土鳖虫调控黏结合蛋白聚糖3改善非酒精性脂肪性肝炎的作用机制
DOI: 10.3969/j.issn.1001-5256.2022.07.012
Mechanism of action of Eupolyphaga steleophaga in improving nonalcoholic steatohepatitis by regulating syndecan 3
-
摘要:
目的 探讨土鳖虫调控黏结合蛋白聚糖3(SDC3)对胆碱缺乏(CDAA)诱导的非酒精性脂肪性肝炎的作用及其机制。 方法 18只雄性C57BL/6小鼠随机分为胆碱充足(CSAA)组、CDAA组和胆碱缺乏加土鳖虫(CDAA+T)组。造模第12周开始,CDAA+T组小鼠给予0.108 g/kg土鳖虫(相当于成人用量10倍量)灌胃,CSAA、CDAA组以等体积生理盐水灌胃,18周末取血清和肝组织,检测小鼠肝功能、TC和TG,观测肝组织病理形态学。实时定量PCR检测转化生长因子(TGF)β1、α-平滑肌肌动蛋白(α-SMA)、Ⅰ型胶原(Col1α1)、SDC3 mRNA表达;检测SDC3在人和小鼠原代肝细胞、肝星状细胞(HSC)、内皮细胞和Kupffer细胞中的表达,应用Si-RNA沉默SDC3,检测SDC3在HSC活化中的作用。蛋白免疫印迹法检测SDC3蛋白表达。计量资料多组间比较采用单因素方差分析, 组内进一步两两比较采用SNK或LSD-t检验。 结果 与CSAA组相比,CDAA组肝功能(ALT、AST)、血清和肝脏TC、TG水平显著升高(P值均<0.01),CDAA+T组小鼠血清ALT、AST、TC、TG水平较CDAA组均显著下降(P值均<0.05)。HE染色显示CDAA组脂肪变明显,炎症细胞浸润增多,CDAA+T组炎症细胞浸润减轻;天狼星红染色显示CDAA组胶原增生显著增加,CDAA+T组胶原增生则减少;油红染色中CDAA组脂肪沉积明显,而CDAA+T组脂肪沉积减少。与CDAA组相比,CDAA+T组肝组织TGFβ、SDC3、α-SMA、COL1α1的mRNA表达和SDC3、α-SMA蛋白表达水平显著降低。免疫组化结果显示:SDC3在CSAA组中表达非常低,CDAA组中SDC3表达显著升高,主要在间质细胞,土鳖虫干预后表达显著降低(P值均<0.05)。PCR结果显示:人和小鼠肝脏各类型细胞中SDC3在HSC细胞表达最高(P值均<0.001)。体外培养LX2细胞,土鳖虫处理能显著降低TGFβ诱导的α-SMA、Col1α1上调,基因沉默SDC3后,土鳖虫不能抑制α-SMA、Col1α1升高(P值均<0.05)。 结论 土鳖虫显著改善CDAA诱导的非酒精性脂肪性肝炎,其机制可能通过调控HSC中SDC3表达实现的。 Abstract:Objective To investigate the effect of Eupolyphaga steleophaga on nonalcoholic steatohepatitis induced by choline-deficient L-amino acid-defined diet (CDAA) and its mechanism by regulating syndecan 3. Methods A total of 18 male C57BL/6 mice were randomly divided into choline-sufficient L-amino acid-defined diet (CSAA) group, CDAA group, and CDAA+Eupolyphaga steleophaga group (CDAA+T group). Since week 12 of modeling, the mice in the CDAA+T group were fed with Eupolyphaga steleophaga 0.108 g/kg (10 times that the dose for adults) by gavage, and those in the CSAA and CDAA groups were given an equal volume of normal saline by gavage. Serum and liver tissue samples were collected at the end of week 18 to measure liver function, total cholesterol (TC), and triglyceride (TG) and observe liver pathology. Quantitative real-time PCR was used to measure the mRNA expression levels of transforming growth factor β (TGFβ), α-smooth muscle actin (α-SMA), collagen type Ⅰ α1 (Col1α1), and SDC3; the mRNA expression of SDC3 was measured in human and mouse primary hepatocytes, hepatic stellate cells (HSCs), liver sinusoidal endothelial cells (LSECs), and Kupffer cells (KCs), and SDC3 was silenced by si-RNA to investigate the role of SDC3 in HSC activation. Western blotting was used to measure the protein expression of SDC3. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the SNK test or the least significant difference t-test was used for further comparison between two groups. Results Compared with the CSAA group, the CDAA group had significant increases in liver function parameters [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] and the levels of TC and TG in serum and the liver (all P < 0.05), and compared with the CDAA group, the CDAA+T group had significant reductions in the serum levels of ALT, AST, TC, and TG (all P < 0.05). HE staining showed that the CDAA group had marked hepatocyte steatosis and increased inflammatory cell infiltration, and the CDAA+T group had alleviated inflammatory cell infiltration; Sirius Red staining showed a significant increase in collagen hyperplasia in the CDAA group and a significant reduction in collagen hyperplasia in the CDAA+T group; oil red staining showed marked fat deposition in the CDAA group and a reduction in fat deposition in the CDAA+T group. Compared with the CDAA group, the CDAA+T group had significant reductions in the mRNA expression levels of TGFβ, SDC3, α-SMA, and COL1α1 and the protein expression levels of SDC3 and α-SMA. Immunohistochemistry showed a very low expression level of SDC3 in the CSAA group and a significant increase in the expression of SDC3 in the CDAA group, mainly in the interstitial cells, and there was a significant reduction after Eupolyphaga steleophaga intervention (all P < 0.05). PCR results showed the highest expression of SDC3 in HSCs of human and mouse liver (all P < 0.001). LX2 cells were cultured in vitro, and Eupolyphaga steleophaga treatment significantly reduced the upregulation of α-SMA and Col1α1 induced by TGFβ, while after SDC3 gene silencing, Eupolyphaga steleophaga did not inhibit the increases in α-SMA and Col1α1 (all P < 0.05). Conclusion Eupolyphaga steleophaga can significantly improve nonalcoholic steatohepatitis induced by CDAA, possibly by regulating the expression of SDC3 in HSCs. -
Key words:
- Eupolyphaga /
- Non-alcoholic Steatohepatitis /
- Syndecan-3
-
原发性肝癌是肝脏最常见的恶性肿瘤之一,其在我国发病率居恶性肿瘤的第4位,肿瘤相关死亡第2位[1]。肝细胞癌(HCC)是肝癌中的最常见的类型,是目前全球第五大恶性肿瘤。慢性肝脏疾病患者,如患有病毒性肝炎、酒精性肝病、非酒精性脂肪性肝炎等,是HCC的主要高危人群。中国的新发病例数及死亡病例数均占了全球的50%左右,因此HCC严重影响我国人民的健康[2]。
对于肝癌患者的根治性治疗,目前主要有肝切除术(liver resection, LR)和肝移植术(liver transplantation, LT)两种方法[3-4],2种方法的选择取决于医院和医生的水平、患者的意愿及身体状况等。为了提高对这2种治疗方法结果的认识,更好的指导临床,本文回顾性分析了首都医科大学附属北京佑安医院171例HCC患者治疗后3年的随访资料,比较LR和LT 2种方法的临床治疗效果。
1. 资料与方法
1.1 研究对象
选取2009年3月—2014年3月在本院首次接受LR或LT的HCC患者171例,依据治疗方式的不同分为LR组(n=83)和LT组(n=88)。LT组患者肝癌的分期依照UICC/AJCC第7版及巴塞罗那分期(BCLC)进行。
1.2 纳入与排除标准
纳入标准:(1)病理确诊符合HCC的诊断标准;(2)LR及LT治疗均在适应证内。排除标准:(1)既往有恶性肿瘤病史;(2)术前曾行抗肿瘤治疗;(3)术后有肿瘤残留;(4)合并严重糖尿病、高血压、心脏病、肾脏病等可能影响生存期的疾病。
1.3 随访情况
患者经过门诊及电话随访,随访主要终点为死亡或随访满3年(36个月),次要终点为检测到肿瘤复发。
1.4 伦理学审查
本研究获得首都医科大学附属北京佑安医院伦理委员会审批,批号:LL-2018-031-K。受试者均签署知情同意书。
1.5 统计学方法
采用SPSS 25.0统计学软件进行数据分析。分类资料组间比较使用χ2检验。比较亚组间无瘤生存期和总生存期的差异使用Kaplan-Meier和log-rank检验。检测影响预后的因素使用单因素和多因素Cox比例风险模型。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
171例HCC患者中男142例(83.04%),女29例(16.96%)。95例(55.56%)患者在50岁以下。3年随访期间共有20例(11.70%)患者失访。3年随访结束时,69例(40.35%)患者肿瘤复发,32例(18.71%)患者发生HCC相关死亡(表 1)。
表 1 肝癌患者的基本资料指标 例数(n=171) 手术类型 χ2值 P值 LR组(n=83) LT组(n=88) 性别(例) 0.192 0.661 男 142 70 72 女 29 13 16 年龄(例) 0.117 0.732 ≤50岁 95 45 50 >50岁 76 38 38 潜在肝病(例) 2.333 0.311 HBV 153 76 77 HCV 17 6 11 其他 1 1 0 肿瘤位置(例) 1.161 0.281 右叶 119 61 58 非右叶或多叶 52 22 30 肿瘤数目(例) 29.649 <0.001 单发 113 38 75 多发 58 45 13 肿瘤大小(例) 46.383 <0.001 <3 cm 72 13 59 3~5 cm 62 43 19 >5 cm 37 27 10 AFP (例) 3.350 0.187 <20 ng/ml 82 42 40 20~400 ng/ml 52 20 32 >400 ng/ml 37 21 16 TNM分期(例) 0.174 0.677 Ⅰ+Ⅱ 117 57 63 Ⅲ+Ⅳ 54 26 25 BCLC肝癌分期(例) 0.057 0.972 A 87 42 45 B 44 22 22 C 40 19 21 Child-Pugh分级(例) 7.833 0.005 C 140 75 65 B+C 31 8 23 病理分级(例) 2.573 0.276 低分化 29 18 11 中分化 113 52 61 高分化 29 13 16 肿瘤复发(例) 4.121 0.042 否 102 43 59 是 69 40 29 HCC所致死亡(例) 0.937 0.333 否 139 65 74 是 32 18 14 从基础数据上可以看出,LT组较LR组的单发肿瘤比例更多、肿瘤更小、Child-Pugh分期更高、复发率更低(P值均<0.05),而其他的指标如性别、年龄、潜在肝病、肿瘤位置、AFP水平、TNM分期、BCLC肝癌分期和病理分级2组间比较差异均无统计学意义(P值均>0.05)(表 1)。
2.2 无瘤生存期和总生存期
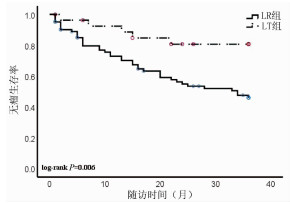
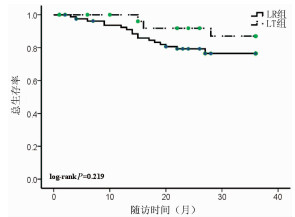
随访3年,LR组患者无瘤生存率是46.02%,而LT组患者的无瘤生存率为80.71%,两组差异具有统计学意义(P=0.006)(图 1);LR组患者的总生存率是76.44%,LT组患者的总生存率为86.99%,两组比较差异无统计学意义(P=0.219)(图 2)。
2.3 HCC患者结局的影响因素分析
使用Cox生存分析方法分析无瘤生存期、总生存期和临床特征之间的相关性。在无瘤生存期的单因素分析中,治疗方法和TNM分期是无瘤生存期的影响因素(P值均<0.05)(表 2)。多因素分析结果显示,治疗方法和TNM期是HCC患者无瘤生存期的独立预后因素[RR(95% CI)分别为0.239(0.093~0.612)、4.834(2.598~8.993),P值分别为0.003、<0.001]。在总生存期的单因素分析中,TNM分期是无瘤生存期的影响因素(P<0.001)(表 2)。多因素分析发现TNM分期是HCC患者总生存期的独立预后因素[RR(95% CI):4.970(2.052~12.037),P<0.001]。
表 2 肝癌患者无瘤生存期和总生存期的单因素分析指标 无瘤生存期 总生存期 RR 95%CI P值 RR 95%CI P值 性别 男 1.000 1.000 女 0.539 0.213~1.367 0.193 0.474 0.110~2.034 0.315 年龄 ≤50岁 1.000 1.000 >50岁 1.023 0.570~1.838 0.939 0.678 0.281~1.636 0.387 肝病 HBV 1.000 1.000 HCV/其他 0.380 0.092~1.569 0.181 0.872 0.203~3.747 0.854 肿瘤位置 肝右叶 1.000 1.000 其他位置 0.816 0.422~1.581 0.548 0.693 0.254~1.893 0.475 肿瘤数目 单发 1.000 1.000 多发 1.513 0.833~2.751 0.174 1.491 0.628~3.542 0.365 肿瘤大小 <3 cm 1.000 1.000 <3 cm 1.000 1.000 3~5 cm 1.360 0.647~2.860 0.418 2.956 0.890~9.819 0.077 >5 cm 3.877 1.924~7.812 <0.001 5.595 1.721~18.189 0.004 AFP <20 ng/ml 1.000 1.000 20~400 ng/ml 1.862 0.861~4.025 0.114 0.982 1.699~12.123 0.003 >400 ng/ml 3.625 1.804~7.285 <0.001 4.539 1.699~12.123 0.003 TNM分期 Ⅰ-Ⅱ 1.000 1.000 Ⅲ 4.084 2.237~7.455 <0.001 4.985 2.058~12.076 <0.001 BCLC HCC分期 A 1.000 1.000 B 1.778 0.887~3.564 0.003 1.235 0.349~4.378 0.001 C 3.008 1.453~6.230 0.105 0.003 5.566 2.052~15.101 0.001 Child-Pugh分级 A 1.000 1.000 B+C 0.446 0.160~1.245 0.123 1.886 0.731~4.864 0.189 病理分期 低分化 1.000 1.000 中/高分化 0.813 0.378~1.750 0.597 0.524 0.203~1.350 0.181 治疗方法 LR 1.000 1.000 LT 3.383 1.334~8.579 0.010 0.474 0.140~1.610 0.232 3. 讨论
肝癌的治疗需要多学科协同、多种方法综合进行。目前临床上常用的肝癌治疗方法包括:LR、LT、局部消融治疗(射频消融治疗术为主)、经导管肝动脉化疗栓塞术、放射治疗、全身治疗(包括分子靶向治疗、免疫治疗、化疗、中医治疗等)等[3-5]。既往认为LT治疗的肝癌患者生存结局更好[5]。但是随着对HCC发生发展的探索、影像诊断水平的提高、局部和全身治疗方法的完善、手术技术的进步和术后多学科诊疗模式的形成,不同治疗下患者的最终结局,也随之而改善,有学者[6-7]提出目前LR治疗肝癌与LT同样取得理想效果。金子铮等[8]研究发现LT较LR治疗肝癌患者的5年生存率的优势,2005年—2011年较1989年—2004年明显缩小(73% vs 61%,77% vs 36%),他们认为这种优势的缩小是由于手术水平的进步、围手术期对肝硬化治疗的优化和对于复发肿瘤多手段的治疗。除了总体变化趋势外,治疗的结果还和医院的水平、患者的状况等多个因素相关。本研究发现LT较LR治疗肝癌3年后肿瘤的复发率明显降低,而总生存率差别不显著。Shen等[9]回顾分析了1218例LT和2068例LR治疗肝癌的患者得出结论,LT比LR在无瘤生存率和总生存率上都有优势。矫学黎等[7]对352例LR和37例LT治疗的患者进行了统计分析后也得到了相似的结论。本研究中LT组较LR组的肿瘤复发率明显降低,与文献一致。另一项纳入9篇关于LT和LR治疗HCC效果研究的Meta分析[10]结果显示,HCC患者术后第1年两种治疗方法的总生存率相似,术后第5年LR组较LT组总生存率明显降低。这与本研究中两组总生存率的结果稍有差别。该研究还认为,与LR组相比,HCC患者术后3年LT组的获益才开始表现出来,而本研究的随访截点正好是3年,因此本研究结果与其他中心的研究结果并不矛盾。肿瘤复发才是病死率增加的开始,如果延长随访时间,本研究的总生存率也将出现显著性差异。故本研究数据结合文献检索的结果,支持LT较LR治疗肝癌更有优势。
从笔者的基础数据上可以看出,LT组较LR组的单发肿瘤更多、肿瘤更小、Child-Pugh分期更高,而其他的指标如性别、年龄、潜在肝病、肿瘤位置、AFP水平、TNM分期、BCLC肝癌分期和病理分级2组比较差异均无统计学差异,这是因为在选择LT治疗的时候,兼顾疗效与公平,平衡考虑肿瘤患者预后,主观限制了总体的肿瘤数目和大小。LT较LR治疗肝癌患者的另一优势是不用考虑患者的肝功能,可以同时解决肿瘤和肝功能衰竭两大问题,本研究入组的LT患者中有2例是Child-Pugh C级。综上所述,LT更倾向于选择肝功能储备较差的早期肝癌患者,3年无瘤生存率令人满意。
LT也存在一定的缺点,比如供体的缺乏、术中并发症多、药物排异等,为了弥补这些缺点,目前已有较多研究在推动LT的进步,包括移植方法上的改进、治疗适应证的拓展、新型抗排异药物的研发和围手术期处理的优化等[11-14]。
本研究结果还得出TNM分期是无瘤生存期和总生存期的独立预测因素,支持TNM分期在临床上的重要作用,不但可以用于指导治疗方法的选择,还能预测术后结果。但是TNM分期也有缺点,淋巴结或远处脏器转移有时术前和术中难以准确评估,因此更好的判断预后的指标和方法也是研究的热点。
另外,本研究是单中心研究,基于现有的临床资料,还存在着样本量较小、随访时间短、纳入因素不全面等因素,而且对于手术切除范围、肝移植方法、供体类型、术后治疗、医疗成本、并发症情况等因素未纳入其中,局限了研究的意义。希望将来设计多中心、样本量更大的研究来弥补以上不足, 将更有利于临床工作的不断提升。
-
表 1 RT-PCR引物序列
Table 1. RT-PCR primer sequence
基因 上游 下游 18 s rRNA 5′-AGTCCCTGCCCTTTGTACACA-3′ 5′-CGATCCGAGGGCCTCACTA-3′ mα-SMA 5′-GTTCAGTGGTGCCTCTGTCA-3′ 5′-ACTGGGACGACATGGAAAAG-3′ mCol1α1 5′-TAGGCCATTGTGTATGCAGC-3′ 5′-ACATGTTCAGCTTTGTGGACC-3′ hSDC3 5′-GGCGCAGTGAGAACTTCG-3′ 5′-CCCCGAGTAGAGGTCATCCAG-3′ mSDC3 5′-GTCCAGCCAGAAAGCTACCAC-3′ 5′-CATCCCCTGTATGCCGGTG-3′ mTGFβ 5′-GCAAAGACCATCTGTCTCACA-3′ 5′-CTCCTCATCGTGTTGGTGG-3′ 注:m,小鼠引物;h,人源引物。 表 2 各组肝功能、血脂、肝脏脂肪表达及病理学改变
Table 2. Changes of liver function, serum lipids, liver fat expression and pathological in each group
指标 CSAA组 CDAA组 CDAA+T组 F值 P值 ALT(U/L) 11.56±2.93 72.79±7.061) 26.17±3.762) 253.68 <0.001 AST(U/L) 18.47±4.85 52.94±7.231) 26.43±2.732) 70.38 <0.001 血清TC(mmol/L) 2.95±0.64 7.97±1.221) 4.24±0.902) 45.21 <0.001 血清TG(mmol/L) 0.27±0.08 0.63±0.101) 0.35±0.072) 27.82 <0.001 肝脏TC(mmol/gprot) 1.57±0.45 2.49±0.671) 1.67±0.412) 5.70 <0.05 肝脏TG(mmol/gprot) 8.63±2.53 19.85±1.661) 16.03±1.492) 51.57 <0.001 NAS评分(分) 0.67±0.52 6.83±0.751) 4.50±1.052) 90.26 <0.001 天狼星红面积(%) 1.01±0.14 6.39±0.651) 3.17±0.262) 344.94 <0.001 油红面积(%) 0.83±0.12 7.90±1.441) 3.34±0.502) 131.45 <0.001 注:与CSAA组比较,1)P<0.01;与CDAA组比较,2)P<0.05。 表 3 纤维化指标和SDC3在CDAA模型中表达
Table 3. Expression of fibrosis index and SDC3 in CDAA model
指标 CSAA组 CDAA组 CDAA+T组 F值 P值 RT-PCR TGFβ mRNA 1.00±0.37 3.12±1.701) 1.50±0.292) 14.25 <0.001 Col1α1 mRNA 0.98±0.28 4.11±1.191) 0.81±0.232) 16.71 <0.001 SDC3 mRNA 1.00±0.57 5.88±1.831) 2.66±1.032) 46.69 <0.001 α-SMA mRNA 1.54±1.88 10.10±3.371) 3.53±0.862) 46.33 <0.001 免疫印迹 SDC3/β-Actin 1.00±0.19 2.52±0.431) 1.29±0.082) 15.92 <0.05 免疫组化 SDC3阳性面积(%) 0.09±0.03 0.40±0.051) 0.25±0.132) 134.37 <0.001 α-SMA阳性面积(%) 1.09±0.20 2.97±0.211) 1.78±0.232) 160.52 <0.001 注:与CSAA组比较,1)P<0.01;与CDAA组比较,2)P<0.01。 表 4 SDC3在肝脏各类细胞中表达
Table 4. Expression of SDC3 in various liver cells
组别 SDC3 mRNA相对表达量 人 鼠 Hepato 0.92±0.24 1.00±0.31 HSC 32.74±1.65 45.67±2.98 LSEC 21.27±1.75 29.47±3.97 Kupffer细胞 26.67±1.87 30.53±3.82 F值 490.38 282.88 P值 <0.001 <0.001 表 5 土鳖虫提取物抑制TGFβ诱导的LX2纤维化
Table 5. The LX2 fibrosis induced by TGFβ was inhibited by the extract of eupolyphaga
组别 α-SMA mRNA Col1α1 mRNA Si-NC-PBS-TGFβ组 1.00±0.11 1.00±0.04 Si-NC-T-TGFβ组 0.53±0.071) 0.34±0.021) Si-SDC3-PBS-TGFβ组 2.57±0.121) 1.79±0.361) Si-SDC3-T-TGFβ组 0.96±0.10 0.89±0.11 F值 157.98 19.69 P值 <0.001 <0.01 注:与Si-NC-PBS-TGFβ组比较,1)P<0.001。 -
[1] LI HS, CHEN SD, YING H, et al. Intervention effect of salidroside on liver fat synthesis and oxidation of non-alcoholic fatty liver in rats induced by high-fat diet[J]. China J Tradit Chin Med Pharma, 2017, 32(10): 4625-4628. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201710084.htm李红山, 陈少东, 应豪, 等. 红景天苷对高脂饮食诱导的大鼠非酒精性脂肪肝肝脏脂肪合成和氧化环节的干预作用[J]. 中华中医药杂志, 2017, 32(10): 4625-4628. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201710084.htm [2] SHI ZH, ZHENG D, GUO J, et al. Effects of scallion extract on PGC-1α and mitochondrial biosynthesis in nonalcoholic fatty liver model rats[J]. Lishizhen Med Mater Med Res, 2018, 29(10): 2320-2322. DOI: 10.3969/j.issn.1008-0805.2018.10.005.时昭红, 郑丁, 郭洁, 等. 葱白提取物对非酒精性脂肪肝模型大鼠PGC-1α和线粒体生物合成的影响[J]. 时珍国医国药, 2018, 29(10): 2320-2322. DOI: 10.3969/j.issn.1008-0805.2018.10.005. [3] LIU C, YUAN X, TAO L, et al. Xia-yu-xue decoction (XYXD) reduces carbon tetrachloride (CCl4)-induced liver fibrosis through inhibition hepatic stellate cell activation by targeting NF-κB and TGF-β1 signaling pathways[J]. BMC Complement Altern Med, 2015, 15: 201. DOI: 10.1186/s12906-015-0733-1. [4] LIU C, CAI J, CHENG Z, et al. Xiayuxue decoction reduces renal injury by promoting macrophage apoptosis in hepatic cirrhotic rats[J]. Genet Mol Res, 2015, 14(3): 10760-10773. DOI: 10.4238/2015.September.9.15. [5] WU L, ZHANG J, MA WT, et al. Xiayuxue Decoction inhibits methionine-choline-deficient-induced nonalcoholic steatohepatitis in mice[J/CD]. Chin J Liver Dis (Electronic Version), 2018, 10(3): 8. DOI: 10.3969/j.issn.1674-7380.2018.03.009.吴柳, 张洁, 马文婷, 等. 下瘀血汤对胆碱蛋氨酸缺乏诱导的小鼠非酒精性脂肪性肝炎的抑制作用[J/CD]. 中国肝脏病杂志(电子版), 2018, 10(3): 8. DOI: 10.3969/j.issn.1674-7380.2018.03.009. [6] WU L, YANG GY, ZHANG J, et al. Xiayuxue Decoction improved HFD-induced-nonalcoholic steatohepatitis mice by down-regulating NLRP3[J]. Chin J Integr Trad West Med, 2020, 40(10): 7. DOI: 10.7661/j.cjim.20200904.336.吴柳, 杨广越, 张洁, 等. 下瘀血汤下调NLRP3改善高脂饮食诱导小鼠非酒精性脂肪性肝炎[J]. 中国中西医结合杂志, 2020, 40(10): 7. DOI: 10.7661/j.cjim.20200904.336. [7] XIE YC, HU GX, PENG YZ, et al. Clinical effect of Dahuang Zhechong capsules combined with entecavir in treatment of chronic hepatitis B patients with liver fibrosis[J]. J Clin Hepatol, 2016, 32(8): 1502-1507. DOI: 10.3969/j.issn.1001-5256.2016.08.014.谢永财, 胡国信, 彭雁忠, 等. 大黄蟅虫胶囊联合恩替卡韦治疗慢性乙型肝炎肝纤维化的效果观察[J]. 临床肝胆病杂志, 2016, 32(8): 1502-1507. DOI: 10.3969/j.issn.1001-5256.2016.08.014. [8] CHEN SL, CHEN DX, DU GL. Experimental study of Xiayuxue Decoction component compatibility aganist immunity hepatic fibrosis rat model[J]. Lishizhen Med Mater Med Res, 2013, 24(6): 3. DOI: 10.3969/j.issn.1008-0805.2013.06.047陈少丽, 陈德兴, 都广礼. 下瘀血汤组分配伍抗免疫性肝纤维化大鼠模型的实验研究[J]. 时珍国医国药, 2013, 24(6): 3. DOI: 10.3969/j.issn.1008-0805.2013.06.047. [9] REIZES O, LINCECUM J, WANG Z, et al. Transgenic expression of syndecan-1 uncovers a physiological control of feeding behavior by syndecan-3[J]. Cell, 2001, 106(1): 105-116. DOI: 10.1016/s0092-8674(01)00415-9. [10] STRADER AD, REIZES O, WOODS SC, et al. Mice lacking the syndecan-3 gene are resistant to diet-induced obesity[J]. J Clin Invest, 2004, 114(9): 1354-1360. DOI: 10.1172/JCI20631. [11] TAO L, MA W, WU L, et al. Glial cell line-derived neurotrophic factor (GDNF) mediates hepatic stellate cell activation via ALK5/Smad signalling[J]. Gut, 2019, 68(12): 2214-2227. DOI: 10.1136/gutjnl-2018-317872. [12] ZHANG W, YANG GY, SHEN DX, et al. Mechanism of action of Xiayuxue decoction in inhibiting liver fibrosis by regulating glial cell line-derived neurotrophic factor[J]. J Clin Hepatol, 2021, 37(3): 575-581. DOI: 10.3969/j.issn.1001-5256.2021.03.015.张玮, 杨广越, 沈东晓, 等. 下瘀血汤抑制胶质细胞源性神经营养因子抗肝纤维化的作用机制[J]. 临床肝胆病杂志, 2021, 37(3): 575-581. DOI: 10.3969/j.issn.1001-5256.2021.03.015. [13] LIU XL, YANG GY, ZHANG W, et al. Therapeutic effect of Taohong Siwu decoction on a mouse model of carbon tetrachloride-induced liver fibrosis and its mechanism[J]. J Clin Hepatol, 2021, 37(11): 2563-2568. DOI: 10.3969/j.issn.1001-5256.2021.11.016.刘旭凌, 杨广越, 张玮, 等. 桃红四物汤对CCl4诱导肝纤维化小鼠模型的干预作用及其机制[J]. 临床肝胆病杂志, 2021, 37(11): 2563-2568. DOI: 10.3969/j.issn.1001-5256.2021.11.016. [14] The Chinese National Workshop on Fatty Liver and Alcoholic Liver Disease for the Chinese Liver Disease Association. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition[J]. J Mod Med Health, 2011, 27(5): 641-644. DOI: 10.3760/cma.j.issn.1007-3418.2011.03.002.中华医学会肝病学分会脂肪肝和酒精性肝病学组. 非酒精性脂肪性肝病诊疗指南(2010年修订版)[J]. 现代医药卫生, 2011, 27(5): 641-644. DOI: 10.3760/cma.j.issn.1007-3418.2011.03.002. [15] ZHENG PY, AN XQ, LIU JC. Role of angiopoietin-like proteins in the development of nonalcoholic fatty liver dis-ease[J]. J Clin Hepatol, 2021, 37(11) : 2680-2683. DOI: 10.3969/j.issn.1001-5256.2021.11.042.郑培玉, 安秀琴, 刘近春. 血管生成素样蛋白在非酒精性脂肪性肝病发展中的作用[J]. 临床肝胆病杂志, 2021, 37(11) : 2680-2683. DOI: 10.3969/j.issn.1001-5256.2021.11.042. [16] MANNE V, HANDA P, KOWDLEY KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis[J]. Clin Liver Dis, 2018, 22(1): 23-37. DOI: 10.1016/j.cld.2017.08.007. [17] LAN T, HU Y, HU F, et al. Hepatocyte glutathione S-transferase mu 2 prevents non-alcoholic steatohepatitis by suppressing ASK1 signaling[J]. J Hepatol, 2022, 76(2): 407-419. DOI: 10.1016/j.jhep.2021.09.040. [18] REPETTO MG, OSSANI G, MONSERRAT AJ, et al. Oxidative damage: the biochemical mechanism of cellular injury and necrosis in choline deficiency[J]. Exp Mol Pathol, 2010, 88(1): 143-149. DOI: 10.1016/j.yexmp.2009.11.002. [19] VETELÄINEN R, van VLIET A, van GULIK TM. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model[J]. J Gastroenterol Hepatol, 2007, 22(9): 1526-1533. DOI: 10.1111/j.1440-1746.2006.04701.x. [20] ZHOU Q, SU J, JI MY. Progress in the treatment of nonalcoholic fatty liver disease[J]. China Med Herald, 2020, 17(6): 26-29. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202006008.htm周谦, 苏娟, 季梦遥. 非酒精性脂肪性肝病的治疗研究进展[J]. 中国医药导报, 2020, 17(6): 26-29. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202006008.htm [21] DONG PP, ZHANG JY, WEI YL, et al. Pharmacokinetics and pharmacodynamics of bioactive peptide LL8 from Steleophaga plancyi in rats[J]. Chin Tradit Herb Drug, 2021, 52(15): 4607-4613. DOI: 10.7501/j.issn.0253-2670.2021.15.019.董萍萍, 张加余, 魏永利, 等. 土鳖虫活性肽LL8在大鼠体内的药动学及药效学研究[J]. 中草药, 2021, 52(15): 4607-4613. DOI: 10.7501/j.issn.0253-2670.2021.15.019. 期刊类型引用(1)
1. 宋林泉,梁志宏. 肝门再阻断法在肝切除术后胆漏中的预防效果. 吉林医学. 2024(02): 364-367 .  百度学术
百度学术其他类型引用(3)
-




 PDF下载 ( 5504 KB)
PDF下载 ( 5504 KB)

 下载:
下载:



 下载:
下载:




 百度学术
百度学术