虫草菌丝通过抑制TLR4/NF-κB信号通路与血管生成素样蛋白4对肝纤维化小鼠模型的保护作用
DOI: 10.3969/j.issn.1001-5256.2022.07.016
Cultured mycelia of Cordyceps sinensis exerts a protective effect on a mouse model of liver fibrosis by inhibiting the Toll-like receptor 4/nuclear transcription factor-κB signaling pathway and angiopoietin-like protein 4
-
摘要:
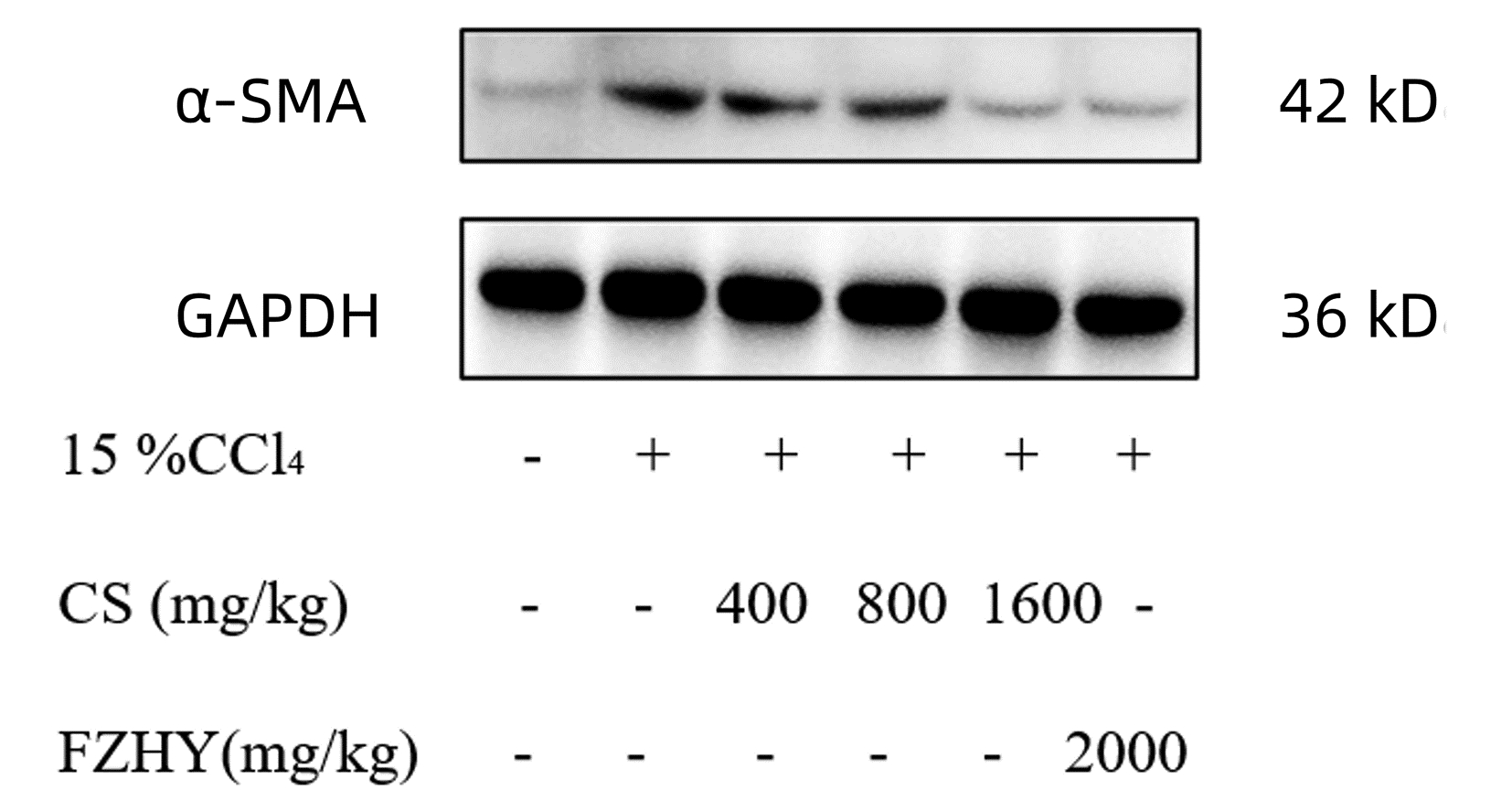
目的 探究虫草菌丝对CCl4诱导的肝纤维化小鼠模型的干预作用,及对Toll样受体4(TLR4)/核转录因子-κB(NF-κB)通路与血管生成素样蛋白4(ANGPTL4)的影响。 方法 将60只SPF级雄性C57/BL6J小鼠随机分为正常组、模型组、扶正化瘀方组及虫草菌丝低、中、高剂量组,每组各10只。模型组和各用药组注射15% CCl4-橄榄油,造模6周。在第4周首日开始给药,连续3周。分别采用血生化法检测小鼠血清ALT、AST、TBil水平或活性,HE和SR染色观察肝组织病理学,碱水解法测定肝组织羟脯氨酸(HYP)含量情况,PCR检测α-SMA、Col-Ⅰ的mRNA表达情况,免疫组化测定肝组织α-SMA、Col-Ⅰ与ANGPTL4蛋白表达,Western Blot检测α-SMA、ANGPTL4、TLR4、NF-κB/P-NF-κB、CD163蛋白的表达。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与正常组比较,模型组小鼠血清ALT、AST、TBil水平均显著增加(P值均<0.01);与模型组比较,虫草菌丝高剂量组显著改善小鼠肝纤维化程度,血清ALT、AST、TBil水平均显著减少(P值均<0.05)。HE染色发现虫草菌丝高剂量组能显著减少肝组织炎性细胞浸润。SR染色发现,虫草菌丝高剂量组明显减轻肝脏胶原沉积。与正常组比较,模型组肝组织HYP含量、SR阳性染色面积比均显著增加(P值均<0.01);与模型组比较,虫草菌丝高剂量组肝组织HYP含量、SR阳性染色面积比均显著减小(P值均<0.05)。与正常组比较,免疫组化、PCR和Western Blot结果显示模型组肝组织中α-SMA和Col-Ⅰ mRNA及蛋白表达显著增加(P值均<0.01);与模型组比较,虫草菌丝高剂量组肝组织中α-SMA、Col-Ⅰ胶原纤维表达均显著减少(P值均<0.05)。与正常组比较,模型组肝组织ANGPTL4、TLR4、P-NF-κB/NF-κB、CD163蛋白表达均显著增加(P值均<0.01);与模型组比较,虫草菌丝高剂量组肝组织ANGPTL4、TLR4、P-NF-κB/NF-κB、CD163蛋白含量均显著降低(P值均<0.05)。 结论 虫草菌丝对CCl4诱导的小鼠肝纤维化具有显著治疗作用,其机制可能与调控TLR4/NF-κB信号通路及抑制肝组织ANGPTL4表达相关。 Abstract:Objective To investigate the interventional effect of cultured mycelia of Cordyceps sinensis on a mouse model of CCl4-induced liver fibrosis and its effect on the Toll-like receptor 4 (TLR4)/nuclear transcription factor-κB (NF-κB) pathway and angiopoietin-like protein 4 (ANGPTL4). Methods A total of 60 specific pathogen-free male C57/BL6J mice were randomly divided into normal group, model group, Fuzheng Huayu group, and low-, middle-, and high-dose Cordyceps sinensis groups, with 10 mice in each group. The mice in the model group and each medication group were intraperitoneally injected with 15% CCl4-olive oil for 6 weeks of modeling, and drug intervention was started on the first day of week 4 and lasted for 3 consecutive weeks. Blood biochemistry was used to measure the serum level or activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBil); HE and SR staining was used to observe liver histopathology; alkaline hydrolysis was used to measure the content of hydroxyproline (HYP) in liver tissue; PCR was used to measure the mRNA expression of alpha-smooth muscle actin (α-SMA) and collagen type Ⅰ (Col-Ⅰ), immunohistochemistry was used to measure the protein expression of α-SMA, Col-Ⅰ, and ANGPTL4 in liver tissue, and Western blot (WB) was used to measure the protein expression of α-SMA, ANGPTL4, TLR4, NF-κB/phosphorylated NF-κB (P-NF-κB), and CD163. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the LSD-t test was used for further comparison between two groups. Results Compared with the normal group, the model group had significant increases in the serum levels of ALT, AST, and TBil (all P < 0.01), and compared with the model group, the high-dose Cordyceps sinensis group had a significant improvement in the degree of liver fibrosis and significant reductions in the serum levels of ALT, AST, and TBil (all P < 0.05). HE staining revealed that the high-dose Cordyceps sinensis group had a significant reduction in inflammatory cell infiltration in liver tissue, and SR staining showed that high-dose Cordyceps sinensis significantly alleviated collagen deposition in the liver tissue. Compared with the normal group, the model group had significant increases in HYP content and Sirius red-positive area ratio in liver tissue (both P < 0.01), and compared with the model group, the high-dose Cordyceps sinensis group had significant reductions in HYP content and Sirius red-positive area ratio (both P < 0.05). Compared with the normal group based on the results of immunohistochemistry, PCR, and WB, the model group had significant increases in the mRNA and protein expression levels of α-SMA and Col-Ⅰ in liver tissue (all P < 0.01), and compared with the model group, the high-dose Cordyceps sinensis group had significant reductions in the expression levels of α-SMA and Col-Ⅰ(P < 0.05). Compared with the normal group, the model group had significant increases in the protein expression of ANGPTL4, TLR4, P-NF-κB/NF-κB, and CD163 in liver tissue (all P < 0.01), and compared with the model group, the high-dose Cordyceps sinensis group had significant reductions in the protein expression of ANGPTL4, TLR4, P-NF-κB/NF-κB, and CD163 in liver tissue (all P < 0.05). Conclusion Cordyceps sinensis mycelia have a marked therapeutic effect on CCl4-induced liver fibrosis in mice, possibly by regulating the TLR4/NF-κB signaling pathway and inhibiting ANGPTL4 expression in liver tissue. -
Key words:
- Liver Cirrhosis /
- Angiopoietins /
- Toll-Like Receptor 4 /
- NF-kappa B
-
表 1 PCR引物序列
Table 1. PCR primer sequences
基因名称 引物序列 GAPDH 上游:5′-CATCACTGCCACCCAGAAGACTG-3′ 下游:5′-ATGCCAGTGAGCTTCCCGTTCAG-3′ α-SMA 上游:5′-ACCATCGGCAATGAGCGTTTCC-3′ 下游:5′-GCTGTTGTAGGTGGTCTCATGG-3′ Col-Ⅰ 上游:5′-CCTCAGGGTATTGCTGGACAAC-3′ 下游:5′-CAGAAGGACCTTGTTTGCCAGG-3′ 表 2 各组小鼠血清生化结果比较
Table 2. Comparison of serum biochemistry results of each group of mice
组别 动物数(只) ALT(U/L) AST(U/L) TBil(μmol/L) 正常组 10 33.67±5.27 176.83±22.57 1.78±0.11 模型组 10 208.67±23.141) 395.72±43.281) 4.43±0.261) 虫草菌丝低剂量组 10 191.06±19.24 317.82±53.293) 4.18±0.36 虫草菌丝中剂量组 10 178.72±25.38 287.04±38.472) 4.06±0.67 虫草菌丝高剂量组 10 133.63±17.672)4)5) 234.57±32.912)4) 3.76±0.115) 扶正化瘀方组 10 142.84±19.372)4) 250.61±61.922) 3.9±0.53 F值 53.021 22.162 28.222 P值 <0.001 <0.001 <0.001 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.01,3)P<0.05;与虫草菌丝低剂量组比较,4)P<0.01;与虫草菌丝中剂量组比较,5)P<0.05。 表 3 HYP含量和SR阳染面积
Table 3. HYP content and SR positive staining area
组别 动物数(只) HYP(μg/mL) SR阳染面积(%) 正常组 10 326.63±41.01 1.63±0.34 模型组 10 684.08±103.541) 6.28±1.321) 虫草菌丝低剂量组 10 628.78±65.42 5.48±0.87 虫草菌丝中剂量组 10 580.57±89.82 4.95±0.742) 虫草菌丝高剂量组 10 500.82±67.362)3) 4.38±0.722) 扶正化瘀方组 10 551.29±46.282) 4.40±0.952) F值 18.393 43.112 P值 <0.001 <0.001 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.05;与虫草菌丝低剂量组比较,3)P<0.05。 表 4 α-SMA和Col-Ⅰ免疫组化阳染面积
Table 4. Immunohistochemical positive staining area of α-SMA and Col-Ⅰ
组别 动物数(只) α-SMA阳染面积(%) Col-Ⅰ阳染面积(%) 正常组 10 1.00±0.31 1.00±0.41 模型组 10 6.11±0.561) 1.74±0.531) 虫草菌丝低剂量组 10 5.62±0.48 1.66±0.52 虫草菌丝中剂量组 10 5.14±0.96 1.51±0.39 虫草菌丝高剂量组 10 4.57±0.282) 1.32±0.482) 扶正化瘀方组 10 4.89±0.57 1.53±0.46 F值 69.413 10.936 P值 <0.001 0.032 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.05。 表 5 α-SMA和Col- Ⅰ mRNA表达
Table 5. α-SMA and Col- Ⅰ mRNA expression
组别 动物数(只) α-SMA Col-Ⅰ 正常组 10 1.00±0.31 1.00±0.24 模型组 10 4.95±0.911) 6.77±0.811) 虫草菌丝低剂量组 10 3.94±0.72 5.62±0.63 虫草菌丝中剂量组 10 3.58±0.532) 5.31±0.532) 虫草菌丝高剂量组 10 3.22±0.582) 4.79±0.472) 扶正化瘀方组 10 3.58±0.71 5.21±0.51 F值 13.903 16.902 P值 0.026 0.019 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.05。 表 6 α-SMA蛋白表达
Table 6. α-SMA protein expression
组别 动物数(只) α-SMA/GAPDH 正常组 10 1.00±0.56 模型组 10 2.73±0.671) 虫草菌丝低剂量组 10 2.33±0.36 虫草菌丝中剂量组 10 2.06±0.48 虫草菌丝高剂量组 10 1.55±0.612)3) 扶正化瘀方组 10 1.68±0.382)3) F值 12.433 P值 0.036 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.01;与虫草菌丝低剂量组比较,3)P<0.05。 表 7 ANGPTL4免疫组化阳染面积
Table 7. Immunohistochemical positive staining area of ANGPTL4
组别 动物数(只) ANGPTL4阳染面积(%) 正常组 10 1.00±0.17 模型组 10 1.82±0.321) 虫草菌丝低剂量组 10 1.73±0.28 虫草菌丝中剂量组 10 1.63±0.25 虫草菌丝高剂量组 10 1.32±0.312) 扶正化瘀方组 10 1.59±0.25 F值 12.874 P值 0.037 注:与正常组比较,1)P<0.05;与模型组比较,2)P<0.05。 表 8 ANGPTL4、TLR4、P-NF-κB、NF-κB、CD163蛋白表达
Table 8. ANGPTL4, TLR4, P-NF-κB, NF-κB, CD163 protein expression
组别 动物数(只) ANGPTL4/GAPDH TLR4/GAPDH P-NF-κB/NF-κB CD163/GAPDH 正常组 10 1.00±0.13 1.00±0.18 1.00±0.19 1.00±0.27 模型组 10 1.76±0.242) 1.69±0.322) 1.90±0.311) 2.12±0.341) 虫草菌丝低剂量组 10 1.63±0.29 1.61±0.21 1.78±0.21 1.95±0.28 虫草菌丝中剂量组 10 1.58±0.31 1.49±0.27 1.67±0.26 1.82±0.31 虫草菌丝高剂量组 10 1.23±0.193) 1.18±0.193) 1.35±0.283) 1.51±0.253) 扶正化瘀方组 10 1.38±0.353) 1.31±0.32 1.62±0.29 1.69±0.30 F值 12.227 22.492 15.103 11.141 P值 0.035 <0.001 0.021 0.039 注:与正常组比较,1)P<0.01,2)P<0.05;与模型组比较,3)P<0.05。 -
[1] LIU CH, LYU J. Hepatic fibrosis 2006: report of the Third AASLD Single Topic Conference[J]. Chin Hepatol, 2007, 12(2): 138-142. DOI: 10.14000/j.cnki.issn.1008-1704.2007.02.024.刘成海, 吕靖. 美国肝病学会肝纤维化专题会议报道[J]. 肝脏, 2007, 12(2): 138-142. DOI: 10.14000/j.cnki.issn.1008-1704.2007.02.024. [2] HERNANDEZ-GEA V, FRIEDMAN SL. Pathogenesis of liver fibrosis[J]. Annu Rev Pathol, 2011, 6: 425-456. DOI: 10.1146/annurev-pathol-011110-130246. [3] ZHAO ZM, LIU CH. Therapeutic insights of traditional Chinese medicine in liver fibrosis[J]. J Prac Hepatol, 2016, 19(1): 12-15. DOI: 10.3969/j.issn.1672-5069.2016.01.004.赵志敏, 刘成海. 中医药治疗肝纤维化研究进展[J]. 实用肝脏病杂志, 2016, 19(1): 12-15. DOI: 10.3969/j.issn.1672-5069.2016.01.004. [4] CHEN GX, WEN B, SUN HT, et al. Effect of Biejiajian Wan on NF-κB Signaling pathway in rat hepatic fibrosis model induced by CCl4[J]. Chin J Exp Med Formul, 2018, 24(10): 161-167. DOI: 10.13422/j.cnki.syfjx.20180834.陈冠新, 文彬, 孙海涛, 等. 鳖甲煎丸对CCl4致大鼠肝纤维化模型中NF-κB信号通路的影响[J]. 中国实验方剂学杂志, 2018, 24(10): 161-167. DOI: 10.13422/j.cnki.syfjx.20180834. [5] YANG HR, XU Y, YANG YS, et al. Anti-hepatic fibrosis effect and mechanism of natural products[J]. Chin J Exp Med Formul, 2018, 24(14): 214-221. DOI: 10.13422/j.cnki.syfjx.20181428.杨华蕊, 徐妍, 杨永寿, 等. 天然产物抗肝纤维化作用及其机制研究进展[J]. 中国实验方剂学杂志, 2018, 24(14): 214-221. DOI: 10.13422/j.cnki.syfjx.20181428. [6] ZHANG DQ, XU Y, YANG HL, et al. Effect of Xiayuxue Tang with different processing method on CCl4-induced hepatic fibrosis in rats[J]. Chin J Exp Med Formul, 2020, 26(5): 18-25. DOI: 10.13422/j.cnki.syfjx.20200540.张定棋, 徐莹, 杨海琳, 等. 不同制法下瘀血汤对CCl4诱导大鼠肝纤维化的影响[J]. 中国实验方剂学杂志, 2020, 26(5): 18-25. DOI: 10.13422/j.cnki.syfjx.20200540. [7] MA YF, YANG JE. Efficacy and mechanism of Rougan Huazhuo Bushen recipe in the treatment of liver fibrosis in elderly patients with chronic hepatitis B[J]. Chin J Gerontol, 2021, 41(18): 3959-3962. DOI: 10.3969/j.issn.1005-9202.2021.18.023.马云峰, 杨俊恩. 柔肝化浊补肾方治疗老年慢性乙型肝炎肝纤维化疗效及机制[J]. 中国老年学杂志, 2021, 41(18): 3959-3962. DOI: 10.3969/j.issn.1005-9202.2021.18.023. [8] WANG XB, LIU P, TANG ZP, et al. Acting mechanism of cordyceps mycelia extract for antagonizing hepatic sinusoidal capillarization in rats with dimethylnitrosamine induced liver cirrhosis[J]. Chin J Integr Trad West Med, 2009, 29(9): 810-815. DOI: 10.3321/j.issn:1003-5370.2009.09.011.王宪波, 刘平, 唐志鹏, 等. 虫草菌丝提取物抗二甲基亚硝胺诱致大鼠肝硬化肝窦毛细血管化作用机制的研究[J]. 中国中西医结合杂志, 2009, 29(9): 810-815. DOI: 10.3321/j.issn:1003-5370.2009.09.011. [9] TANG ZP, LIU P, WANG XB, et al. Effect of Cordyceps mycelia extract on hepatic sinusoidal endothelial cells permeability in dimethylnitrosamine-induced liver fibrosis in rats[J]. Chin J New Drugs Clin Remed, 2006, 25(9): 713-717. DOI: 10.3969/j.issn.1007-7669.2006.09.017.唐志鹏, 刘平, 王宪波, 等. 虫草菌丝提取物对DMN诱导肝纤维化大鼠肝窦内皮细胞通透性影响[J]. 中国新药与临床杂志, 2006, 25(9): 713-717. DOI: 10.3969/j.issn.1007-7669.2006.09.017. [10] SINGH A, KODURU B, CARLISLE C, et al. NADPH oxidase 4 modulates hepatic responses to lipopolysaccharide mediated by Toll-like receptor-4[J]. Sci Rep, 2017, 7(1): 14346. DOI: 10.1038/s41598-017-14574-8. [11] WEI M, LI B, WANG YF, et al. Gentian bitterside attenuates acute liver injury in mice by inhibiting TLR4/MyD88/NF-κB signaling pathway[J]. Chin J Exp Med Formul, 2021, 27(22): 76-83. DOI: 10.13422/j.cnki.syfjx.20212103.韦敏, 李波, 王跃峰, 等. 龙胆苦苷通过抑制TLR4/MyD88/NF-κB信号通路减轻小鼠急性肝损伤的作用[J]. 中国实验方剂学杂志, 2021, 27(22): 76-83. DOI: 10.13422/j.cnki.syfjx.20212103. [12] ARYAL B, ROTLLAN N, ARALDI E, et al. ANGPTL4 deficiency in haematopoietic cells promotes monocyte expansion and atherosclerosis progression[J]. Nat Commun, 2016, 7: 12313. DOI: 10.1038/ncomms12313. [13] ATTA HM. Reversibility and heritability of liver fibrosis: Implications for research and therapy[J]. World J Gastroenterol, 2015, 21(17): 5138-5148. DOI: 10.3748/wjg.v21.i17.5138. [14] GROOTAERT C, van de WIELE T, VERSTRAETE W, et al. Angiopoietin-like protein 4: health effects, modulating agents and structure-function relationships[J]. Expert Rev Proteomics, 2012, 9(2): 181-199. DOI: 10.1586/epr.12.12. [15] SIMILLION C, SEMMO N, IDLE JR, et al. Robust regression analysis of GCMS data reveals differential rewiring of metabolic networks in hepatitis B and C patients[J]. Metabolites, 2017, 7(4): 51. DOI: 10.3390/metabo7040051. [16] TERATANI T, TOMITA K, WADA A, et al. Angiopoietin-like protein 4 deficiency augments liver fibrosis in liver diseases such as nonalcoholic steatohepatitis in mice through enhanced free cholesterol accumulation in hepatic stellate cells[J]. Hepatol Res, 2021, 51(5): 580-592. DOI: 10.1111/hepr.13603. [17] PRYSTUPA A, KICIŃSKI P, LUCHOWSKA-KOCOT D, et al. Analysis of novel serum markers of fibrosis and angiogenesis in patients with alcoholic liver cirrhosis[J]. Ann Agric Environ Med, 2020, 27(4): 568-573. DOI: 10.26444/aaem/127621. [18] ZHANG X, YUAN S, ZHANG X, et al. ANGPTL4 regulates CD163 expression and Kupffer cell polarization induced cirrhosis via TLR4/NF-κB pathway[J]. Exp Cell Res, 2021, 405(2): 112706. DOI: 10.1016/j.yexcr.2021.112706. [19] PORCHERAY F, VIAUD S, RIMANIOL AC, et al. Macrophage activation switching: an asset for the resolution of inflammation[J]. Clin Exp Immunol, 2005, 142(3): 481-489. DOI: 10.1111/j.1365-2249.2005.02934.x. [20] KRENKEL O, TACKE F. Liver macrophages in tissue homeostasis and disease[J]. Nat Rev Immunol, 2017, 17(5): 306-321. DOI: 10.1038/nri.2017.11. [21] AKAHORI H, KARMALI V, POLAVARAPU R, et al. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury[J]. Nat Commun, 2015, 6: 7792. DOI: 10.1038/ncomms8792. [22] LI R, GUO Y, ZHANG Y, et al. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-κB and MAPK signaling pathways[J]. Int J Mol Sci, 2019, 20(5): 1103. DOI: 10.3390/ijms20051103. [23] YU Y, LI MP, XU B, et al. A study of regulatory effects of TLR4 and NF-κB on primary biliary cholangitis[J]. Eur Rev Med Pharmacol Sci, 2019, 23(9): 3951-3959. DOI: 10.26355/eurrev_201905_17824. [24] FIOROTTO R, SCIRPO R, TRAUNER M, et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-κB-mediated inflammatory response in mice[J]. Gastroenterology, 2011, 141(4): 1498-1508.e1-5. DOI: 10.1053/j.gastro.2011.06.052. [25] LIN L, LI R, CAI M, et al. Andrographolide ameliorates liver fibrosis in mice: Involvement of TLR4/NF-κB and TGF-β1/Smad2 signaling pathways[J]. Oxid Med Cell Longev, 2018, 2018: 7808656. DOI: 10.1155/2018/7808656. [26] YOUNIS NS, GHANIM A, ELMORSY MA, et al. Taurine ameliorates thioacetamide induced liver fibrosis in rats via modulation of toll like receptor 4/nuclear factor kappa B signaling pathway[J]. Sci Rep, 2021, 11(1): 12296. DOI: 10.1038/s41598-021-91666-6. -




 PDF下载 ( 6282 KB)
PDF下载 ( 6282 KB)


 下载:
下载: