线粒体功能障碍与肝细胞癌的关系
DOI: 10.3969/j.issn.1001-5256.2022.06.045
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:高小强负责资料分析,撰写论文;左石、贾晓东负责提出具体修改意见并进行修改;陆荫英负责拟定撰写思路,指导文章写作并最终定稿。
-
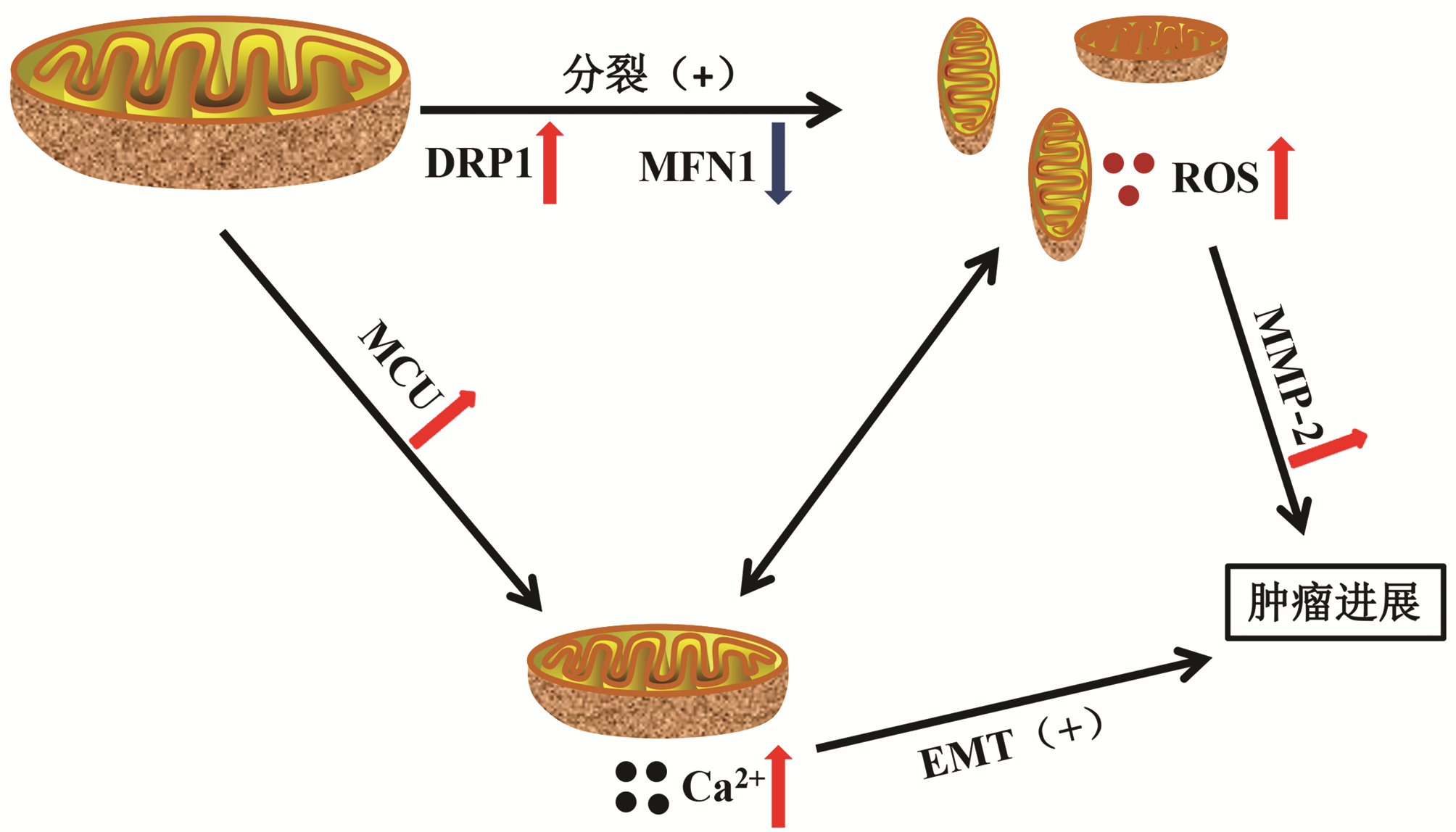
摘要: 肝细胞癌(HCC)是一种发病率高、早诊率低、预后差的肿瘤,其发生与发展涉及多方面因素。线粒体是细胞的“能量工厂”,是体内活性氧自由基产生的主要部位之一,并参与调控细胞凋亡,是一种重要的细胞器。在HCC发生、发展过程中,线粒体膜、氧化呼吸链、线粒体动力学、线粒体DNA、线粒体钙稳态等均发生不同程度的变化,且这种改变会影响HCC的进展。系统阐述线粒体与HCC的关系,为HCC的诊治提供新方向。Abstract: Hepatocellular carcinoma (HCC) is a type of tumor with a high incidence rate, a low rate of early diagnosis, and poor prognosis, and its development and progression involve many factors. As an important organelle in cells, mitochondria is the "energy factory" of cells and is one of the main sites for the production of reactive oxygen species in vivo, and it also participates in the regulation of cell apoptosis. There are varying degrees of changes in mitochondrial membrane, oxidation respiratory chain, mitochondrial dynamics, mitochondrial DNA, and mitochondrial calcium homeostasis during the development and progression of HCC, and such changes may affect the progression of HCC. This article systematically elaborates on the association between mitochondria and HCC, so as to provide a new direction for the diagnosis and treatment of HCC.
-
Key words:
- Mitochondrion /
- Carcinoma, Hepatocellular /
- Therapeutics
-
[1] LOOSEN SH, CASTOLDI M, JÖRDENS MS, et al. Serum levels of circulating microRNA-107 are elevated in patients with early-stage HCC[J]. PLoS One, 2021, 16(3): e0247917. DOI: 10.1371/journal.pone.0247917. [2] GAO Y, LYU L, FENG Y, et al. A review of cutting-edge therapies for hepatocellular carcinoma (HCC): Perspectives from patents[J]. Int J Med Sci, 2021, 18(14): 3066-3081. DOI: 10.7150/ijms.59930. [3] XING M, WANG X, KIKEN RA, et al. Immunodiagnostic biomarkers for hepatocellular carcinoma (HCC): The first step in detection and treatment[J]. Int J Mol Sci, 2021, 22(11) : 6139. DOI: 10.3390/ijms22116139. [4] SUPINSKI GS, SCHRODER EA, CALLAHAN LA. Mitochondria and critical illness[J]. Chest, 2020, 157(2): 310-322. DOI: 10.1016/j.chest.2019.08.2182. [5] MIDDLETON P, VERGIS N. Mitochondrial dysfunction and liver disease: Role, relevance, and potential for therapeutic modulation[J]. Therap Adv Gastroenterol, 2021, 14: 17562848211031394. DOI: 10.1177/17562848211031394. [6] LIAO ZB, TAN XL, DONG KS, et al. miRNA-448 inhibits cell growth by targeting BCL-2 in hepatocellular carcinoma[J]. Dig Liver Dis, 2019, 51(5): 703-711. DOI: 10.1016/j.dld.2018.09.021. [7] YIN PH, LEE HC, CHAU GY, et al. Alteration of the copy number and deletion of mitochondrial DNA in human hepatocellular carcinoma[J]. Br J Cancer, 2004, 90(12): 2390-2396. DOI: 10.1038/sj.bjc.6601838. [8] HERNÁNDEZ-ALVAREZ MI, ZORZANO A. Mitochondrial dynamics and liver cancer[J]. Cancers (Basel), 2021, 13(11): 2571. DOI: 10.3390/cancers13112571. [9] LI Y, GUO X, GUO S, et al. Next generation sequencing-based analysis of mitochondrial DNA characteristics in plasma extracellular vesicles of patients with hepatocellular carcinoma[J]. Oncol Lett, 2020, 20(3): 2820-2828. DOI: 10.3892/ol.2020.11831. [10] JIN M, WANG J, JI X, et al. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma[J]. J Exp Clin Cancer Res, 2019, 38(1): 136. DOI: 10.1186/s13046-019-1135-x. [11] SUN H, YU J, WEN Z, et al. Decreased expression of Beclin-1 in patients with hepatocellular carcinoma[J]. J BUON, 2019, 24(2): 634-641. [12] FUNK K, CZAUDERNA C, KLESSE R, et al. BAX redistribution induces apoptosis resistance and selective stress sensitivity in human HCC[J]. Cancers (Basel), 2020, 12(6) : 1437. DOI: 10.3390/cancers12061437. [13] LE PH, HUANG SC, LIM SN, et al. Complex Ⅳ subunit 1 defect predicts postoperative survival in hepatocellular carcinoma[J]. Oncol Lett, 2014, 7(5): 1430-1438. DOI: 10.3892/ol.2014.1966. [14] XIAO MH, LIN YF, XIE PP, et al. Downregulation of a mitochondrial micropeptide, MPM, promotes hepatoma metastasis by enhancing mitochondrial complex I activity[J]. Mol Ther, 2022, 30(2): 714-725. DOI: 10.1016/j.ymthe.2021.08.032. [15] WANG T, XIE X, LIU H, et al. Pyridine nucleotide-disulphide oxidoreductase domain 2 (PYROXD2): Role in mitochondrial function[J]. Mitochondrion, 2019, 47: 114-124. DOI: 10.1016/j.mito.2019.05.007. [16] LI Y, LIN S, LI L, et al. PDSS2 Deficiency Induces hepatocarcinogenesis by decreasing mitochondrial respiration and reprogramming glucose metabolism[J]. Cancer Res, 2018, 78(16): 4471-4481. DOI: 10.1158/0008-5472.CAN-17-2172. [17] ZHANG Z, LI TE, CHEN M, et al. MFN1-dependent alteration of mitochondrial dynamics drives hepatocellular carcinoma metastasis by glucose metabolic reprogramming[J]. Br J Cancer, 2020, 122(2): 209-220. DOI: 10.1038/s41416-019-0658-4. [18] WANG X, LIU Y, SUN J, et al. Mitofusin-2 acts as biomarker for predicting poor prognosis in hepatitis B virus related hepatocellular carcinoma[J]. Infect Agent Cancer, 2018, 13: 36. DOI: 10.1186/s13027-018-0212-7. [19] ANDERSON S, BANKIER AT, BARRELL BG, et al. Sequence and organization of the human mitochondrial genome[J]. Nature, 1981, 290(5806): 457-465. DOI: 10.1038/290457a0. [20] GUO ZS, JIN CL, YAO ZJ, et al. Analysis of the mitochondrial 4977 bp deletion in patients with hepatocellular carcinoma[J]. Balkan J Med Genet, 2017, 20(1): 81-86. DOI: 10.1515/bjmg-2017-0006. [21] HUNG WY, LIN JC, LEE LM, et al. Tandem duplication/triplication correlated with poly-cytosine stretch variation in human mitochondrial DNA D-loop region[J]. Mutagenesis, 2008, 23(2): 137-142. DOI: 10.1093/mutage/gen002. [22] HIGURASHI M, MARUYAMA T, NOGAMI Y, et al. High expression of FOXM1 critical for sustaining cell proliferation in mitochondrial DNA-less liver cancer cells[J]. Exp Cell Res, 2020, 389(1): 111889. DOI: 10.1016/j.yexcr.2020.111889. [23] ROMERO-GARCIA S, PRADO-GARCIA H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review)[J]. Int J Oncol, 2019, 54(4): 1155-1167. DOI: 10.3892/ijo.2019.4696. [24] LI CJ, LIN HY, KO CJ, et al. A novel biomarker driving poor-prognosis liver cancer: overexpression of the mitochondrial calcium gatekeepers[J]. Biomedicines, 2020, 8(11): 451. DOI: 10.3390/biomedicines8110451. [25] KOIKE K. Molecular basis of hepatitis C virus-associated hepatocarcinogenesis: Lessons from animal model studies[J]. Clin Gastroenterol Hepatol, 2005, 3(10 Suppl 2): S132-S135. DOI: 10.1016/s1542-3565(05)00700-7. [26] ZHANG R, ZHANG F, WANG C, et al. Identification of sequence polymorphism in the D-Loop region of mitochondrial DNA as a risk factor for hepatocellular carcinoma with distinct etiology[J]. J Exp Clin Cancer Res, 2010, 29: 130. DOI: 10.1186/1756-9966-29-130. [27] JIN Y, YU Q, ZHOU D, et al. The mitochondrial DNA 9-bp deletion polymorphism is a risk factor for hepatocellular carcinoma in the Chinese population[J]. Genet Test Mol Biomarkers, 2012, 16(5): 330-334. DOI: 10.1089/gtmb.2011.0208. [28] AHMED HS, WAHAB EA, ELHADY HA, et al. Association of genetic polymorphism of BCL-2 (rs2279115) with susceptibility to HCV-related hepatocellular carcinoma[J]. Immunol Res, 2020, 68(4): 189-197. DOI: 10.1007/s12026-020-09143-7. [29] HUANG Q, ZHAN L, CAO H, et al. Increased mitochondrial fission promotes autophagy and hepatocellular carcinoma cell survival through the ROS-modulated coordinated regulation of the NFKB and TP53 pathways[J]. Autophagy, 2016, 12(6): 999-1014. DOI: 10.1080/15548627.2016.1166318. [30] REN T, ZHANG H, WANG J, et al. MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells[J]. Oncogene, 2017, 36(42): 5897-5909. DOI: 10.1038/onc.2017.167. [31] HU W, FU J, LU SX, et al. Decrease of Bcl-xL/Bcl-2-associated death promoter in hepatocellular carcinoma indicates poor prognosis[J]. Am J Cancer Res, 2015, 5(5): 1805-1813. [32] LI S, WAN P, PENG T, et al. Associations between sequence variations in the mitochondrial DNA D-loop region and outcome of hepatocellular carcinoma[J]. Oncol Lett, 2016, 11(6): 3723-3728. DOI: 10.3892/ol.2016.4466. [33] JIAN C, FU J, CHENG X, et al. Low-dose sorafenib acts as a mitochondrial uncoupler and ameliorates nonalcoholic steatohepatitis[J]. Cell Metab, 2020, 31(5): 892-908. e11. DOI: 10.1016/j.cmet.2020.04.011. [34] CHIOU JF, TAI CJ, WANG YH, et al. Sorafenib induces preferential apoptotic killing of a drug- and radio-resistant Hep G2 cells through a mitochondria-dependent oxidative stress mechanism[J]. Cancer Biol Ther, 2009, 8(20): 1904-1913. DOI: 10.4161/cbt.8.20.9436. [35] LI Y, XIA J, SHAO F, et al. Sorafenib induces mitochondrial dysfunction and exhibits synergistic effect with cysteine depletion by promoting HCC cells ferroptosis[J]. Biochem Biophys Res Commun, 2021, 534: 877-884. DOI: 10.1016/j.bbrc.2020.10.083. [36] ZHANG C, HU J, SHENG L, et al. Metformin delays AKT/c-Met-driven hepatocarcinogenesis by regulating signaling pathways for de novo lipogenesis and ATP generation[J]. Toxicol Appl Pharmacol, 2019, 365: 51-60. DOI: 10.1016/j.taap.2019.01.004. [37] YE RR, PENG W, CHEN BC, et al. Mitochondria-targeted artesunate conjugated cyclometalated iridium(iii) complexes as potent anti-HepG2 hepatocellular carcinoma agents[J]. Metallomics, 2020, 12(7): 1131-1141. DOI: 10.1039/d0mt00060d. [38] PITTALA S, KRELIN Y, SHOSHAN-BARMATZ V. Targeting liver cancer and associated pathologies in mice with a mitochondrial VDAC1-based peptide[J]. Neoplasia, 2018, 20(6): 594-609. DOI: 10.1016/j.neo.2018.02.012. -



 PDF下载 ( 2104 KB)
PDF下载 ( 2104 KB)

 下载:
下载:


