2型糖尿病及空腹胰岛素水平对肝硬化腹水患者发生自发性细菌性腹膜炎的影响
DOI: 10.3969/j.issn.1001-5256.2022.07.017
Influence of type 2 diabetes mellitus and fasting insulin level on the risk of spontaneous peritonitis in patients with cirrhotic ascites
-
摘要:
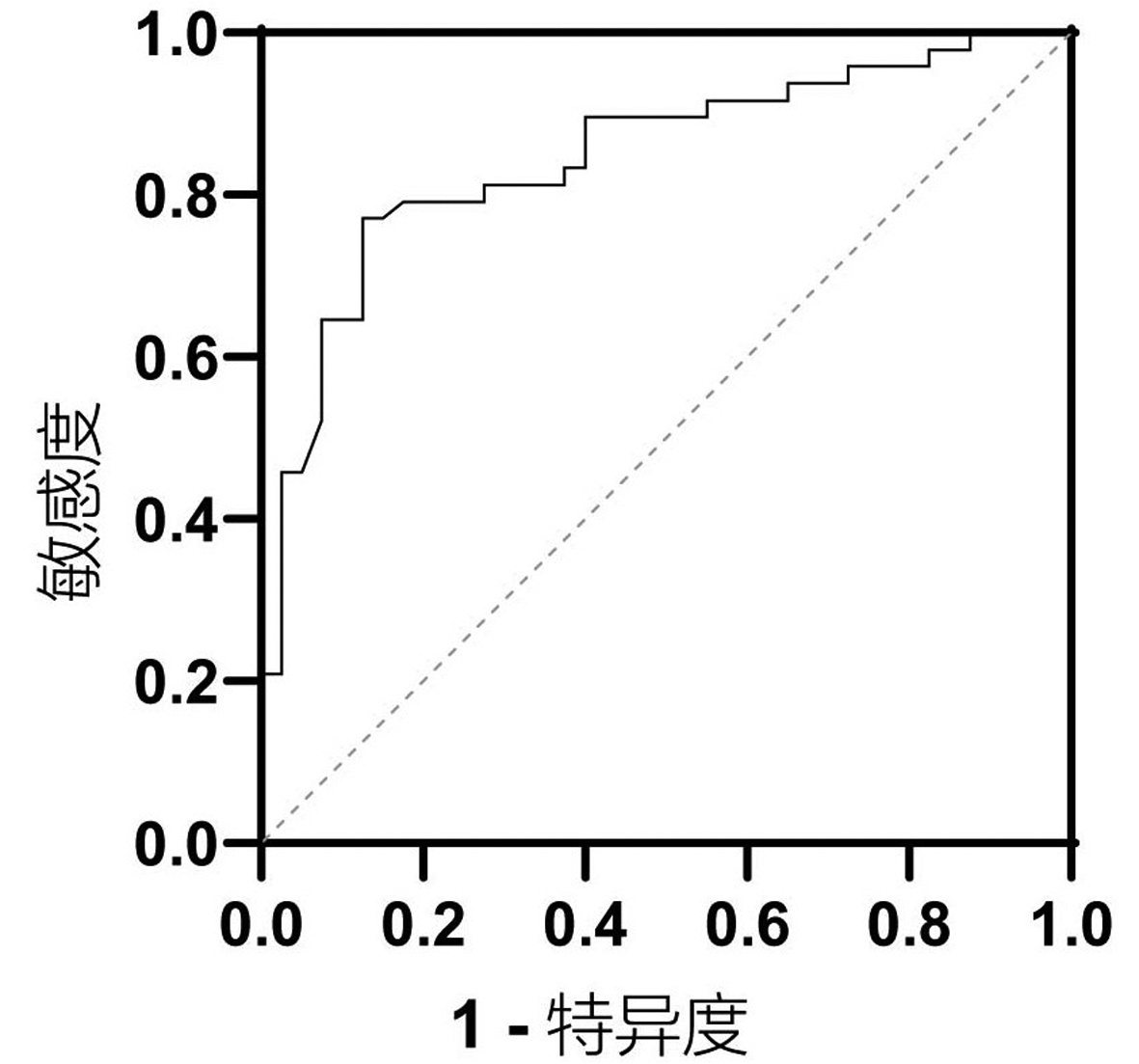
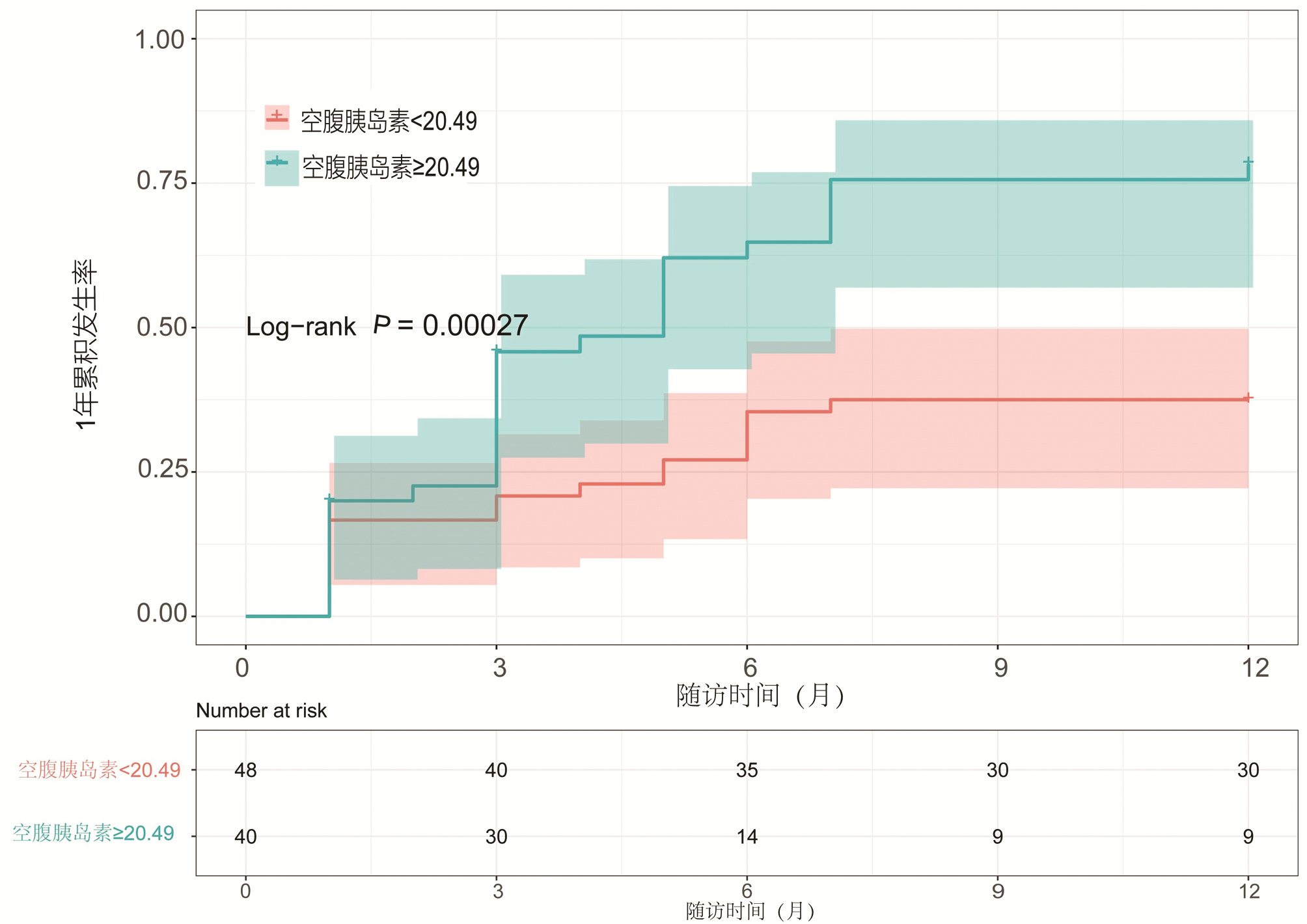
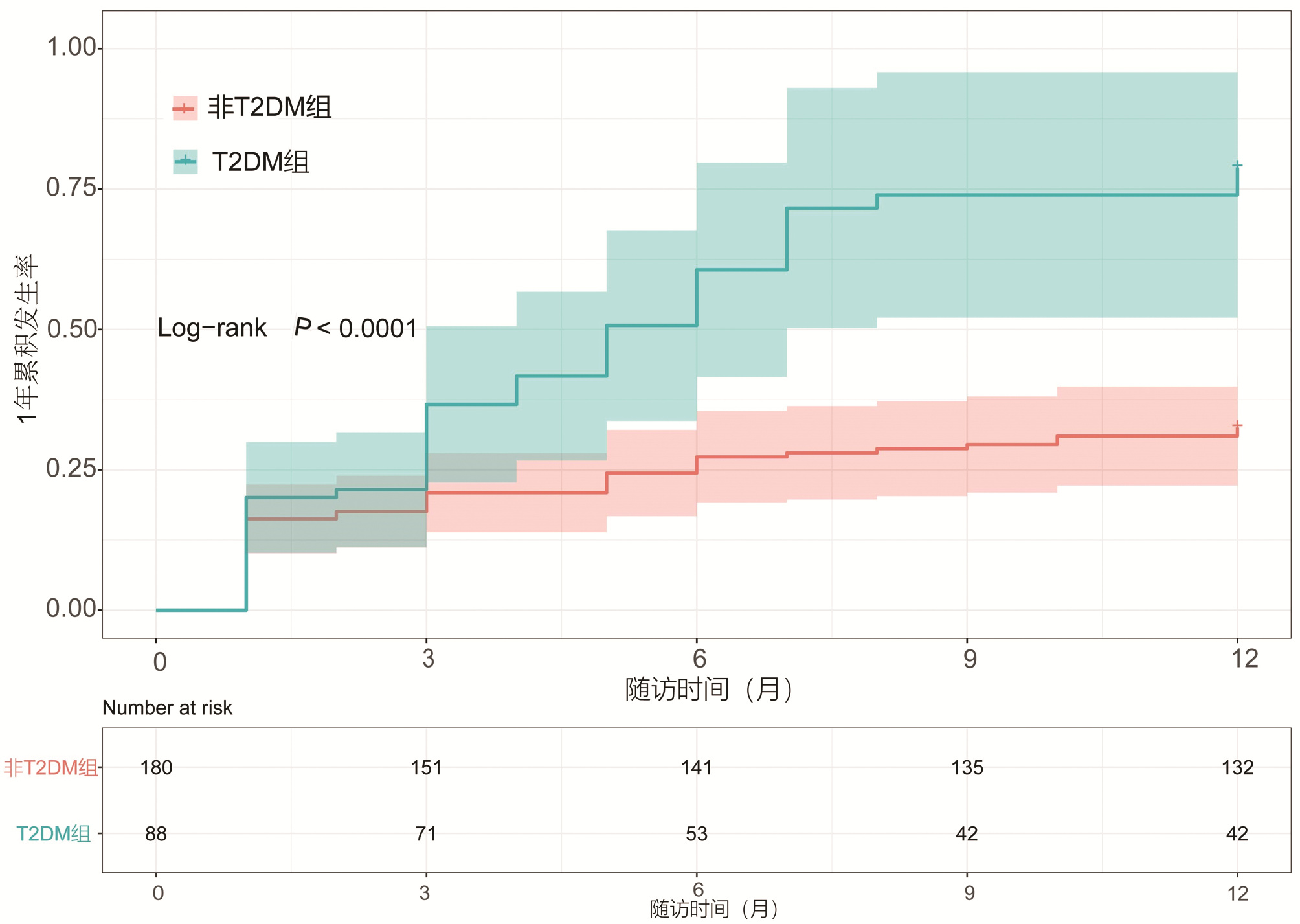
目的 本研究拟讨论失代偿期肝硬化腹水患者发生自发性细菌性腹膜炎(SBP)的预测因素及2型糖尿病(T2DM)与空腹胰岛素水平对患者发生SBP风险的影响。 方法 选取2013年1月—2018年10月于天津市第二人民医院临床诊断为肝硬化伴腹水患者,回顾性分析其基本临床资料,并随访1年,记录发生SBP时间或随访终止时间,数据获取方式为查询患者的住院病历。计量资料两组间比较采用t检验或Mann-Whitney U检验,计数资料两组间比较采用χ2检验或Wilcoxon秩和检验。Cox回归分析肝硬化伴腹水患者SBP发生的相关因素。用Kaplan-Meier分析方法计算生存曲线,log-rank检验分析差异性,采用ROC曲线计算空腹胰岛素的最佳临界值。 结果 共纳入肝硬化腹水患者268例,发生SBP者98例(36.6%),1年内发生SBP的独立预测因素为基线时合并T2DM(HR=2.848,95%CI:1.470~4.195), TBil水平(HR=1.004,95%CI:1.001~1.007)与中性粒细胞百分比(N%)(HR=1.032,95%CI:1.010~1.055)(P值均<0.05)。Kaplan-Meier生存曲线分析表明,T2DM患者1年内SBP累积发生率明显高于非T2DM患者(χ2=16.821, P<0.05)。合并T2DM者88例(32.8%),基线空腹胰岛素≥20.49 μU/mL (HR=2.757,95%CI:1.499~5.071)可显著增加发生SBP的风险(P<0.05)。Kaplan-Meier生存曲线分析表明,空腹胰岛素≥20.49 μU/mL组比<20.49 μU/mL组1年内发生SBP的风险显著增高(χ2=13.297, P<0.05)。 结论 当肝硬化腹水患者出现无法解释的TBil与N%升高时,或合并T2DM及空腹胰岛素≥20.49 μU/mL时,应警惕SBP的发生,必要时可行进一步的干预措施,从而延缓疾病进展,改善预后。 Abstract:Objective To investigate the predictive factors for spontaneous bacterial peritonitis (SBP) in patients with decompensated cirrhotic ascites and the influence of type 2 diabetes mellitus (T2DM) and fasting insulin level on the risk of SBP. Methods A retrospective analysis was performed for the clinical data of patients who were diagnosed with liver cirrhosis and ascites in Tianjin Second People's Hospital from January 2013 to October 2018, and the patients were followed up for 1 year to record the onset time of SBP and the ending time of follow-up. Related data were obtained by searching the patients' medical records. The t-test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test or the Wilcoxon test was used for comparison of categorical data between two groups; a Cox regression analysis was used to investigate the factors for SBP in patients with cirrhotic ascites. The Kaplan-Meier method was used to plot survival curves, the log-rank test was used for survival difference analysis, and the receiver operating characteristic (ROC) curve was used to calculate the optimal cut-off value of fasting insulin. Results A total of 268 patients with cirrhotic ascites were enrolled, among whom 98 (36.6%) developed SBP. T2DM at baseline (hazard ratio [HR]=2.848, 95% confidence interval [CI]: 1.470-4.195, P < 0.05), baseline total bilirubin (TBil) (HR=1.004, 95%CI: 1.001-1.007, P < 0.05), and baseline percentage of neutrophils (N%) (HR=1.032, 95%CI: 1.010-1.055, P < 0.05) were independent predictive factors for SBP within 1 year. The Kaplan-Meier survival curve analysis showed that the patients with T2DM had a significantly higher 1-year cumulative incidence rate of SBP than those without T2DM (χ2=16.821, P < 0.05). Of all 268 patients, 88 (32.8%) had T2DM, and baseline fasting insulin ≥20.49 μU/mL (HR=2.757, 95%CI: 1.499-5.071, P < 0.05) significantly increased the risk of SBP. The Kaplan-Meier survival curve analysis showed that the fasting insulin ≥20.49 μU/mL group had a significantly higher risk of SBP within 1 year than the < 20.49 μU/mL group (χ2=13.297, P < 0.05). Conclusion The onset of SBP should be considered when patients with cirrhotic ascites have unexplained increases in TBil and N% or have T2DM or fasting insulin ≥20.49 μU/mL, and intervention measures can be adopted when necessary to delay disease progression and improve prognosis. -
Key words:
- Liver Cirrhosis /
- Ascites /
- Spontaneous Bacterial Peritonitis /
- Diabetes Mellitus, Type 2 /
- Insulin
-
表 1 肝硬化腹水患者发生SBP预测因素单因素分析
Table 1. Univariate analysis of SBP in cirrhotic ascites patients
项目 SBP组(n=98) 非SBP组(n=170) 统计值 P值 男性[例(%)] 63(64.3) 118(69.4) χ2=0.745 0.388 年龄(岁) 56.3±11.1 54.1±10.9 t=1.613 0.108 病因[例(%)] χ2=8.481 0.270 乙型肝炎 48(49) 100(58.8) 丙型肝炎 10(10.1) 8(4.7) 酒精性 18(18.4) 31(18.2) 乙型肝炎+酒精性 7(7.1) 6(3.5) 丙型肝炎+酒精性 1(1.0) 1(0.6) 乙型肝炎+丙型肝炎 1(1.0) 4(2.4) 其他 7(7.1) 6(3.5) 隐源性 6(6.1) 14(8.2) 腹水程度分级[例(%)] Z=4.077 <0.001 1级 6(6.1) 32(18.8) 2级 56(57.1) 108(63.5) 3级 36(36.7) 30(17.6) 高血压[例(%)] 29(29.6) 45(26.5) χ2=0.303 0.582 T2DM[例(%)] 48(49.0) 40(23.5) χ2=18.257 <0.001 并发症[例(%)] 上消化道出血 19(19.4) 11(6.5) χ2=10.434 0.001 肝性脑病 12(12.2) 12(7.1) χ2=2.051 0.152 肝衰竭 16(16.3) 11(6.5) χ2=6.665 0.010 Child-Pugh分级[例(%)] Z=3.969 <0.001 A级 3(3.1) 16(9.4) B级 30(30.6) 83(48.8) C级 65(66.3) 71(41.8) 转归[例(%)] 好转 42(42.9) 104(61.2) χ2=8.412 0.004 上消化道出血 14(14.3) 32(18.8) χ2=0.900 0.343 肝性脑病 17(17.3) 22(12.9) χ2=0.970 0.325 肝衰竭 24(24.5) 16(9.4) χ2=11.130 0.001 肝癌 11(11.2) 13(7.6) χ2=0.976 0.323 死亡 6(6.1) 10(5.9) χ2=0.006 0.936 空腹血糖(mmol/L) 5.7(4.8~7.6) 5.6(5.0~6.5) Z=1.051 0.293 ALT(U/L) 26(15~62) 28(17~66) Z=1.442 0.149 AST(U/L) 44(25~105) 49(32~119) Z=1.015 0.310 ALP(μ/L) 97(70~144) 100(69~161) Z=0.058 0.954 GGT(U/L) 54(25~119) 59(32~148) Z=1.072 0.284 TBil(μmol/L) 59(22~125) 36(19~68) Z=2.391 0.017 Alb(g/L) 28.9(24.9~33.1) 29.1(26.2~34.6) Z=1.659 0.097 ChE(U/L) 2430(1830~3117) 2643(2075~3598) Z=3.042 0.002 BUN(mmol/ L) 6.0(4.5~8.1) 5.7(4.3~7.3) Z=2.667 0.008 SCr(μmol/ L) 72(57~88) 69(57~85) Z=1.970 0.049 Na+(mmol/ L) 136(133~139) 138(136~141) Z=4.348 <0.001 TC(mmol/ L) 3.0(2.1~3.8) 3.2(2.6~3.8) Z=2.464 0.014 TG(mmol/ L) 0.9(0.7~1.5) 0.9(0.6~1.2) Z=1.675 0.094 WBC(×109/ L) 5.7(4.0~7.6) 3.9(2.7~5.5) Z=4.817 <0.001 PLT(×109/ L) 85(49~142) 75(56~112) Z=0.735 0.462 N% 70.4(62.8~81.0) 60.6(54.0~69.0) Z=6.262 <0.001 INR 1.5(1.3~1.9) 1.4(1.2~1.6) Z=2.959 0.003 PT(s) 17.8(15.7~20.9) 16.9(15.2~19.1) Z=2.452 0.014 PTA(%) 50.5(41.0~66.0) 61.0(49.6~73.8) Z=3.092 0.002 PCT(ng/mL) 0.4(0.2~0.8) 0.3(0.1~0.4) Z=3.241 0.001 CRP(mg/L) 15.8(8.5~34.6) 15.1(6.0~17.9) Z=2.895 0.004 AFP(ng/mL) 5.0(2.4~14.5) 5.1(2.7~18.2) Z=1.468 0.142 腹水蛋白(g/L) 10.9(7.1~14.5) 12.1(9.0~15.9) Z=1.294 0.196 腹水WBC(×106/L) 190(117~270) 158(117~260) Z=1.005 0.315 PMN(×106/L) 25(9~59) 16(8~25) Z=2.971 0.003 表 2 肝硬化腹水患者发生SBP的多因素Cox回归分析
Table 2. Multivariate Cox regression analysis of SBP in cirrhotic ascites patients
变量 HR 95%CI P值 糖尿病 2.484 1.470~4.195 0.001 TBil 1.004 1.001~1.007 0.020 N% 1.032 1.010~1.055 0.005 表 3 T2DM组与无T2DM组基线资料的比较
Table 3. Comparison of baseline date between T2DM group and non-T2DM group
项目 无T2DM组(n=180) T2DM组(n=88) 统计值 P值 男性[例(%)] 127(70.6) 54(61.4) χ2=2.278 0.131 年龄(岁) 53.5±11.1 57.7±10.5 t=2.963 0.003 高血压[例(%)] 40(22.2) 34(38.6) χ2=7.967 0.005 SBP [例(%)] 50(27.8) 48(54.5) χ2=18.257 <0.001 冠心病[例(%)] 14(7.8) 16(18.2) χ2=6.436 0.011 空腹血糖(mmol/L) 5.2(4.8~6.1) 6.9(5.7~9.3) Z=7.178 <0.001 ALT(U/L) 30.5(18.0~74.8) 21.0(13.0~41.5) Z=2.332 0.020 AST(U/L) 61.5(32.0~127.3) 38.0(22.0~80.5) Z=2.666 0.008 TBil(μmol/L) 47.1(22.8~93.7) 31.7(17.5~73.4) Z=2.114 0.035 TG(mmol/L) 0.8(0.6~1.2) 1.0(0.7~1.3) Z=2.052 0.040 N% 64.5(55.4~71.2) 69.5(60.6~76.4) Z=2.308 0.021 INR 1.5(1.3~1.8) 1.3(1.1~1.6) Z=3.247 0.001 PT(s) 17.9(15.9~20.9) 16.3(14.4~18.2) Z=3.769 <0.001 PTA(%) 54(43~67) 64(51~81) Z=3.092 0.002 AFP(ng/mL) 6.5(2.8~23.3) 4.1(2.4~7.7) Z=2.446 0.014 腹水WBC(×106/L) 148.5(113.5~223.5) 259.0(133.5~390.5) Z=3.485 <0.001 PMN(×106/L) 16.5(8.0~28.0) 25.0(8.5~61.5) Z=2.401 0.016 表 4 空腹胰岛素≥20.49 μU/mL组与空腹胰岛素<20.49 μU/mL组基线资料的比较
Table 4. Comparison of baseline date between fasting insulin ≥20.49 μU/mL and fasting insulin < 20.49 μU/mL group
项目 空腹胰岛素<20.49 μU/mL组(n=48) 空腹胰岛素≥20.49 μU/mL组(n=40) 统计值 P值 腹水程度分级[例(%)] Z=2.531 0.011 1级 7(14.6) 5(12.5) 2级 35(72.9) 18(45.0) 3级 6(12.5) 17(42.5) 高血压[例(%)] 14(29.2) 20(50.0) χ2=3.994 0.046 SBP[例(%)] 12(25) 36(90) χ2=37.180 <0.001 ChE(U/L) 3492.9±1527.9 2729.1±919.9 t=2.890 0.005 WBC(×109/ L) 4.6(3.4~5.3) 5.7(3.6~7.1) Z=3.243 0.001 N% 63.8(55.5~74.9) 71.1(63.3~79.5) Z=2.372 0.018 Na+(mmol/L) 137.7(132.9~141.1) 137.3(134.9~139.8) Z=2.636 0.008 表 5 T2DM患者发生SBP的多因素Cox回归分析
Table 5. Multivariate Cox regression analysis of SBP in T2DM patients
变量 HR 95%CI P值 PMN 1.001 0.997~1.009 0.117 空腹血糖 0.968 0.902~1.038 0.362 空腹胰岛素≥20.49 μU/mL 2.757 1.499~5.071 0.001 -
[1] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of ascites and complications in cirrhosis[J]. J Clin Hepatol, 2017, 33(10): 1847-1863. DOI: 10.3969/j.issn.1001-5256.2017.10.003.中华医学会肝病学分会. 肝硬化腹水及相关并发症的诊疗指南[J]. 临床肝胆病杂志, 2017, 33(10): 1847-1863. DOI: 10.3969/j.issn.1001-5256.2017.10.003. [2] PIANO S, TONON M, ANGELI P. Changes in the epidemiology and management of bacterial infections in cirrhosis[J]. Clin Mol Hepatol, 2021, 27(3): 437-445. DOI: 10.3350/cmh.2020.0329. [3] PIANO S, ANGELI P. Bacterial infections in cirrhosis as a cause or consequence of decompensation?[J]. Clin Liver Dis, 2021, 25(2): 357-372. DOI: 10.1016/j.cld.2021.01.006. [4] SCHULTALBERS M, TERGAST TL, SIMON N, et al. Frequency, characteristics and impact of multiple consecutive nosocomial infections in patients with decompensated liver cirrhosis and ascites[J]. United European Gastroenterol J, 2020, 8(5): 567-576. DOI: 10.1177/2050640620913732. [5] CRISMALE JF, FRIEDMAN SL. Acute liver injury and decompensated cirrhosis[J]. Med Clin North Am, 2020, 104(4): 647-662. DOI: 10.1016/j.mcna.2020.02.010. [6] FERNÁNDEZ J, PRADO V, TREBICKA J, et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe[J]. J Hepatol, 2019, 70(3): 398-411. DOI: 10.1016/j.jhep.2018.10.027. [7] ELKRIEF L, RAUTOU PE, SARIN S, et al. Diabetes mellitus in patients with cirrhosis: clinical implications and management[J]. Liver Int, 2016, 36(7): 936-948. DOI: 10.1111/liv.13115. [8] TANASE DM, GOSAV EM, COSTEA CF, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD)[J]. J Diabetes Res, 2020, 2020: 3920196. DOI: 10.1155/2020/3920196. [9] ZUWALA-JAGIELLO J, PAZGAN-SIMON M, SIMON K, et al. Serum endocan level in diabetes mellitus of patients with cirrhosis and risk of subsequent development of spontaneous bacterial peritonitis[J]. J Physiol Pharmacol, 2019, 70(3): 399-405. DOI: 10.26402/jpp.2019.3.06. [10] COMAN LI, COMAN OA, BǍDǍRǍU IA, et al. Association between liver cirrhosis and diabetes mellitus: A review on hepatic outcomes[J]. J Clin Med, 2021, 10(2): 262. DOI: 10.3390/jcm10020262. [11] LABENZ C, NAGEL M, KREMER WM, et al. Association between diabetes mellitus and hepatic encephalopathy in patients with cirrhosis[J]. Aliment Pharmacol Ther, 2020, 52(3): 527-536. DOI: 10.1111/apt.15915. [12] TAN ZJ, ZHAO J, LIU WX, et al. Analysis of pathogenic bacteris and factors affecting spontaneous bacterial peritonitis in patients with cirrhosis ascites[J]. Chin J Nosocomiol, 2019, 29(19): 2953-2956. DOI: 10.11816/cn.ci.2019-183023.谭志洁, 赵静, 刘文兴, 等. 肝硬化腹水患者并发自发性细菌性腹膜炎病原菌与影响因素分析[J]. 中华医院感染学杂志, 2019, 29(19): 2953-2956. DOI: 10.11816/cn.ci.2019-183023. [13] LI YT, HUANG JR, PENG ML. Current status and prospects of spontaneous peritonitis in patients with cirrhosis[J]. Biomed Res Int, 2020, 2020: 3743962. DOI: 10.1155/2020/3743962. [14] LEE WG, WELLS CI, MCCALL JL, et al. Prevalence of diabetes in liver cirrhosis: A systematic review and meta-analysis[J]. Diabetes Metab Res Rev, 2019, 35(6): e3157. DOI: 10.1002/dmrr.3157. [15] TERGAST TL, LASER H, GERBEL S, et al. Association between type 2 diabetes mellitus, HbA1c and the risk for spontaneous bacterial peritonitis in patients with decompensated liver cirrhosis and ascites[J]. Clin Transl Gastroenterol, 2018, 9(9): 189. DOI: 10.1038/s41424-018-0053-0. [16] ABU-FREHA N, MICHAEL T, POUPKO L, et al. Spontaneous bacterial peritonitis among cirrhotic patients: Prevalence, clinical characteristics, and outcomes[J]. J Clin Med, 2021, 11(1): 227. DOI: 10.3390/jcm11010227. [17] JIANG P, DOU RC, CUI ZJ, et al. Value of albumin-bilirubin score combined with neutrophil count and procalcitonin in ascites in predicting spontaneous bacterial peritonitis in patients with cirrhotic ascites[J]. J Clin Hepatol, 2021, 37(9): 2097-2101. DOI: 10.3969/j.issn.1001-5256.2021.09.019.江萍, 豆仁成, 崔子瑾, 等. 白蛋白-胆红素评分联合腹水中性粒细胞计数及降钙素原对肝硬化腹水患者发生自发性细菌性腹膜炎的预测价值[J]. 临床肝胆病杂志, 2021, 37(9): 2097-2101. DOI: 10.3969/j.issn.1001-5256.2021.09.019. [18] LIU H, ZHU P, NIE C, et al. The value of ascitic neutrophil gelatinase-associated lipocalin in decompensated liver cirrhosis with spontaneous bacterial peritonitis[J]. J Clin Lab Anal, 2020, 34(6): e23247. DOI: 10.1002/jcla.23247. [19] ROSENBLATT R, ATTEBERRY P, TAFESH Z, et al. Uncontrolled diabetes mellitus increases risk of infection in patients with advanced cirrhosis[J]. Dig Liver Dis, 2021, 53(4): 445-451. DOI: 10.1016/j.dld.2020.10.022. [20] TIAN CY, HU H, ZHANG GY, et al. Influence of glucose metabolism disorder on complications associated with liver cirrhosis[J]. J Clin Hepatol, 2021, 37(5): 1197-1200. DOI: 10.3969/j.issn.1001-5256.2021.05.046.田彩云, 胡晗, 张国远, 等. 糖代谢紊乱对肝硬化相关并发症的影响[J]. 临床肝胆病杂志, 2021, 37(5): 1197-1200. DOI: 10.3969/j.issn.1001-5256.2021.05.046. -



 PDF下载 ( 2419 KB)
PDF下载 ( 2419 KB)

 下载:
下载: