HBV感染者外周血自然杀伤样B淋巴细胞、自然杀伤细胞、B淋巴细胞亚群的变化及临床意义
DOI: 10.3969/j.issn.1001-5256.2022.07.008
Changes and clinical significance of natural killer-like B cells, natural killer cells, and B cells in peripheral blood of patients with hepatitis B virus infection
-
摘要:
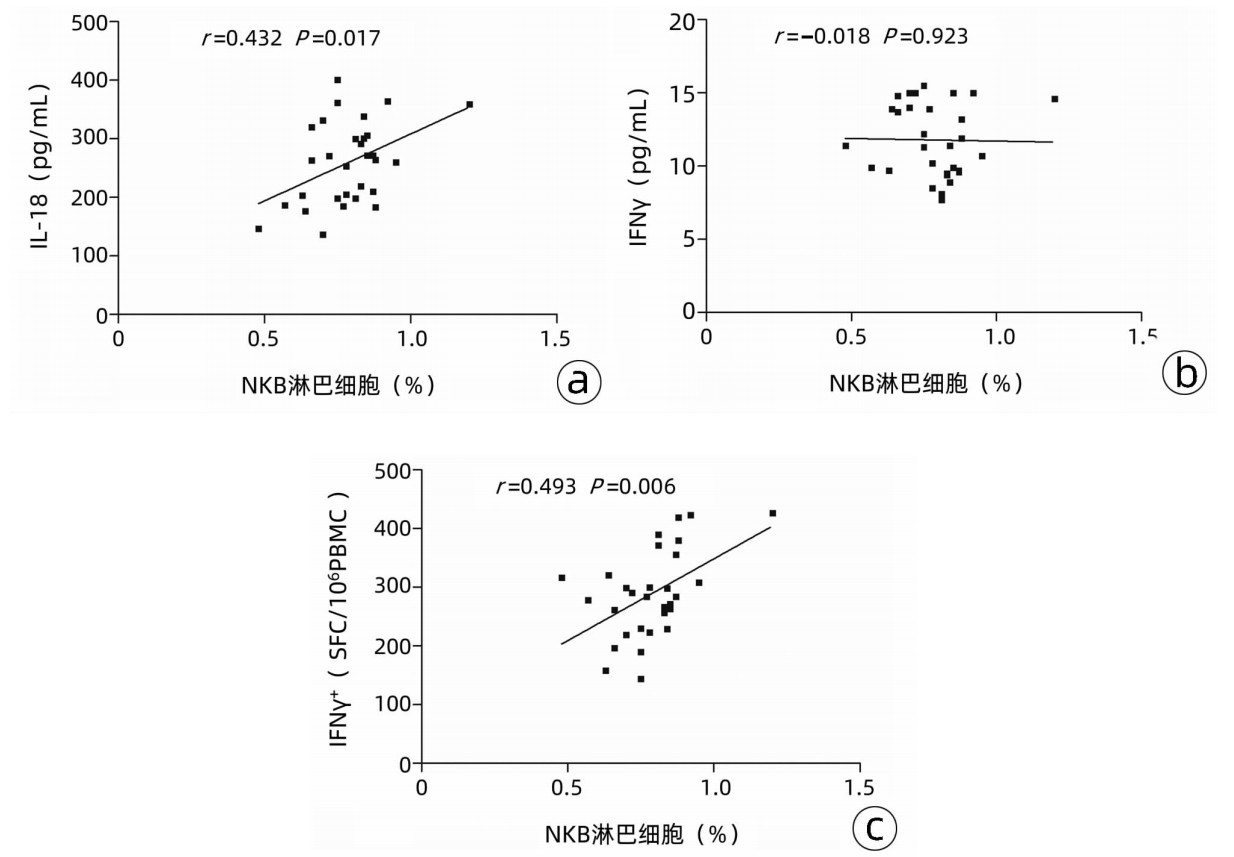
目的 观察HBV感染者自然杀伤样B淋巴细胞(NKB淋巴细胞)、NK细胞和B淋巴细胞亚群的变化,分析其与临床指标的相关性。 方法 选取2017年1月—2018年12月在唐都医院就诊的急性乙型肝炎(AHB)患者15例、慢性乙型肝炎(CHB)患者30例、无症状HBV携带者(ASC)29例和对照者12例。采集外周血,分离血浆和外周血单个核细胞。酶联免疫吸附试验检测血浆IL-18和IFNγ水平。酶联斑点吸附试验检测HBV特异性CD8+T淋巴细胞分泌IFNγ水平。流式细胞术检测CD3-CD19+CD56+CD16+NKB淋巴细胞、不同NK细胞亚群(CD3-CD19-CD56highCD16-NK细胞、CD3-CD19-CD56+CD16+NK细胞、CD3-CD19-CD56-CD16+NK细胞)、CD3-CD19+B淋巴细胞比例,分析与病毒复制、肝脏炎症指标的相关性。符合正态分布的计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。不符合正态分布的计量资料多组间比较采用Kruskal-Wallis H检验。相关性分析采用Pearson相关性检验。 结果 CD3-CD19+CD16+CD56+NKB淋巴细胞占淋巴细胞的比例在AHB患者、CHB患者、ASC和对照者之间的差异有统计学意义(F=16.42,P<0.000 1),CHB患者NKB淋巴细胞比例[(0.79±0.13)%]显著低于AHB患者[(0.94±0.15)%]、ASC[(1.02±0.12)%]和对照者[(1.11±0.27)%](P值均<0.001)。血浆IL-18水平在AHB患者、CHB患者、ASC和对照者之间的差异有统计学意义(F=5.733,P=0.001),CHB患者IL-18水平[(259.30±70.09)pg/mL]显著低于AHB患者[(336.00±103.00)pg/mL]和对照者[(319.30±64.80)pg/mL](P值均<0.05),ASC IL-18水平[(258.60±59.82)pg/mL]亦显著低于AHB患者和对照者(P值均<0.01),但血浆IL-18在CHB患者和ASC之间的差异无统计学意义(P=0.965)。CD3-CD19-CD56highCD16-NK细胞、CD3-CD19-CD56+CD16+NK细胞、CD3-CD19-CD56-CD16+NK细胞以及B淋巴细胞比例在4组之间的差异均无统计学意义(P值均>0.05)。CHB患者血浆IFNγ水平显著低于AHB患者、ASC者和对照者(P值均<0.01)。NKB淋巴细胞比例和IL-18与HBV DNA定量、ALT水平均无显著相关性(P值均>0.05)。CHB患者中NKB淋巴细胞占淋巴细胞的比例与血浆IL-18水平呈正相关(r=0.432,P=0.017),与病毒特异性CD8+T淋巴细胞分泌IFNγ水平呈正相关(r=0.493,P=0.006)。 结论 NKB淋巴细胞和IL-18可能参与HBV感染慢性化,与慢性HBV感染自然史相关。 Abstract:Objective To investigate the changes of natural killer-like B (NKB) cells, natural killer (NK) cells, and B cells and their correlation with clinical indices in patients with hepatitis B virus (HBV) infection. Methods A total of 15 patients with acute hepatitis B (AHB), 30 patients with chronic hepatitis B (CHB), 29 asymptomatic HBV carriers (ASCs), and 12 controls who attended Tangdu Hospital from January 2017 and December 2018 were enrolled. Peripheral blood samples were collected, and plasma and peripheral blood mononuclear cells (PBMCs) were isolated. ELISA was used to measure the plasma levels of interleukin-18 (IL-18) and interferon-γ (IFNγ), and enzyme-linked immunospot assay was used to measure the level of IFNγ secreted by HBV-specific CD8+ T cells; flow cytometry was used to measure the percentages of CD3-CD19+CD56+CD16+ NKB cells, different NK cell subsets (including CD3-CD19-CD56highCD16- NK cells, CD3-CD19-CD56+CD16+ NK cells, and CD3-CD19-CD56-CD16+ NK cells), and their correlation with viral replication and liver inflammation markers was analyzed. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups; non-normally distributed continuous data were expressed as M(P25-P75), and the Kruskal-Wallis H test was used for comparison between multiple groups; the Pearson correlation test was used for correlation analysis. Results There was a significant difference in the percentage of CD3-CD19+CD16+CD56+ NKB cells between the AHB patients, CHB patients, ASCs, and controls (F = 16.42, P < 0.000 1), and the CHB patients had a significantly lower percentage of NKB cells than the AHB patients, ASCs, and controls (0.79%±0.13% vs 0.94%±0.15%/1.02%±0.12%/1.11%±0.27%, all P < 0.001). There was a significant difference in plasma IL-18 level between the AHB patients, CHB patients, ASCs, and controls (F = 5.733, P = 0.001); the CHB patients had a significantly lower IL-18 level than the AHB patients and the controls (259.30±70.09 pg/mL vs 336.00±103.00 pg/mL and 319.30±64.80 pg/mL, both P < 0.05), and ASCs had a significantly lower IL-18 level (258.60±59.82 pg/mL) than the AHB patients and the controls (both P < 0.01), while there was no significant difference in plasma IL-18 level between the CHB patients and ASCs (P = 0.965). There were no significant differences in the percentage of CD3-CD19-CD56highCD16- NK cells, CD3-CD19-CD56+CD16+ NK cells, CD3-CD19-CD56-CD16+ NK cells, and B cells between the four groups (all P > 0.05). The CHB patients had a significantly lower plasma IFNγ level than the AHB patients, ASCs, and controls (all P < 0.01). The percentage of NKB cells and the level of IL-18 were not significantly correlated with HBV DNA quantification or alanine aminotransferase level (all P > 0.05). In the CHB patients, the percentage of NKB cells was positively correlated with plasma IL-18 level (r = 0.432, P = 0.017) and the level of IFNγ secreted by HBV-specific CD8+ T cells (r =0.493, P=0.006). Conclusion NKB cells and IL-18 might be involved in the chronicity of HBV infection and is associated with the natural history of chronic HBV infection. -
Key words:
- Hepatitis B virus /
- Natural Killer T-Cells /
- B-Lymphocytes
-
急性HBV感染可诱导多克隆病毒特异性细胞毒性T淋巴细胞增殖分化,杀伤病毒感染的肝细胞,在清除HBV的同时可造成严重肝损伤。慢性HBV感染可诱导机体免疫耐受,造成免疫逃逸,无法有效清除病毒,导致感染慢性化[1]。因此,HBV感染的临床结局是病毒感染与机体免疫应答复杂相互作用的结果[2]。自然杀伤(natural killer,NK)样B淋巴细胞是一类新鉴定的免疫细胞亚群,NKB淋巴细胞兼具NK细胞和B淋巴细胞的表型,通过分泌IL-12和IL-18调控NK细胞和固有淋巴样细胞功能,在病原体感染早期即可发挥有效免疫调控和抗感染活性[3]。但有关NKB淋巴细胞在HBV感染中的分布和临床意义罕见相关报道。本研究分析了HBV感染者中NKB淋巴细胞、NK细胞和B淋巴细胞亚群的变化和临床意义。
1. 资料与方法
1.1 研究对象
选取2017年1月—2018年12月就诊于唐都医院传染科的HBV感染者,包括急性乙型肝炎(acute hepatitis B,AHB)患者、慢性乙型肝炎(chronic hepatitis B,CHB)患者、无症状HBV携带者(asymptomatic HBV carrier,ASC)。AHB纳入标准:(1)既往无乙型肝炎病史;(2)HBsAg阳性、HBV DNA阳性;(3)ALT水平升高超过5倍正常值上限;(4)抗-HBc IgM阳性;(5)6个月内HBsAg和HBV DNA均阴转。CHB患者纳入标准符合《慢性乙型肝炎防治指南(2015更新版)》[4]的诊断标准:(1)HBsAg阳性大于6个月;(2)HBV DNA阳性;(3)ALT水平超过2倍正常值上限。ASC纳入标准:(1)HBsAg阳性、HBeAg阳性、HBV DNA阳性;(2)ALT水平在正常范围内。所有患者既往均未接受抗病毒治疗,年龄>18岁。对照组纳入标准:(1)同时期在唐都医院行健康查体者;(2)年龄>18岁;(3)既往无HBV感染病史和乙型肝炎家族史。排除标准:(1)合并其他嗜肝病毒感染或HIV感染者;(2)合并自身免疫性疾病、酒精性肝硬化、药物性肝损伤者;(3)合并恶性肿瘤者;(4)合并失代偿期肝硬化、肝衰竭、肝癌等终末期肝病者;(5)妊娠或哺乳期妇女。
1.2 研究方法
1.2.1 主要试剂和仪器
淋巴细胞分离液购自美国Sigma公司。小鼠抗人CD3-FITC、小鼠抗人CD19-APCH7、小鼠抗人CD16-APC、小鼠抗人CD56-PerCP Cy5.5均购自美国BD公司。人IL-18和IFNγ酶联免疫吸附试验(ELISA)检测试剂盒购自武汉华美生物技术公司。IFNγ酶联斑点吸附试验(ELISPOT)检测试剂盒购自瑞典Mabtech公司。FACS Aria Ⅱ流式细胞仪为美国BD公司产品。ELISA微孔读板仪为美国伯乐公司产品。ELISPOT读板仪为德国AID公司产品。
1.2.2 血浆和外周血单个核细胞(PBMC)的分离
清晨、空腹采集EDTA抗凝外周血静脉血10 mL,于4 ℃、1000 r/min离心10 min分离血浆,冻存于-80 ℃备用。剩余血细胞立即使用淋巴细胞分离液、采用Ficoll密度梯度离心法分离PBMC。使用0.4%台盼蓝对PBMC计数,以5×106个/管分装在冻存管中,加入90%胎牛血清+10%二甲基亚砜冻存于液氮中备用。
1.2.3 流式细胞检测免疫细胞亚群比例
复苏PBMC,取106个PBMC,转入FACS管中,洗涤2次后加入小鼠抗人CD3-FITC、小鼠抗人CD19-APC H7、小鼠抗人CD16-APC、小鼠抗人CD56- PerCP Cy5.5进行表面染色,4 ℃避光孵育30 min,洗涤后进行流式检测。使用FACS Aria Ⅱ流式细胞仪检测,BD FACS Diva软件获取细胞,FlowJo V10软件分析结果。NKB淋巴细胞、NK细胞和B淋巴细胞的流式分析策略:首先根据前向角散射和侧向角散射对淋巴细胞进行圈门,在淋巴细胞门内分析CD3-CD19+细胞,即为B淋巴细胞。在CD3-CD19+门内,分析CD16+CD56+细胞,即为NKB淋巴细胞。在淋巴细胞门内分析CD3-CD19-细胞,在CD3-CD19-门内,分析CD16和CD56表达,分析不同NK细胞亚群,包括:CD3-CD19-CD56highCD16- NK细胞、CD3-CD19-CD56+CD16+NK细胞、CD3-CD19-CD56-CD16+NK细胞(图 1)。
1.2.4 ELISA检测血浆IL-18和IFNγ水平
使用商品化的ELISA试剂盒对血浆中IL-18和IFNγ水平进行检测,操作按说明书要求进行。
1.2.5 ELISPOT检测HBV特异性CD8+T淋巴细胞分泌IFNγ水平
使用抗IFNγ(0.5 μg/mL)包被96孔PVDF膜板,4 ℃孵育过夜,向每孔中加入105个PBMC(100 μL)和HBV全基因组多肽(15肽,每条多肽之间有5个氨基酸重叠,共313条;10 μg/mL)[5],37 ℃、5%CO2条件下刺激培养16 h,洗涤后每孔加入100 μL生物素标记的抗IFNγ单抗,室温孵育60 min,洗涤后每孔加入100 μL碱性磷酸酶标记的链球菌亲和素,室温避光孵育45 min,洗板后加入显色剂反应15 min,终止反应后读板,以斑点形成细胞数(spot forming cells, SFC)/106 PBMC为单位计数产生IFNγ的细胞。
1.3 统计学方法
根据临床试验设计样本含量估计方法[6],使用PASS 15.0软件,对横断面研究的样本量进行估算,检验水平为0.05,检验效能为0.10,组数为4,计算样本例数。采用SPSS 23.0统计软件处理数据。符合正态分布的计量资料以x±s表示,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。不符合正态分布的计量资料使用M(P25~P75)表示,组间比较采用Kruskal-Wallis H检验。相关性分析采用Pearson相关性检验。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入AHB患者15例,CHB患者30例,ASC 29例,对照者12例。入组受试者一般资料见表 1。
表 1 入组受试者一般资料比较Table 1. Comparison of clinical data of enrolled subjects项目 AHB患者
(n=15)CHB患者
(n=30)ASC
(n=29)对照者
(n=12)统计值 P值 男/女(例) 13/2 23/7 22/7 10/2 χ2=0.429 0.286 年龄(岁) 33.50±8.54 32.20±8.86 26.70±8.43 31.20±3.74 F=3.311 0.024 ALT(U/L) 384(250~822) 114(81~172) 32(24~39) 16(9~31)1) H=22.160 <0.000 1 HBV DNA (log10IU/mL) 4.24±1.44 7.85±1.38 7.60±0.79 F=51.070 <0.000 1 HBeAg阳性 11 21 29 抗-HBe阳性 4 9 0 注:1)此结果为10例对照者的结果,2例对照者未行ALT检测。 2.2 HBV感染者外周血NKB淋巴细胞、NK细胞、B淋巴细胞比例和IL-18、IFNγ水平变化
CD3-CD19+CD16+CD56+NKB淋巴细胞占淋巴细胞的比例在AHB患者、CHB患者、ASC和对照者之间的差异有统计学意义(F=16.42,P<0.000 1),CHB患者NKB淋巴细胞比例[(0.79±0.13)%]显著低于AHB患者[(0.94±0.15)%]、ASC[(1.02±0.12)%]和对照者[(1.11±0.27)%](P值均<0.001)(图 2a)。CD3-CD19-CD56highCD16-NK细胞、CD3-CD19-CD56+CD16+NK细胞、CD3-CD19-CD56-CD16+NK细胞以及B淋巴细胞占淋巴细胞的比例在AHB患者、CHB患者、ASC和对照者之间的差异均无统计学意义(F值分别为0.598、0.505、1.950、1.676,P值均>0.05)(图 2b~e)。血浆IL-18水平在AHB患者、CHB患者、ASC和对照者之间的差异有统计学意义(F=5.733,P=0.001),CHB患者IL-18水平[(259.30±70.09)pg/mL]显著低于AHB患者[(336.00±103.00)pg/mL]和对照者[(319.30±64.80)pg/mL](P值均<0.05),ASC IL-18水平[(258.60±59.82) pg/mL]亦显著低于AHB患者和对照者(P值均<0.01),但血浆IL-18水平在CHB患者和ASC之间的差异无统计学意义(P=0.965)(图 2f)。血浆IFNγ水平在AHB患者、CHB患者、ASC和对照者之间的差异有统计学意义(F=4.737,P=0.004),CHB患者IFNγ水平[(11.79±2.47)pg/mL]显著低于AHB患者[(14.55±3.81)pg/mL]、ASC[(13.79±2.82)pg/mL]和对照者[(14.50±2.42)pg/mL](P<0.01)(图 2g)。
 图 2 HBV感染者和对照者外周血NKB淋巴细胞、NK细胞、B淋巴细胞比例和IL-18、IFNγ水平比较注:a,CD3-CD19+CD16+CD56+NKB淋巴细胞;b,CD3-CD19-CD56highCD16- NK细胞;c,CD3-CD19-CD56+CD16+NK细胞;d,CD3-CD19-CD56-CD16+ NK细胞;e,B淋巴细胞;f,IL-18;g,IFNγ。Figure 2. Comparison of peripheral NKB cells, NK cells, and B cells percentage and IL-18, IFNγlevel among HBV infected patients and controls
图 2 HBV感染者和对照者外周血NKB淋巴细胞、NK细胞、B淋巴细胞比例和IL-18、IFNγ水平比较注:a,CD3-CD19+CD16+CD56+NKB淋巴细胞;b,CD3-CD19-CD56highCD16- NK细胞;c,CD3-CD19-CD56+CD16+NK细胞;d,CD3-CD19-CD56-CD16+ NK细胞;e,B淋巴细胞;f,IL-18;g,IFNγ。Figure 2. Comparison of peripheral NKB cells, NK cells, and B cells percentage and IL-18, IFNγlevel among HBV infected patients and controls2.3 HBV感染者中NKB淋巴细胞与临床指标相关性
AHB患者、CHB患者和ASC中NKB淋巴细胞和IL-18与HBV DNA定量、ALT水平均无相关性(P值均>0.05)。AHB患者和ASC中NKB淋巴细胞与IL-18水平无相关性(P>0.05),CHB患者中NKB淋巴细胞占淋巴细胞的比例与血浆IL-18水平呈正相关(r=0.432,P=0.017)(图 3a),CHB患者中NKB淋巴细胞占淋巴细胞的比例与血浆IFNγ水平无相关性(r=-0.018,P=0.923)(图 3b),但CHB患者中NKB淋巴细胞比例与病毒特异性CD8+T淋巴细胞分泌IFNγ水平呈显著正相关(r=0.493,P=0.006)(图 3c)。
3. 讨论
本研究首次对新近鉴定的淋巴细胞亚群——NKB淋巴细胞在HBV感染者中的变化进行分析。NKB淋巴细胞虽然兼具NK细胞和B淋巴细胞的标记,在小鼠中的表型为CD3-CD19+NK1.1+[3],在人类中的表型为CD3-CD19+(或CD20+)NKp46+[3, 7-8],但NKB淋巴细胞的功能却尚未完全阐明。Kerdiles等[9]认为,虽然NKB淋巴细胞具有NK细胞的表型,但在功能上与传统B淋巴细胞基本相似。但Wang等[3]则认为,NKB淋巴细胞主要通过分泌IL-18和IL-12在微生物感染早期阶段活化NK细胞和固有淋巴样细胞,发挥抗感染免疫活性,这一功能活性亦在猴免疫缺陷病毒感染中得到证实[10]。本课题组前期研究[8]发现,NKB淋巴细胞在牙周炎患者外周血和牙周组织中的水平均显著升高,并主要通过分泌IL-18发挥促进牙周组织炎症应答的作用。新近的研究[11]发现,原发性肝癌患者外周血和肿瘤浸润NKB淋巴细胞比例及其分泌的IL-18水平均显著降低。但有关NKB淋巴细胞在HBV感染中的数量和功能的变化尚未见相关报道。本研究应用经典NK细胞表型(CD3-CD16+CD56+)和B淋巴细胞表型(CD3-CD19+)对HBV感染者外周血中CD3-CD19+CD16+CD56+NKB淋巴细胞进行了检测,结果发现,AHB患者和ASC外周血NKB淋巴细胞比例与对照者无显著差异,但CHB患者中NKB淋巴细胞比例则显著低于对照者。在对NKB淋巴细胞的标志性细胞因子IL-18的表达水平进行分析时亦发现,CHB患者和ASC中IL-18表达水平下降,这与既往研究[12]基本一致。
NKB淋巴细胞比例和IL-18水平与病毒复制、肝脏炎症均无显著相关性。由于既往的研究主要集中在NKB淋巴细胞在急性病毒感染中的作用,本研究未发现AHB患者中存在NKB淋巴细胞数量变化,提示NKB淋巴细胞可能并未直接参与急性HBV感染的病程。而ASC和CHB是慢性HBV感染自然史的2个不同阶段,多数患者在青少年阶段处于免疫耐受期,病毒处于高水平复制,肝脏炎症应答轻,随着疾病进展,机体免疫系统对感染病毒的肝细胞进行杀伤,清除病毒的同时诱导肝脏炎症损伤[13-14]。因此,本研究入组的ASC较CHB患者年龄小,但NKB淋巴细胞和IL-18与年龄无显著相关性。NKB淋巴细胞仅在CHB患者中降低,在ASC中无明显变化,而IL-18在CHB患者和ASC中均显著降低,但仅在CHB患者中NKB淋巴细胞与IL-18呈显著正相关。这可能说明由于ASC患者处于免疫耐受阶段,NKB淋巴细胞活性尚未受到抑制,ASC患者中其他分泌IL-18的细胞(如单核细胞、角质形成细胞等)亦可能被抑制,导致IL-18水平下降。而进入免疫清除期后,长期病毒持续感染仍可能导致免疫细胞失能,造成NKB淋巴细胞水平下降,其分泌的IL-18水平亦降低,不能清除病原体和诱导抗病毒免疫应答,加重病毒持续感染。因此,NKB淋巴细胞水平下降可能与HBV感染自然史密切相关。此外,CHB患者外周血中NKB淋巴细胞降低亦可能与疾病过程中NKB淋巴细胞向肝脏的募集浸润有关。新近的研究[15]发现,肝癌患者中NKB淋巴细胞可通过分泌IL-18调控CD8+T淋巴细胞活性。本研究发现,NKB淋巴细胞比例与分泌IFNγ的HBV特异性CD8+T淋巴细胞水平呈显著正相关,提示在CHB疾病过程中,NKB淋巴细胞也可能参与了病毒特异性CD8+T淋巴细胞功能调控。但本研究未纳入ASC和CHB患者的肝穿刺标本,仍需要使用HBV转基因小鼠模型进行研究加以证实。
本课题组既往研究[16]发现,HBV质粒高压尾静脉注射诱导的HBV感染小鼠模型肝脏中NK细胞比例与对照小鼠无显著差异,本研究在HBV感染者中亦发现了相似的结果。NK细胞根据CD16和CD56表达水平可分为CD56highCD16-NK细胞、CD56+CD16+NK细胞、CD56-CD16+NK细胞。CD56highCD16-NK细胞通过分泌多种细胞因子参与机体免疫调控;CD56+CD16+NK细胞是成熟的、具有细胞毒性的NK细胞亚群,通过CD16发挥抗体依赖的细胞介导的毒性作用;CD56-CD16+NK细胞则是失能的NK细胞,分泌细胞因子和细胞毒性作用均显著降低,增殖能力亦低下[17]。本研究发现,HBV感染者中三类NK细胞亚群比例与对照者均无显著差异,这与既往发现NK细胞在AHB患者和CHB患者中受到抑制的趋势不尽相同[18],这可能与分析NK细胞不同亚型时采用的细胞表面标志不同有关。NK细胞通过分泌IFNγ介导后续适应性免疫活化促进HBV清除[19],因此本研究亦对血清中IFNγ水平进行检测,结果发现,CHB患者血浆IFNγ水平显著降低,这与本课题组既往研究[20]结果一致,但与NK细胞亚群变化趋势不尽相同,这可能由于NK细胞并非为血浆中IFNγ的唯一细胞来源有关。既往有研究[21-22]发现HBsAg和HBeAg可诱导成熟的NK细胞功能活性下降,导致HBV感染者疾病进展,促进失代偿期肝硬化、肝癌等终末期肝病发生。B淋巴细胞在HBV感染过程中发挥诱导抗病毒抗体产生、抗原提呈等多种功能[23],但本研究中亦未发现HBV感染者和对照者外周血B淋巴细胞比例存在明显差异。因此,HBV感染者中NK细胞和B淋巴细胞功能的变化仍有待进一步研究。同时,本研究为横断面研究,针对AHB患者和CHB患者未进行治疗前后不同免疫细胞亚群的比较,这也是本研究的薄弱环节之一。
综上所述,慢性HBV感染可能诱导NKB淋巴细胞水平下降,NKB淋巴细胞及其分泌的IL-18参与HBV感染慢性化,与慢性HBV感染自然史相关。
-
注:a,CD3-CD19+CD16+CD56+NKB淋巴细胞;b,CD3-CD19-CD56highCD16- NK细胞;c,CD3-CD19-CD56+CD16+NK细胞;d,CD3-CD19-CD56-CD16+ NK细胞;e,B淋巴细胞;f,IL-18;g,IFNγ。
图 2 HBV感染者和对照者外周血NKB淋巴细胞、NK细胞、B淋巴细胞比例和IL-18、IFNγ水平比较
Figure 2. Comparison of peripheral NKB cells, NK cells, and B cells percentage and IL-18, IFNγlevel among HBV infected patients and controls
表 1 入组受试者一般资料比较
Table 1. Comparison of clinical data of enrolled subjects
项目 AHB患者
(n=15)CHB患者
(n=30)ASC
(n=29)对照者
(n=12)统计值 P值 男/女(例) 13/2 23/7 22/7 10/2 χ2=0.429 0.286 年龄(岁) 33.50±8.54 32.20±8.86 26.70±8.43 31.20±3.74 F=3.311 0.024 ALT(U/L) 384(250~822) 114(81~172) 32(24~39) 16(9~31)1) H=22.160 <0.000 1 HBV DNA (log10IU/mL) 4.24±1.44 7.85±1.38 7.60±0.79 F=51.070 <0.000 1 HBeAg阳性 11 21 29 抗-HBe阳性 4 9 0 注:1)此结果为10例对照者的结果,2例对照者未行ALT检测。 -
[1] LIN CL, KAO JH. Natural history of acute and chronic hepatitis B: The role of HBV genotypes and mutants[J]. Best Pract Res Clin Gastroenterol, 2017, 31(3): 249-255. DOI: 10.1016/j.bpg.2017.04.010. [2] WU J, HAN M, LI J, et al. Immunopathogenesis of HBV infection[J]. Adv Exp Med Biol, 2020, 1179: 71-107. DOI: 10.1007/978-981-13-9151-4_4. [3] WANG S, XIA P, CHEN Y, et al. Natural killer-like B cells prime innate lymphocytes against microbial infection[J]. Immunity, 2016, 45(1): 131-144. DOI: 10.1016/j.immuni.2016.06.019. [4] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. [5] DONG J, YANG XF, WANG LX, et al. Modulation of tim-3 expression by antigen-dependent and -independent factors on T cells from patients with chronic hepatitis B virus infection [J]. Front Cell Infect Microbiol, 2017, 7: 98. DOI: 10.3389/fcimb.2017.00098. [6] LI L, TANG YX, HE W, et al. Estimated sample content of commonly used clinical trial designs [J]. Systems Med, 2018, 3(9): 191-193. DOI: 10.19368/j.cnki.2096-1782.2018.09.191.李黎, 唐雨欣, 何伟, 等. 常用临床试验设计的样本含量估计[J]. 系统医学, 2018, 3(9): 191-193. DOI: 10.19368/j.cnki.2096-1782.2018.09.191. [7] MANICKAM C, NWANZE C, RAM DR, et al. Progressive lentivirus infection induces natural killer cell receptor-expressing B cells in the gastrointestinal tract[J]. AIDS, 2018, 32(12): 1571-1578. DOI: 10.1097/QAD.0000000000001855. [8] ZHANG Y, KUANG W, LI D, et al. Natural killer-like B cells secreting interleukin-18 induces a proinflammatory response in periodontitis[J]. Front Immunol, 2021, 12: 641562. DOI: 10.3389/fimmu.2021.641562. [9] KERDILES YM, ALMEIDA FF, THOMPSON T, et al. Natural-killer-like B cells display the phenotypic and functional characteristics of conventional B cells[J]. Immunity, 2017, 47(2): 199-200. DOI: 10.1016/j.immuni.2017.07.026. [10] RASCLE P, JACQUELIN B, PETITDEMANGE C, et al. NK-B cell cross talk induces CXCR5 expression on natural killer cells[J]. iScience, 2021, 24(10): 103109. DOI: 10.1016/j.isci.2021.103109. [11] LIU S, YANG L, JIA S, et al. Interleukin-35 suppresses the activity of natural killer-like B cells in patients with hepatocellular carcinoma[J]. Int Immunopharmacol, 2021, 100: 108161. DOI: 10.1016/j.intimp.2021.108161. [12] LU Y, BAO JG, DENG Y, et al. Role of IL-18 gene promoter polymorphisms, serum IL-18 levels, and risk of hepatitis B virus-related liver disease in the Guangxi Zhuang Population: a retrospective case-control study[J]. Asian Pac J Cancer Prev, 2015, 16(14): 6019-6026. DOI: 10.7314/apjcp.2015.16.14.6019. [13] CHEN YX, HUANG R, WU C. Influence of pregnancy on the natural history of chronic hepatitis B virus infection[J]. J Clin Hepatol, 2019, 35(7): 1430-1434. DOI: 10.3969/j.issn.1001-5256.2019.07.004.陈雨欣, 黄睿, 吴超. 妊娠对慢性HBV感染自然史的影响[J]. 临床肝胆病杂志, 2019, 35(7): 1430-1434. DOI: 10.3969/j.issn.1001-5256.2019.07.004. [14] LIN CL, KAO JH. Natural history of acute and chronic hepatitis B: The role of HBV genotypes and mutants [J]. Best Pract Res Clin Gastroenterol, 2017, 31(3): 249-255. DOI: 10.1016/j.bpg.2017.04.010. [15] LIU S, YANG L, JIA S, et al. Interleukin-35 suppresses the activity of natural killer-like B cells in patients with hepatocellular carcinoma[J]. Int Immunopharmacol, 2021, 100: 108161. DOI: 10.1016/j.intimp.2021.108161. [16] PENG MJ, YANG XF, WEI X, et al. Influence of hepatitis B virus infection on intrahepatic natural killer cells and innate lymphoid cell 22[J]. J Clin Hepatol, 2016, 32(10): 1883-1887. DOI: 10.3969/j.issn.1001-5256.2016.10.012.彭梅娟, 杨晓飞, 魏欣, 等. HBV感染对肝脏自然杀伤细胞和固有淋巴样细胞22的影响[J]. 临床肝胆病杂志, 2016, 32(10): 1883-1887. DOI: 10.3969/j.issn.1001-5256.2016.10.012. [17] LIANG HJ, CUI YH, WANG YP, et al. Matrix metallopeoteinase regulates natural killer cells function in methicili-resistant staphylococcus aureus sepsis [J]. Chin J Emerg Med, 2020, 29(6): 835-840. DOI: 10.3760/cma.j.issn.1671-0282.2020.06.019.梁海军, 崔艳慧, 王燕平, 等. 基质金属蛋白酶对MRSA脓毒症患者NK细胞的调控作用[J]. 中华急诊医学杂志, 2020, 29(6): 835-840. DOI: 10.3760/cma.j.issn.1671-0282.2020.06.019. [18] QU XJ, LI MH, CAO WH, et al. Study the change of NK cells in HBV infection [J]. Chin J Exp Clin Virol, 2016, 30(5): 439-443. DOI: 10.3760/cma.j.issn.1003-9279.2016.05.006.屈晓晶, 李明慧, 曹卫华, 等. NK细胞在HBV感染中的作用研究[J]. 中华实验和临床病毒学杂志, 2016, 30(5): 439-443. DOI: 10.3760/cma.j.issn.1003-9279.2016.05.006. [19] CAI HN, XU Y, CHEN XY, et al. Effect of HBV infection on biological activity of NK cells cultured in vitro[J/CD]. Chin J Hepat Surg(Electronic Edition), 2020, 9(3): 274-277. DOI: 10.3877/cma.j.issn.2095-3232.2020.03.016.蔡惠宁, 许燕, 陈晓燕, 等. HBV感染对体外培养NK细胞生物活性影响的研究[J/CD]. 中华肝脏外科手术学电子杂志, 2020, 9(3): 274-277. DOI: 10.3877/cma.j.issn.2095-3232.2020.03.016. [20] LIAN JQ, YANG XF, ZHAO RR, et al. Expression profiles of circulating cytokines, chemokines and immune cells in patients with hepatitis B virus infection[J]. Hepat Mon, 2014, 14(6): e18892. DOI: 10.5812/hepatmon.18892. [21] JIANG Y, CHEN Y, CHEN L, et al. Impaired circulating CD56dim NK cells are associated with decompensation of HBV-related cirrhosis[J]. Hum Immunol, 2020, 81(1): 32-40. DOI: 10.1016/j.humimm.2019.11.006. [22] CHEN Y, TIAN Z. HBV-Induced Immune Imbalance in the Development of HCC[J]. Front Immunol, 2019, 10: 2048. DOI: 10.3389/fimmu.2019.02048. [23] CAI Y, YIN W. The multiple functions of B cells in chronic HBV infection[J]. Front Immunol, 2020, 11: 582292. DOI: 10.3389/fimmu.2020.582292. 期刊类型引用(5)
1. 吕丽媛,杨冬明,倪超,钱方,王鹏飞. 肺癌化疗患者血清TBNK淋巴细胞亚群水平变化与预后的相关性研究. 中国肿瘤临床. 2024(06): 302-307 .  百度学术
百度学术2. 刘伶,王栋,贺松,赵偲,韩凌飞. 乙丙型肝炎病毒双重感染外周血淋巴细胞亚群表达. 中华医院感染学杂志. 2024(14): 2099-2102 .  百度学术
百度学术3. 侯环荣,康谊. 成人传染性单核细胞增多症相关肝损伤患者自然杀伤细胞亚群的比例和功能变化. 中国实验血液学杂志. 2023(04): 1217-1223 .  百度学术
百度学术4. 郭丽颖,李晓燕,苏瑞,曹宇,王静,雷金艳,李澎,任玮,宋涛涛,贾建伟,赵洁,伍喜良,苗静. 治疗后慢性乙型肝炎病毒感染者中低病毒血症人群外周血淋巴细胞状态分析. 中华微生物学和免疫学杂志. 2023(07): 525-533 .  百度学术
百度学术5. 于雅丽. IL-33、IL-37、NLRP3及NK/DC比值与慢性乙型肝炎患者病情相关性研究. 广州医药. 2023(10): 52-57 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2862 KB)
PDF下载 ( 2862 KB)


 下载:
下载:


 下载:
下载:

 百度学术
百度学术