扩大慢性乙型肝炎抗病毒治疗应重视ALT正常、年龄≤30岁的HBeAg阴性慢性HBV感染者
DOI: 10.3969/j.issn.1001-5256.2022.07.006
HBeAg-negative chronic HBV-infected individuals with normal alanine aminotransferase and an age of ≤30 years should be taken seriously when expanding anti-HBV treatment for chronic hepatitis B
-
摘要:
目的 拟对2022年2月发布的《扩大慢性乙型肝炎抗病毒治疗的专家意见》的推荐意见进行分析验证和完善。 方法 本研究为单中心回顾性研究,连续纳入2014年1月—2020年10月于中国人民解放军总医院第五医学中心接受肝穿刺活组织病理检查且ALT正常的成人慢性HBV感染者,并分析≤30岁和>30岁亚组中不同HBeAg状态人群存在中、重度肝损伤人群比例。 结果 共纳入慢性HBV感染者290例,HBeAg阳性者121例(41.7%),HBeAg阴性者169例(58.3%)。进一步按年龄分组,在HBeAg阳性中,≤30岁37例,>30岁84例;在HBeAg阴性感染者中,≤30岁24例,>30岁145例。4组比较,年龄(H=151.539)、性别(χ2=9.959)、ALT(H=29.041)、AST(H=11.127)、Alb(H=23.538)、HBV DNA(H=187.982)、HBsAg(H=132.520)组间比较差异均有统计学意义(P值均<0.05)。年龄>30岁和≤30岁组均有近50%的患者存在中度及以上肝损伤(50.22% vs 47.54%)。根据HBeAg状态和年龄进一步将患者分为HBeAg阳性≤30岁(n=37)和>30岁(n=84)以及HBeAg阴性≤30岁(n=24)和>30岁(n=145)共4组。在HBeAg阳性亚组中,>30岁与≤30岁者存在中度及以上肝损伤的差异无统计学意义(42.9% vs 37.8%,P=0.605);≤30岁的HBeAg阴性亚组与>30岁的HBeAg阴性亚组存在中度及以上肝损伤的差异亦无统计学意义(62.5% vs 54.5%,P=0.464)。在无创指标无法决策的队列(LSM<9.0 kPa者和LSM数据缺失但FIB-4<3.25者,n=269)中,≤30岁的HBeAg阴性亚组与>30岁的HBeAg阴性/阳性亚组存在中度及以上肝损伤的差异无统计学意义(59.1% vs 50.7%、59.1% vs 41.8%,P值分别为0.468、0.149)。 结论 在扩大抗HBV治疗时,也应关注HBeAg阴性且年龄≤30岁的慢性HBV感染者。条件允许时,依照本研究总结修订的抗病毒治疗启动线路图,或可进一步提高个体化抗病毒治疗的精准性。 Abstract:Objective To validate and refine the recommendations in the recently published expert opinion on expanding anti-HBV treatment for chronic hepatitis B. Methods Adult individuals with chronic HBV infection and normal alanine aminotransferase (ALT) who underwent liver biopsy in The Fifth Medical Center of Chinese PLA General Hospital from January 2014 to October 2020 were enrolled in this single-center retrospective study, and the proportion of individuals with moderate or severe liver injury was analyzed in the population with different HBeAg status in the ≤30 years and > 30 years subgroups. Results A total of 290 individuals with chronic HBV infection were included, among whom 121 (41.7%) were HBeAg positive and 169 (58.3%) were HBeAg negative. The HBeAg-positive group and the HBeAg-negative group were further divided into subgroups according to age: in the HBeAg-positive group, 37 were aged ≤30 years and 84 were aged > 30 years; in the HBeAg-negative group, 24 were aged ≤30 years and 145 were aged > 30 years. There were significant differences between the four groups in age (H=151.539, P < 0.05), sex (χ2=9.959, P < 0.05), ALT (H=29.041, P < 0.05), aspartate aminotransferase (H=11.127, P < 0.05), albumin (H=23.538, P < 0.05), HBV DNA (H=187.982, P < 0.05), and HBsAg (H=132.520, P < 0.05). In both > 30 years and ≤30 years groups, nearly 50% of the patients had a moderate or higher grade of liver injury (50.22% vs 47.54%). According to HBeAg status and age, the patients were further divided into HBeAg-positive ≤30 years group with 37 patients, HBeAg-positive > 30 years group with 84 patients, HBeAg-negative ≤30 years group with 24 patients, and HBeAg-negative > 30 years group with 145 patients. In the HBeAg-positive group, there was no significant difference in the proportion of patients with a moderate or higher grade of liver injury between the patients aged > 30 years and those aged ≤30 years (42.9% vs 37.8%, P=0.605), and there was also no significant difference in such proportion between the HBeAg-negative ≤30 years group and the HBeAg-negative/positive > 30 years groups (62.5% vs 54.5%, P=0.464). In the cohort for which a decision could not be made based on noninvasive indices (269 patients with liver stiffness measurement < 9.0 kPa or without the data of liver stiffness measurement but with fibrosis-4 < 3.25), there was no significant difference in the proportion of patients with a moderate or higher grade of liver injury between the HBeAg-negative ≤30 years group and the HBeAg-negative/positive > 30 years groups (59.1% vs 50.7% and 59.1% vs 41.8%, P=0.468 and 0.149). Conclusion HBeAg-negative chronic HBV-infected individuals with an age of ≤30 years should be taken into consideration when expanding anti-HBV treatment for chronic hepatitis B. If conditions permit, the revised flow chart for the initiation of anti-HBV treatment based on this study can be applied to further improve the precision of individualized anti-HBV treatment. -
Key words:
- Hepatitis B, Chronic /
- Hepatitis B e Antigens /
- Age Factors /
- Alanine Transaminase /
- Antiviral Treatment
-
2022年2月,由中华医学会肝病学分会发布的《扩大慢性乙型肝炎抗病毒治疗的专家意见》[1](以下简称为《专家意见》)总结了目前对扩大慢性乙型肝炎患者抗病毒治疗适应证的临床研究证据,并针对国内未合并肝硬化与肝癌的慢性乙型肝炎患者抗病毒治疗的推荐意见进行了更新和补充。《专家意见》推荐“血清HBV DNA阳性,无论ALT高低,只要符合年龄>30岁,建议抗病毒治疗”。庄辉教授[2]近期也提出了“ALT正常、HBeAg阳性慢性HBV感染患者,如年龄>30岁,或有肝细胞癌(HCC)或肝硬化家族史,可考虑治疗;ALT正常、HBV DNA≥2000 IU/mL、HBeAg阴性慢性HBV感染患者,如年龄>30岁,或有HCC或肝硬化家族史,可考虑治疗”的观点。欧洲肝病学会[3]和我国最新的慢性乙型肝炎诊疗指南[4]均将>30岁作为慢性HBV感染者启动抗病毒治疗的参考条件之一。
为了对上述推荐意见和观点进行验证,笔者在一个单中心回顾性队列中,以30岁为界值,并将国内外主流指南共同推荐的“存在中度及以上肝损伤为启动抗病毒治疗的病理指证”作为标准进行分析。由于我国普及乙型肝炎疫苗接种后HBeAg阴性慢性乙型肝炎呈逐年递增趋势,至2006年全国HBV感染流行病学调查时,慢性HBV感染及乙型肝炎患者中HBeAg阴性的占比已达66.7%[5]。因此,本研究将重点着眼于HBeAg阴性慢性HBV感染者,以期提供更多临床证据,完善《专家意见》。
1. 资料与方法
1.1 研究对象
回顾性选取2014年1月—2021年10月于中国人民解放军总医院第五医学中心接受肝穿刺活组织病理检查且ALT正常的成人慢性HBV感染者。纳入标准:(1)年龄≥18岁,性别不限;(2)HBsAg和/或HBV DNA阳性6个月以上;(3)接受肝组织学检查,且有明确病理诊断;(4)肝组织学检查同期ALT正常(定义为ALT≤40 U/L)。排除标准:(1)合并其他肝炎病毒感染;(2)合并其他肝脏疾病,如酒精性或非酒精性脂肪肝、药物性肝损伤、自身免疫性肝病、胆汁淤积性肝炎等;(3)疾病进展为肝硬化、肝癌、肝衰竭等;(4)6个月内服用过抗病毒药物或应用干扰素;(5)1个月内服用过保肝降酶药物或免疫调剂药物;(6)妊娠或哺乳期妇女。
1.2 肝穿刺活组织病理检查
所有入组者均在超声引导下行肝穿刺活组织病理检查,肝活检组织样本长度要求≥1.5 cm,至少在镜下包括≥5个汇管区。肝活检组织样本采用4%甲醛固定,石蜡包埋后连续切片,厚度为4 μm,并行苏木精-伊红染色、网状纤维和Masson三色染色。肝组织病理学诊断按照国内通用的2000年西安修订的病毒性肝炎防治方案标准[6](纤维化程度分期S0~S4,炎症分级为G0~G4),由2位病理学专家进行评估。显著肝损伤定义为经病理诊断存在中度以上肝脏炎症(≥G2)和/或中度及以上肝纤维化(≥S2)。
1.3 肝纤维化无创诊断指标
参照我国2018年《瞬时弹性成像技术诊断肝纤维化专家共识》[7]中的推荐意见[肝硬度值(LSM)≥9.0 kPa考虑进展期肝纤维化]和既往研究结论(FIB-4≥3. 25诊断为进展期肝纤维化),将可通过无创指标提示存在明确肝损伤者剔除,仅纳入无法决策者,结合肝组织活检病理检查结果分析不同年龄分组中符合抗病毒治疗指征者占比。
1.4 统计学方法
采用SPSS 24.0软件进行统计学分析。符合正态分布的计量资料以x±s表示,组间比较采用方差分析;不符合正态分布的计量资料以M(P25~P75)表示,组间比较采用Kruskal-Wallis H检验。计数资料组间比较采用χ2或Fisher确切概率检验。P<0.05为差异均有统计学意义。
2. 结果
2.1 一般资料
共纳入慢性HBV感染者290例,其中HBeAg阳性者121例(41.7%),HBeAg阴性者169例(58.3%)。进一步按年龄分组,在HBeAg阳性中,≤30岁37例,>30岁84例;在HBeAg阴性感染者中,≤30岁24例,>30岁145例。4组比较,年龄、性别、ALT、AST、Alb、HBV DNA、HBsAg组间比较差异均有统计学意义(P值均<0.05)。其他一般资料见表 1。
表 1 HBeAg阳性与阴性慢性HBV感染者一般资料比较Table 1. Comparison of general data between HBeAg positive and HBeAg negative chronic HBV infection指标 HBeAg阳性(n=121) HBeAg阴性(n=169) 统计值 P值 ≤30岁(n=37) >30岁(n=84) ≤30岁(n=24) >30岁(n=145) 年龄(岁) 26(23~29)1)2) 39(34~47)2)3)4) 27(24~29)1)2) 43(38~49)1)3)4) H=151.539 <0.001 性别[例(%)] χ2=9.959 0.019 男 22(59.5) 38(45.2) 18(75.0)2) 64(44.1)4) 女 15(40.5) 46(54.8) 6(25.0)2) 81(55.9)4) ALT(U/L) 30(22~36)2)4) 25(19~32)2) 20(16~28)3) 20(15~27)1)3) H=29.041 <0.001 AST(U/L) 25(21~31)2) 24(20~28) 21(18~25) 21(18~27)3) H=11.127 0.011 ALP(U/L) 70(56~89) 72(59~92) 71(63~88) 72(59~89) H=0.667 0.881 GGT(U/L) 14(11~19) 17(13~21) 17(14~25) 16(12~23) H=4.941 0.176 Alb(U/L) 41(39~44) 39(37~42)2)4) 43(41~45)1) 41(39~44)1) H=23.538 <0.001 PA(mg/L) 199.6±44.2 194.0±42.3 215.6±40.7 205.2±44.7 F=1.988 0.116 PLT(×109/L) 204(173~237) 200(158~230) 184(150~217) 188(158~225) H=3.816 0.282 HBV DNA(log10 IU/mL) 8.0(7.0~8.4)2)4) 8.0(7.4~8.3)2)4) 2.7(2.3~3.5)1)3) 3.2(2.5~4.1)1)3) H=187.982 <0.001 HBsAg(log10 IU/mL) 4.5(3.9~4.7)2)4) 4.6(3.9~4.9)2)4) 3.3(3.1~3.6)1)3) 3.2(2.6~3.6)1)3 H=132.520 <0.001 炎症分期[例(%)] 0.070 G0~G1 33(89.2) 64(76.2) 18(75.0) 127(87.6) G2~G4 4(10.8) 20(23.8) 6(25.0) 18(12.4) 肝纤维化分期[例(%)] χ2=6.459 0.091 S0~S1 24(64.9) 50(59.5) 10(41.7) 69(47.6) S2~S4 13(35.1) 34(40.5) 14(58.3) 76(52.4) 存在明显肝损伤[例(%)] χ2=6.556 0.087 否 23(62.2) 48(57.1) 9(37.5) 66(45.5) 是 14(37.8) 36(42.9) 15(62.5) 79(54.5) 注:与HBeAg阳性(>30岁)者比较,1)P<0.05;与HBeAg阴性(>30岁)者比较,2)P<0.05;与HBeAg阳性(≤30岁)者比较,3)P<0.05;与HBeAg阴性(≤30岁)者比较,4)P<0.05。 2.2 不同年龄分组中符合抗病毒治疗指征者占比
在不考虑HBeAg状态时,>30岁和≤30岁组均有50%左右慢性HBV感染者存在中度及以上肝损伤(50.22% vs 47.54%,P=0.710)。按照HBeAg状态进行亚组分析发现,在HBeAg阳性亚组中,>30岁与≤30岁者存在中度及以上肝损伤的差异无统计学意义(42.9% vs 37.8%,P=0.605);≤30岁的HBeAg阴性亚组与>30岁的HBeAg阴性/阳性亚组存在中度及以上肝损伤的差异亦无统计学意义(62.5% vs 54.5%、62.5% vs 42.9%,P值分别为0.464、0.089)。
2.3 无创指标无法决策队列中需抗病毒治疗者占比
在无创指标无法决策的队列中,105例慢性HBV感染者具有LSM数据,遂将其中LSM<9.0 kPa者和其余185例LSM数据缺失但FIB-4<3.25者纳入分析。最终纳入269例,其中HBeAg阳性者108例(42%),HBeAg阴性者144例(58%)。结果显示,≤30岁的HBeAg阴性亚组与>30岁的HBeAg阴性/阳性亚组存在中度及以上肝损伤的差异无统计学意义(59.1% vs 50.7%、59.1% vs 41.8%,P值分别为0.468、0.149) (表 2)。
表 2 无创指标无法决策抗病毒治疗者肝损伤情况比较Table 2. Comparison of liver injury in patients with non-invasive indicators unable to determine antiviral therapy是否存在明显肝损伤 HBeAg阳性 HBeAg阴性 χ2值 P值 ≤30岁(n=34) >30岁(n=79) ≤30岁(n=22) >30岁(n=134) 否[例(%)] 23(67.6) 46(58.2) 9(40.9) 66(49.3) 5.819 0.121 是[例(%)] 11(32.4) 33(41.8) 13(59.1) 68(50.7) 3. 讨论
积极的抗病毒治疗通过抑制病毒复制可显著减缓慢性乙型肝炎患者的疾病进展,并降低肝硬化和HCC的发生风险。本研究通过单中心回顾性队列,针对国内近期发布的《扩大慢性乙型肝炎抗病毒治疗的专家意见》[1]中对于ALT正常的慢性HBV感染者启动抗病毒治疗的推荐意见进行了分析和验证。本研究结果显示,对于ALT正常但已发生HBeAg阴转的慢性HBV感染者,即使年龄≤30岁,也应积极启动抗病毒治疗。
截至目前,在以肝组织病理活检作为金标准判断肝损伤情况的ALT正常成年慢性HBV感染者队列中分析不同年龄和HBeAg状态亚组中存在中重肝损伤的研究很少。一项来自国内的回顾性单中心研究[9]纳入300例ALT≤2倍正常值上限的慢性HBV感染者,结果表明,在HBeAg阴性且≤30岁亚组中,18.2%存在中度及以上肝脏炎症(G2~4),45.5%有显著及以上肝纤维化(S2~4);而在HBeAg阳性且≤30岁亚组中,上述比例分别降至3.1%和16.7%。尽管该研究并未对ALT<1倍正常值上限者开展亚组分析,但也提示HBeAg阴性慢性HBV感染者的肝损伤发生早于同年龄层的HBeAg阳性者。慢性HBV感染的疾病进程是长期的病毒与宿主免疫博弈过程,HBeAg阴转或血清学转换提示宿主免疫对HBV复制的控制。本研究中,≤30岁的HBeAg阴性者的HBV DNA水平低于其他3组(P<0.001)。在年龄上,HBeAg阴性组的中位年龄显著高于HBeAg阳性组(42岁vs 35岁,P<0.001);在>30岁亚组中,HBeAg阳性者中位年龄大于阴性者(39岁vs 43岁,P=0.001),符合预期;而在≤30岁亚组中,HBeAg阳性/阴性者中位年龄相近,提示该组更早进入了免疫清除期,加速了HBeAg的消失。上述现象说明,在宿主免疫清除感染肝细胞以控制HBV复制的过程中,更强的抗HBV免疫应答不仅加速了HBeAg的阴转和血清学转换,也带来了活跃的炎症反应和更多的肝组织炎症损伤。考虑到肝脏炎症活动度所致肝损伤及再生过程中的丢失(稀释)HBV cccDNA及新生肝细胞对再感染一定的抵抗作用有利于更好的抗病毒应答[10],未来或许应推荐对≤30岁但已经发生HBeAg阴转者开展抗病毒治疗。
笔者建议对于HBeAg阴转者积极抗病毒治疗的另一个原因是其中的非活动性HBsAg携带状态者仍具有较高的疾病进展风险。据报道[11-13],14%~24%的非活动性HBsAg携带状态者进展为HBeAg阴性慢性乙型肝炎,而近20%逆转为HBeAg阳性慢性乙型肝炎。我国台湾地区的队列研究[14-15]显示,非活动性HBsAg携带状态者25年累积肝硬化发生率高达15%;随访13.1年,这类人群HCC和肝病相关死亡风险分别是健康人群的4.6倍和2.1倍。因此,对于《专家意见》中可能未覆盖到的<30岁的HBeAg阴性的HBV感染者,临床医生在作出是否启动抗病毒治疗的决策时,应给予关注。
本研究存在单中心、小样本的局限,上述建议是否成立需要更多的临床证据。且由于患者来源于三甲医院而非社区,可能存在入组患者本身的偏倚,因此该队列中重度及以上肝损伤的比例可能比自然人群偏高。但由于肝活检有限的可及性,具有肝穿病理结果且来自社区人群的未经治疗、ALT正常的慢性乙型肝炎患者队列研究很少,尚无来自国内的研究。一项美国研究[16]从社区中纳入未经治疗且有肝穿病理结果的慢性HBV感染者,在ALT正常的亚组(n=57)中,≤35岁、36~50、>50岁的患者存在中重度肝损伤的比例分别为0、22%、45%(P=0.033),该结果与本研究队列数据存在很大的差异,提示对于不同来源(如社区医院、三甲医院)的患者,在疾病严重程度上可能存在较大差异。在实际临床应用中,应结合患者来源和其他临床资料(包括年龄和HBeAg状态等),对是否启动抗病毒治疗作出综合判断。
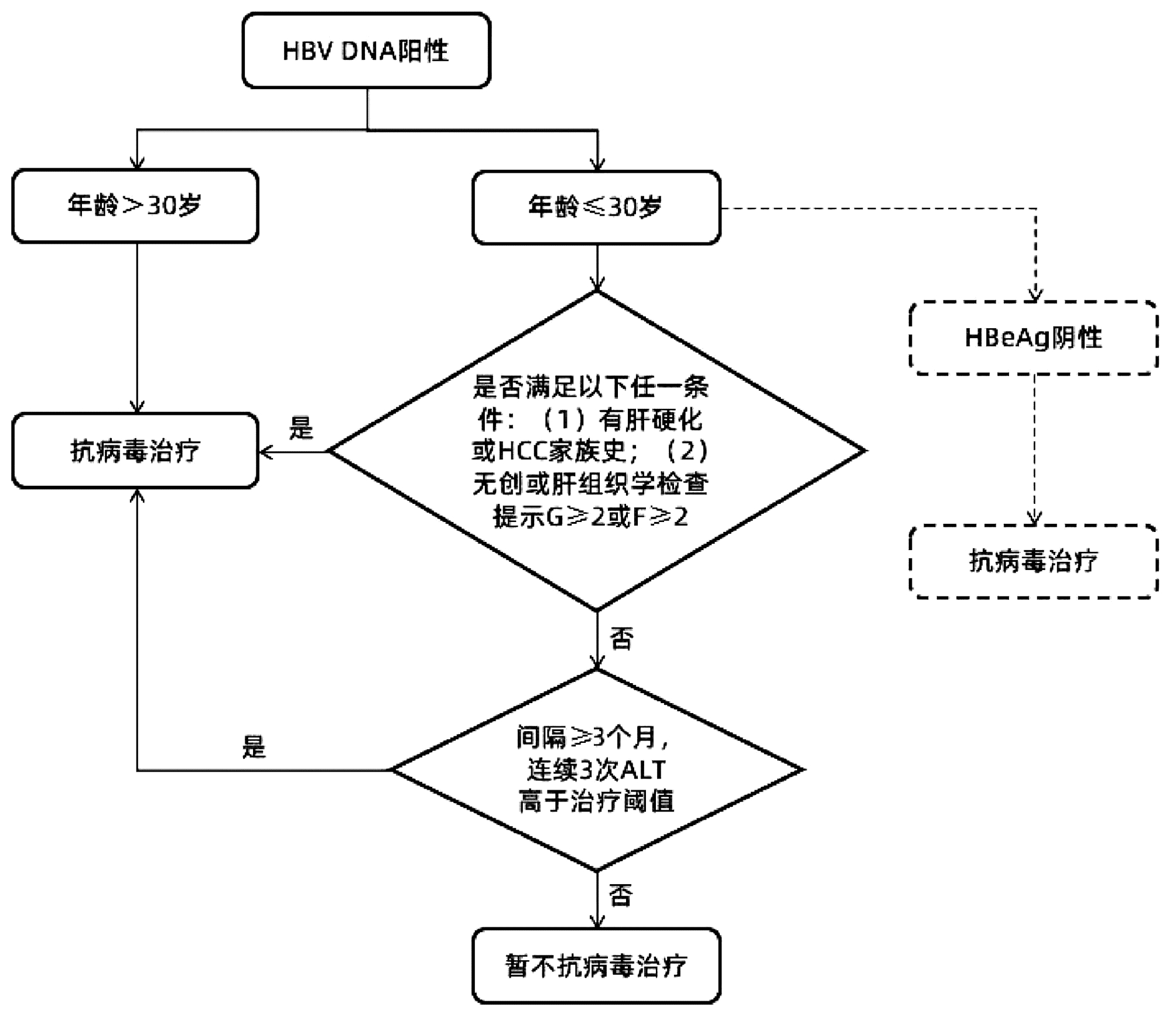
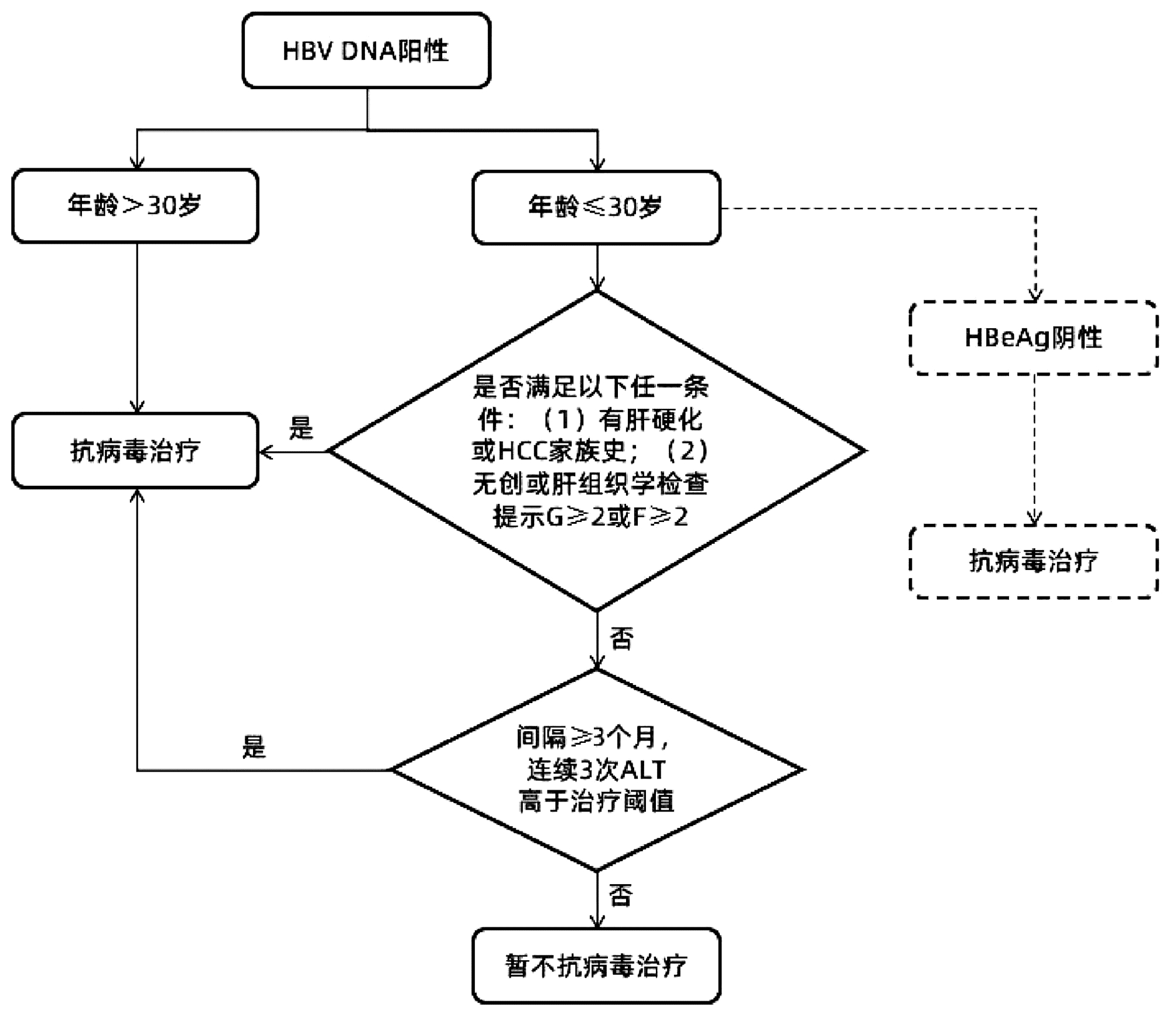
综上所述,笔者基于前期队列的分析结果,对《专家意见》中针对慢性HBV感染者启动抗病毒治疗的流程进行了归纳。同时,依据扩大治疗的专家意见精神,建议对HBeAg阴性且≤30岁的慢性HBV感染者更积极地开展抗病毒治疗,总结抗病毒治疗启动线路图如图 1所示,以期以进一步提高个体化抗病毒治疗的精准性。
 图 1 依据《专家意见》总结修订的抗病毒治疗启动线路图注:在参考文献[1]基础上补充修订。虚线表示笔者新增部分。本流程不包括“不确定期”慢性乙型肝炎患者启动抗病毒治疗的意见。Figure 1. The revised flow chart from the expert opinion for initiation of anti-HBV treatment for chronic hepatitis B
图 1 依据《专家意见》总结修订的抗病毒治疗启动线路图注:在参考文献[1]基础上补充修订。虚线表示笔者新增部分。本流程不包括“不确定期”慢性乙型肝炎患者启动抗病毒治疗的意见。Figure 1. The revised flow chart from the expert opinion for initiation of anti-HBV treatment for chronic hepatitis B -
注:在参考文献[1]基础上补充修订。虚线表示笔者新增部分。本流程不包括“不确定期”慢性乙型肝炎患者启动抗病毒治疗的意见。
图 1 依据《专家意见》总结修订的抗病毒治疗启动线路图
Figure 1. The revised flow chart from the expert opinion for initiation of anti-HBV treatment for chronic hepatitis B
表 1 HBeAg阳性与阴性慢性HBV感染者一般资料比较
Table 1. Comparison of general data between HBeAg positive and HBeAg negative chronic HBV infection
指标 HBeAg阳性(n=121) HBeAg阴性(n=169) 统计值 P值 ≤30岁(n=37) >30岁(n=84) ≤30岁(n=24) >30岁(n=145) 年龄(岁) 26(23~29)1)2) 39(34~47)2)3)4) 27(24~29)1)2) 43(38~49)1)3)4) H=151.539 <0.001 性别[例(%)] χ2=9.959 0.019 男 22(59.5) 38(45.2) 18(75.0)2) 64(44.1)4) 女 15(40.5) 46(54.8) 6(25.0)2) 81(55.9)4) ALT(U/L) 30(22~36)2)4) 25(19~32)2) 20(16~28)3) 20(15~27)1)3) H=29.041 <0.001 AST(U/L) 25(21~31)2) 24(20~28) 21(18~25) 21(18~27)3) H=11.127 0.011 ALP(U/L) 70(56~89) 72(59~92) 71(63~88) 72(59~89) H=0.667 0.881 GGT(U/L) 14(11~19) 17(13~21) 17(14~25) 16(12~23) H=4.941 0.176 Alb(U/L) 41(39~44) 39(37~42)2)4) 43(41~45)1) 41(39~44)1) H=23.538 <0.001 PA(mg/L) 199.6±44.2 194.0±42.3 215.6±40.7 205.2±44.7 F=1.988 0.116 PLT(×109/L) 204(173~237) 200(158~230) 184(150~217) 188(158~225) H=3.816 0.282 HBV DNA(log10 IU/mL) 8.0(7.0~8.4)2)4) 8.0(7.4~8.3)2)4) 2.7(2.3~3.5)1)3) 3.2(2.5~4.1)1)3) H=187.982 <0.001 HBsAg(log10 IU/mL) 4.5(3.9~4.7)2)4) 4.6(3.9~4.9)2)4) 3.3(3.1~3.6)1)3) 3.2(2.6~3.6)1)3 H=132.520 <0.001 炎症分期[例(%)] 0.070 G0~G1 33(89.2) 64(76.2) 18(75.0) 127(87.6) G2~G4 4(10.8) 20(23.8) 6(25.0) 18(12.4) 肝纤维化分期[例(%)] χ2=6.459 0.091 S0~S1 24(64.9) 50(59.5) 10(41.7) 69(47.6) S2~S4 13(35.1) 34(40.5) 14(58.3) 76(52.4) 存在明显肝损伤[例(%)] χ2=6.556 0.087 否 23(62.2) 48(57.1) 9(37.5) 66(45.5) 是 14(37.8) 36(42.9) 15(62.5) 79(54.5) 注:与HBeAg阳性(>30岁)者比较,1)P<0.05;与HBeAg阴性(>30岁)者比较,2)P<0.05;与HBeAg阳性(≤30岁)者比较,3)P<0.05;与HBeAg阴性(≤30岁)者比较,4)P<0.05。 表 2 无创指标无法决策抗病毒治疗者肝损伤情况比较
Table 2. Comparison of liver injury in patients with non-invasive indicators unable to determine antiviral therapy
是否存在明显肝损伤 HBeAg阳性 HBeAg阴性 χ2值 P值 ≤30岁(n=34) >30岁(n=79) ≤30岁(n=22) >30岁(n=134) 否[例(%)] 23(67.6) 46(58.2) 9(40.9) 66(49.3) 5.819 0.121 是[例(%)] 11(32.4) 33(41.8) 13(59.1) 68(50.7) -
[1] Chinese Society of Hepatology, Chinese Medical Association. Expert opinion on expanding anti-HBV treatment for chronic hepatitis B[J]. Chin J Hepatol, 2022, 30(2): 131-136. DOI: 10.3760/cma.j.cn501113-20220209-00060中华医学会肝病学分会. 扩大慢性乙型肝炎抗病毒治疗的专家意见[J]. 中华肝脏病杂志, 2022, 30(2): 131-136. DOI: 10.3760/cma.j.cn501113-20220209-00060 [2] ZHUANG H. Should both patient's age and family history be necessary as the criteria for initiating treatment of HBeAg-positive or negative patients with chronic hepatitis B virus infection and normal alanine aminotransferase?[J]. Chin J Hepatol, 2022, 30(2): 230-232. DOI: 10.3760/cma.j.cn501113-20220129-00051.庄辉. ALT正常HBeAg阳性或阴性慢性乙型肝炎病毒感染患者治疗是否必须同时兼备年龄和家族史?[J]. 中华肝脏病杂志, 2022, 30(2): 230-232. DOI: 10.3760/cma.j.cn501113-20220129-00051. [3] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. [4] Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [5] CUI FQ, BI SL, ZHANG Y, et al. Combination profiles of hepatitis B marks for Chinese in serosurvey in 2006[J]. Chin J Vaccines Immunization, 2009, 15(4): 294-299. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGJM200904005.htm崔富强, 毕胜利, 张勇, 等. 中国人群大样本调查乙型肝炎病毒感染血清学标志物检出模式分析[J]. 中国疫苗和免疫, 2009, 15(4): 294-299. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGJM200904005.htm [6] Chinese Society of Infectious and Parasitic Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Prevention and Control of Viral Hepatitis[J]. Chin J Intern Med, 2001, 40(1): 62-68. DOI: 10.3760/j.issn:0578-1426.2001.01.034.中华医学会传染病与寄生虫病学分会, 中华医学会肝病学分会. 病毒性肝炎防治方案[J]. 中华内科杂志, 2001, 40(1): 62-68. DOI: 10.3760/j.issn:0578-1426.2001.01.034. [7] Chinese Foundation for Hepatitis Prevention and Control; Chinese Society of Infectious Disease and Chinese Society of Hepatology, Chinese Medical Association; Liver Disease Committee of Chinese Research Hospital Association. Consensus on clinical application of transient elastography detecting liver fibrosis: a 2018 update[J]. Chin J Hepatol, 2019, 27(3): 182-191. DOI: 10.3760/cma.j.issn.1007-3418.2019.03.004.中国肝炎防治基金会, 中华医学会感染病学分会, 中华医学会肝病学分会和中国研究型医院学会肝病专业委员会. 瞬时弹性成像技术诊断肝纤维化专家共识(2018年更新版)[J]. 中华肝脏病杂志, 2019, 27(3): 182-191. DOI: 10.3760/cma.j.issn.1007-3418.2019.03.004. [8] KIM WR, BERG T, ASSELAH T, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients[J]. J Hepatol, 2016, 64(4): 773-780. DOI: 10.1016/j.jhep.2015.11.012. [9] GONG X, YANG J, TANG J, et al. A mechanistic assessment of the discordance between normal serum alanine aminotransferase levels and altered liver histology in chronic hepatitis B[J]. PLoS One, 2015, 10(7): e0134532. DOI: 10.1371/journal.pone.0134532. [10] YAN Y, ALLWEISS L, YANG D, et al. Down-regulation of cell membrane localized NTCP expression in proliferating hepatocytes prevents hepatitis B virus infection[J]. Emerg Microbes Infect, 2019, 8(1): 879-894. DOI: 10.1080/22221751.2019.1625728. [11] CHU CM, HUNG SJ, LIN J, et al. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels[J]. Am J Med, 2004, 116(12): 829-834. DOI: 10.1016/j.amjmed.2003.12.040. [12] MCMAHON BJ, HOLCK P, BULKOW L, et al. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus[J]. Ann Intern Med, 2001, 135(9): 759-768. DOI: 10.7326/0003-4819-135-9-200111060-00006. [13] HSU YS, CHIEN RN, YEH CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B[J]. Hepatology, 2002, 35(6): 1522-1527. DOI: 10.1053/jhep.2002.33638. [14] CHU CM, LIAW YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up[J]. Hepatology, 2007, 45(5): 1187-1192. DOI: 10.1002/hep.21612. [15] CHEN JD, YANG HI, ILOEJE UH, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death[J]. Gastroenterology, 2010, 138(5): 1747-1754. DOI: 10.1053/j.gastro.2010.01.042. [16] NGUYEN MH, GARCIA RT, TRINH HN, et al. Histological disease in Asian-Americans with chronic hepatitis B, high hepatitis B virus DNA, and normal alanine aminotransferase levels[J]. Am J Gastroenterol, 2009, 104(9): 2206-2213. DOI: 10.1038/ajg.2009.248. 期刊类型引用(13)
1. 陈刚,许婷婷,余阿娜. HCMV感染对HBeAg阴性乙肝病毒感染孕妇病毒激活及血清免疫功能指标的影响. 中华医院感染学杂志. 2025(01): 86-90 .  百度学术
百度学术2. 王庆玲,石明月. 肝脂肪变对慢性乙型肝炎抗病毒治疗的影响. 中国城乡企业卫生. 2024(01): 94-96 .  百度学术
百度学术3. 王秀和. 乙型肝炎病毒与丙型肝炎病毒合并感染患者的生化免疫结果分析. 中外医疗. 2024(07): 48-50+75 .  百度学术
百度学术4. 丁洋,窦晓光. 关注慢性乙型肝炎特殊人群抗病毒治疗时机及治疗策略. 临床肝胆病杂志. 2024(05): 861-865 .  本站查看
本站查看5. 唐情容,赖长祥,王方,卢瑾,徐春华,李向军,徐逸洲. 年龄≤30岁转氨酶正常的慢性乙型肝炎肝纤维化患者无创诊断模型的建立. 临床肝胆病杂志. 2024(09): 1790-1795 .  本站查看
本站查看6. 刘新,杜欣雨,鲁凤民. 慢性乙型肝炎患者长期核苷(酸)类似物治疗后仍然发生肝癌的原因探讨. 中华肝脏病杂志. 2024(11): 1032-1036 .  百度学术
百度学术7. 易宇,陈应华,罗亚文. HBeAg阴性ALT<2 ULN的慢性HBV感染者的肝脏炎症及纤维化临床特点分析. 海南医学. 2024(24): 3529-3532 .  百度学术
百度学术8. 周召,阿卜杜热西提·阿卜来提,顾智强,常靖,刘新,鲁凤民. 尽早启动抗病毒治疗降低慢性HBV感染者肝细胞癌发生风险. 临床肝胆病杂志. 2023(01): 31-36 .  本站查看
本站查看9. 纪冬,杨永平. 慢性乙型肝炎临床热点问题解析. 解放军医学杂志. 2023(02): 132-137 .  百度学术
百度学术10. 李桂馨,周召,吴丽丽,高志良,鲁凤民. 论慢性乙型肝炎临床治愈. 肝脏. 2023(03): 269-272 .  百度学术
百度学术11. 刘世恒,李文聪,张莹,李静,任伟光,韩芳,张潇潇,赵素贤,南月敏. 谷丙转氨酶正常未接受抗病毒治疗的慢性乙型肝炎患者临床及病理学特点分析. 中华微生物学和免疫学杂志. 2023(07): 534-540 .  百度学术
百度学术12. 付宝云,熊渝婷,王文畅,邓亚,郭畅,杨武才,王春艳,纪冬,王建军. 丙氨酸转氨酶正常的慢性乙型肝炎患者肝组织学改变的关联因素分析. 解放军医学院学报. 2023(11): 1218-1223 .  百度学术
百度学术13. 鲁凤民,于乐成,高林,姜倩倩,陈香梅,庄辉. HBeAg阴性慢性乙型肝炎患者停药后HBsAg可阴转:停药-治愈策略或许正在来临. 中华医学杂志. 2022(40): 3160-3166 .  百度学术
百度学术其他类型引用(6)
-




 PDF下载 ( 2010 KB)
PDF下载 ( 2010 KB)

 下载:
下载:
 下载:
下载:
 百度学术
百度学术