 5(45.45) 16(69.57) 病灶形态[例(%)] 2.531 0.113 规则 7(63.64) 8(34.78) 不规则 4(36.36) 15(65.22) 是否伴有液化[例(%)] 2.168 0.141 是 0 4(17.39) 否 11(100) 19(82.61) 胰管扩张或截断[例(%)] 11.089 0.001 是 2(18.18) 18(78.26) 否 9(81.82) 5(21.74) 血流信号[例(%)] 0.818 0.366 伴有 4(36.36) 5(21.74) 不伴有 7(63.64) 18(78.26) 表 2 肿块型AIP与PDCA超声造影表现
Table 2. Contrast-enhanced ultrasonography between focal autoimmune pancreatitis and pancreatic ductal adenocarcinoma
期别 肿块型AIP(n=11) PDAC(n=23) χ2值 P值 高增强 等增强 低增强 高增强 等增强 低增强 动脉期(例) 7 4 0 0 1 22 30.345 <0.001 静脉期(例) 5 6 0 0 1 22 30.084 <0.001 -
[1] GOYAL S, SAKHUJA P. Autoimmune pancreatitis: Current perspectives[J]. Indian J Pathol Microbiol, 2021, 64(Supplement): S149-S159. DOI: 10.4103/ijpm.ijpm_59_21. [2] SHIMOSEGAWA T, CHARI ST, FRULLONI L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology[J]. Pancreas, 2011, 40(3): 352-358. DOI: 10.1097/MPA.0b013e3182142fd2. [3] PODDIGHE D. Autoimmune pancreatitis and pancreatic cancer: Epidemiological aspects and immunological considerations[J]. World J Gastroenterol, 2021, 27(25): 3825-3836. DOI: 10.3748/wjg.v27.i25.3825. [4] HAGHBIN H, CHUANG J, FATIMA R, et al. Correlation of autoimmune pancreatitis and malignancy: Systematic review and Meta-analysis[J]. Dig Dis Sci, 2021. DOI: 10.1007/s10620-021-07179-9. [5] ZHANG Q, YANG DH, YU LY, et al. Clinical value of contrast-enhanced ultrasound in diagnosis of isolated autoimmune pancreatitis[J]. Chin J Ultrasound Med, 2019, 35(1): 35-38. DOI: 10.3969/j.issn.1002-0101.2019.01.013.张琪, 杨道辉, 于凌云, 等. 超声造影在诊断肿块型自身免疫性胰腺炎中的临床价值[J]. 中国超声医学杂志, 2019, 35(1): 35-38. DOI: 10.3969/j.issn.1002-0101.2019.01.013. [6] NAITOH I, KAMISAWA T, TANAKA A, et al. Clinical characteristics of immunoglobulin IgG4-related sclerosing cholangitis: Comparison of cases with and without autoimmune pancreatitis in a large cohort[J]. Dig Liver Dis, 2021, 53(10): 1308-1314. DOI: 10.1016/j.dld.2021.02.009. [7] CHEN ZK, ZHANG XJ, QIAN QF, et al. Ultrasonic features and diagnosis of autoimmune pancreatitis[J]. Chin J Med Imaging Technol, 2019, 35(2): 310-311. DOI: 10.13929/j.1003-3289.201807102.陈志奎, 张秀娟, 钱清富, 等. 自身免疫性胰腺炎的超声表现与诊断[J]. 中国医学影像技术, 2019, 35(2): 310-311. DOI: 10.13929/j.1003-3289.201807102. [8] ZHANG XL, YANG PP, QIAN LX. Ultrasound manifestations and characteristics analysis of IgG4-related autoimmune pancreatitis[J]. J Clin Exp Med, 2019, 18(19): 2127-2129. DOI: 10.3969/j.issn.1671-4695.2019.19.034.张晓丽, 杨沛沛, 钱林学. IgG4相关自身免疫性胰腺炎的超声表现及特征分析[J]. 临床和实验医学杂志, 2019, 18(19): 2127-2129. DOI: 10.3969/j.issn.1671-4695.2019.19.034. [9] MACINGA P, BAJER L, DEL CHIARO M, et al. Pancreatic cancer in patients with autoimmune pancreatitis: A scoping review[J]. Pancreatology, 2021, 21(5): 928-937. DOI: 10.1016/j.pan.2021.03.007. [10] ZHOU CX, ZHU XH, HUANG Y, et al. Value of contrast-enhance ultrasound and color doppler ultrasonography in the diagnosis of pancreatic tumors[J]. Pract J Cancer, 2017, 32(5): 841-843. DOI: 10.3969/j.issn.1001-5930.2017.05.045.周成香, 朱小虎, 黄杨, 等. 超声造影联合彩色多普勒超声对胰腺肿瘤的诊断价值[J]. 实用癌症杂志, 2017, 32(5): 841-843. DOI: 10.3969/j.issn.1001-5930.2017.05.045. [11] HAN YC. Comparison of ultrasonography characteristics and clinical manifestations between autoimmune pancreatitis and pancreatic cancer[J]. Clin Res Pract, 2019, 4(8): 98-99. DOI: 10.19347/j.cnki.2096-1413.201908038.韩玉彩. 自身免疫性胰腺炎与胰腺癌的超声特点及临床表现比较[J]. 临床医学研究与实践, 2019, 4(8): 98-99. DOI: 10.19347/j.cnki.2096-1413.201908038. [12] CONTI CB, CEREATTI F, DRAGO A, et al. Focal autoimmune pancreatitis: A simple flow chart for a challenging diagnosis[J]. Ultrasound Int Open, 2020, 6(3): E67-E75. DOI: 10.1055/a-1323-4906. [13] GAO F, DU LF. The review of ultrasound mediated micrbubble for pancreatic cancer diagnosis and treatment[J]. Chin J Ultrasonogr, 2020, 29(10): 911-915. DOI: 10.3760/cma.j.cn131148-20200514-00397.高峰, 杜联芳. 超声介导微泡在胰腺癌诊疗中的研究进展[J]. 中华超声影像学杂志, 2020, 29(10): 911-915. DOI: 10.3760/cma.j.cn131148-20200514-00397. [14] ZHANG LP. Differential diagnosis of pancreatic cancer and mass pancreatitis by contrast-enhanced ultrasound[J]. Modern Med Imagel Bimonthly, 2019, 28(12): 2712-2713. https://www.cnki.com.cn/Article/CJFDTOTAL-XDYY201912062.htm张丽萍. 超声造影对胰腺癌与肿块型胰腺炎的鉴别诊断效果分析[J]. 现代医用影像学, 2019, 28(12): 2712-2713. https://www.cnki.com.cn/Article/CJFDTOTAL-XDYY201912062.htm [15] WANG Y, AIDIBAI MHMT, GENG C, et al. Differential diagnostic value of pancreatic cancer and mass pancreatitis by contrast-enhanced ultrasound[J]. J Clin Ultrasound in Med, 2017, 19(8): 526-530. https://www.cnki.com.cn/Article/CJFDTOTAL-LCCY201708006.htm王燕, 艾迪拜·木合买提, 耿诚, 等. 超声造影对胰腺癌与肿块型胰腺炎的鉴别诊断价值[J]. 临床超声医学杂志, 2017, 19(8): 526-530. https://www.cnki.com.cn/Article/CJFDTOTAL-LCCY201708006.htm [16] XU XH, GUO WH, LI Y. Diagnostic value of EUS-EG combined with CEH-EUS in pancreatic space-occupying lesions[J]. Guangdong Med J, 2020, 41(20): 2146-2149. DOI: 10.13820/j.cnki.gdyx.20192312.许晓辉, 郭卫红, 李艳. 超声内镜弹性成像联合谐波造影增强超声内镜在胰腺占位性病变中的诊断价值[J]. 广东医学, 2020, 41(20): 2146-2149. DOI: 10.13820/j.cnki.gdyx.20192312. -



 PDF下载 ( 3738 KB)
PDF下载 ( 3738 KB)

 下载:
下载: