超声剪切波频散成像对代偿期肝硬化高风险食管胃静脉曲张的诊断价值
DOI: 10.3969/j.issn.1001-5256.2022.07.018
Value of ultrasonic shear-wave dispersion imaging in diagnosis of high-risk esophageal and gastric varices in compensated cirrhosis
-
摘要:
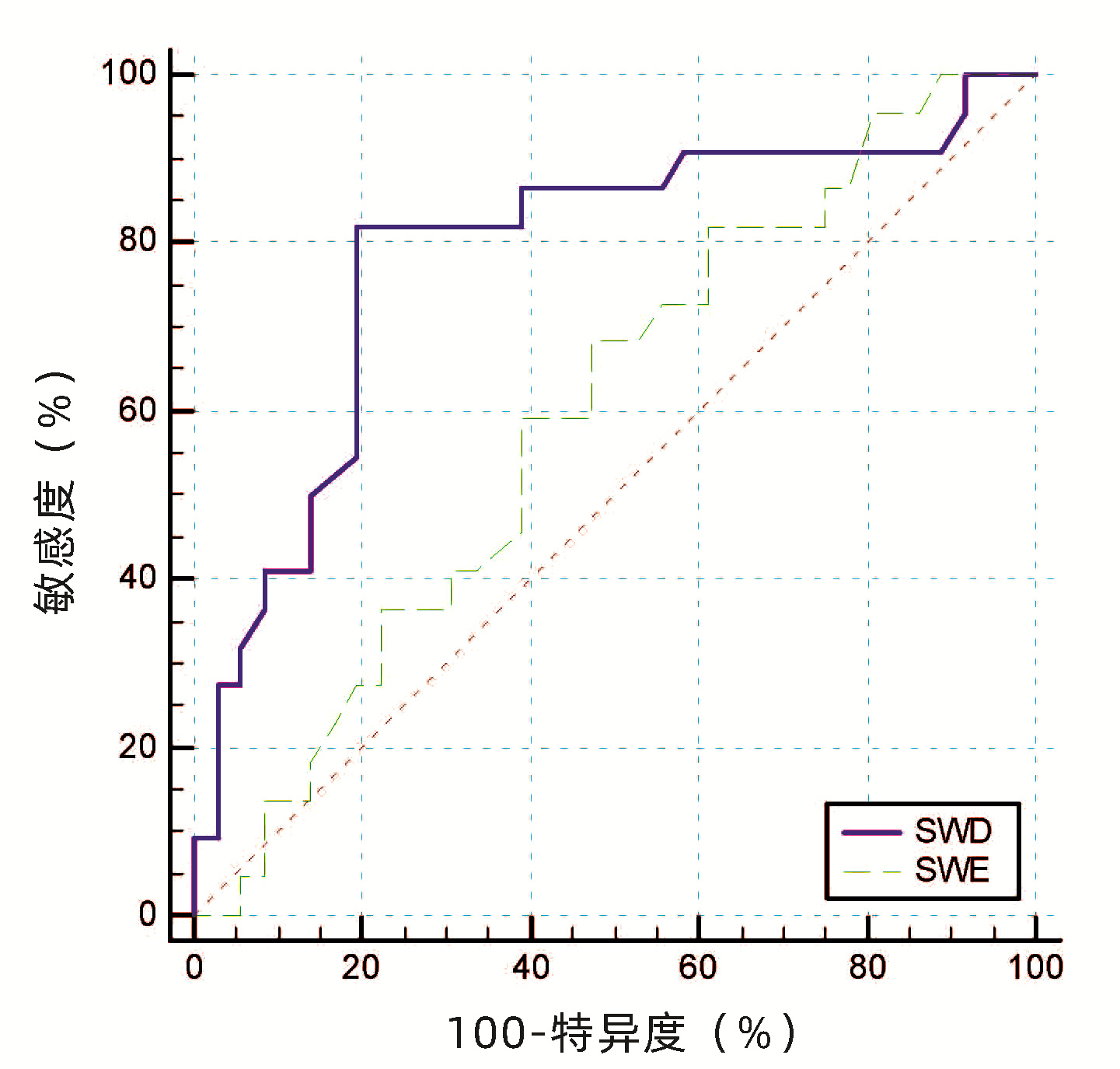
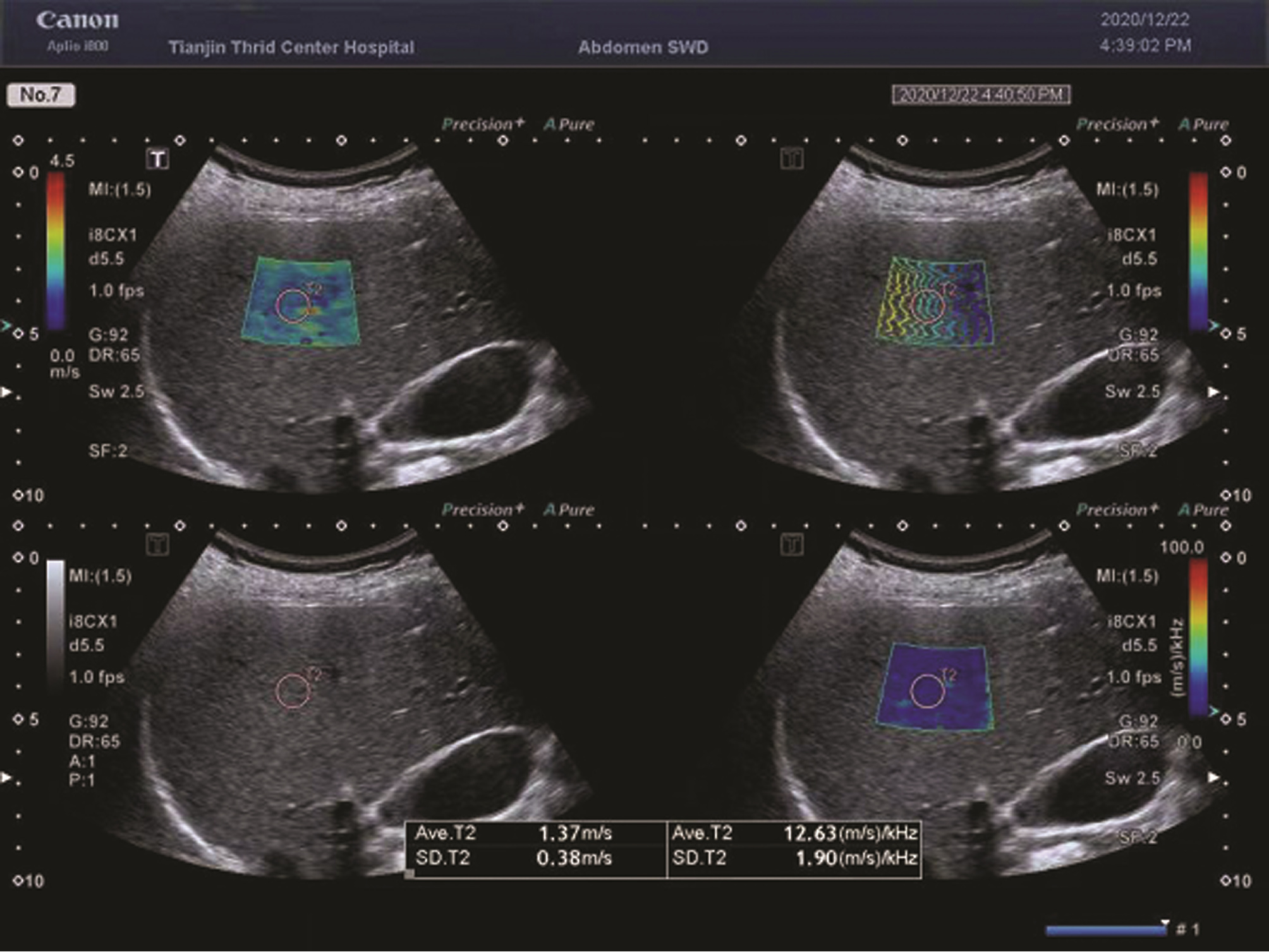
目的 探讨佳能二维超声剪切波弹性成像(SWE)及频散成像(SWD)在诊断代偿期肝硬化高风险食管胃静脉曲张中的临床价值。 方法 选取2020年2月—2021年2月就诊于天津市第三中心医院接受电子胃镜检查的各种病因代偿期肝硬化患者58例,采用佳能Aplio i800彩色超声仪同期检查肝脏SWE及SWD。以胃镜结果将患者分为高风险食管胃静脉曲张(HREGV)组(n=22)及非高风险食管胃静脉曲张(NHREGV)组(n=36)。正态分布的计量资料两组间比较采用独立样本t检验,非正态分布的计量资料两组间比较采用Mann-Whitney U非参数检验。计数资料两组间比较采用χ2检验。采用多因素logistic回归分析筛选预测肝硬化高风险食管胃静脉曲张的无创指标。采用受试者工作特征曲线分析肝脏SWD及SWE对高风险食管静脉曲张的临床诊断价值。 结果 HREGV组与NHREGV组相比,SWD(t=-3.84,P<0.001)、病因(χ2=9.67,P=0.022)、TBil(Z=-2.00,P=0.045)脾脏直径(t=-2.44,P=0.018)、门静脉直径(Z=-1.96, P=0.005)差异均有统计学意义。代偿期肝硬化患者肝脏SWD平均(15.17±2.45) m·s-1·kHz-1,其中HREGV组肝脏SWD为(16.59±2.66)m·s-1·kHz-1,显著高于NHREGV组的(14.31±1.86) m·s-1·kHz-1(t=-3.84,P<0.001);HREGV组与NHREGV组的SWE差异无统计学意义(Z=-1.21, P=0.223)。SWD为代偿期肝硬化患者发生HREGV的独立危险因素(OR=1.67,95%CI:1.17~2.39,P=0.005)。SWD诊断HREGV的曲线下面积(AUC)为0.786,最佳临界值为15.35 m·s-1·kHz-1,特异度80.56%,敏感度81.82%;SWE诊断HREGV的AUC为0.596,特异度52.78%,敏感度68.18%,最佳临界值为9.25 kPa。 结论 佳能Aplio i800彩色超声测定的SWD有望作为一种新的无创检测方法,在一定程度上预测代偿期肝硬化患者HREGV的存在,SWE则诊断价值有限。 Abstract:Objective To investigate the clinical value of Canon two-dimensional ultrasound shear wave elastography (SWE) and shear wave dispersion (SWD) in the diagnosis of high-risk esophageal and gastric varices in compensated cirrhosis. Methods A total of 58 patients with compensated cirrhosis of various etiologies who received electronic gastroscopy in Tianjin Third Central Hospital from February 2020 to February 2021, and Canon Aplio i800 color ultrasound instrument was used to perform SWE and SWD of the liver. According to the results of gastroscopy, the patients were divided into high-risk esophageal and gastric varices group (HREGV group) with 22 patients and non-high-risk esophageal and gastric varices group (NHREGV group) with 36 patients. The independent samples t-test was used for comparison of normally distributed continuous data between two groups, and the non-parametric Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. A multivariate logistic regression analysis was used to analyze and screen out the noninvasive indicators for predicting high-risk esophageal and gastric varices, and the receiver operating characteristic (ROC) curve was used to evaluate the clinical value of liver SWD and SWE in the diagnosis of high-risk esophageal and gastric varices. Results There were significant differences between the HREGV group and the NHREGV group in SWD (t=-3.84, P < 0.001), etiology (χ2=9.67, P=0.022), total bilirubin (Z=-2.00, P=0.045), spleen diameter (t=-2.44, P=0.018), and portal vein diameter (Z=-1.96, P=0.005). The patients with compensated cirrhosis had a mean liver SWD of 15.17±2.45 m·s-1·kHz-1, and the HREGV group had a significantly higher liver SWD than the NHREGV group (16.59±2.66 m·s-1·kHz-1 vs 14.31±1.86 m·s-1·kHz-1, t=-3.84, P < 0.001), while there was no significant difference in SWE between the two groups (Z=-1.21, P=0.223). SWD was an independent risk factor for high-risk esophageal and gastric varices in patients with compensated liver cirrhosis (odds ratio=1.67, 95% confidence interval: 1.17-2.39, P=0.005). In the diagnosis of high-risk esophageal and gastric varices, SWD had an area under the ROC curve (AUC) of 0.786, with a specificity of 80.56% and a sensitivity of 81.82% at the optimal cut-off value of 15.35 m·s-1·kHz-1; SWE had an AUC of 0.596, with a specificity of 52.78% and a sensitivity of 68.18% at the optimal cut-off value of 9.25 kPa. Conclusion Liver SWD measured by Canon Aplio i800 color ultrasound is excepted to become a new noninvasive method to predict the presence of high-risk esophageal and gastric varices in patients with compensated cirrhosis, while SWE has a limited diagnostic value. -
表 1 NHREGV组与HREGV组一般资料比较
Table 1. General information of patients in NHREGV group and HREGV group
指标 总人群(n=58) NHREGV组(n=36) HREGV组(n=22) 统计值 P值 年龄(岁) 53.48±10.91 54.67±10.15 51.55±12.05 t=1.06 0.295 性别[例(%)] χ2=0.91 0.340 男 35(60.34) 20(55.56) 15(68.18) 女 23(39.66) 16(44.44) 7(31.82) 病因[例(%)] χ2=9.67 0.022 乙型肝炎 39(67.24) 29(80.56) 10(45.45) 丙型肝炎 7(12.07) 4(11.11) 3(13.64) ALD 5(8.62) 1(2.78) 4(18.18) 其他 7(12.07) 2(5.56) 5(22.73) CTP分级[例(%)] χ2=1.11 0.292 A 55(94.83) 35(97.22) 20(90.91) B 3(5.17) 1(2.78) 2(9.09) ALT(U/L) 28.00(11.00~101.70) 30.00(11.00~89.00) 24.00(15.00~101.70) Z=-0.34 0.728 AST(U/L) 24.0(14.0~184.2) 26.0(14.0~15.0) 29.0(15.0~184.2) Z=-1.33 0.184 TBil(μmol/L) 18.95(7.60~80.80) 16.10(7.60~80.80) 19.80(12.00~66.60) Z=-2.00 0.045 SWD(m·s-1·kHz-1) 15.17±2.45 14.31±1.86 16.59±2.66 t=-3.84 <0.001 SWE(kPa) 9.45(4.70~34.20) 9.20(4.70~34.20) 10.45(5.80~19.00) Z=-1.21 0.223 脾脏直径(mm) 122.62±23.53 116.82±20.68 131.85±25.27 t =-2.44 0.018 门静脉直径(mm) 12.4(8.4~23.8) 12.0(8.4~16.0) 13.0(10.0~23.8) Z=-1.96 0.005 注:ALD,酒精性肝病。 表 2 代偿期肝硬化患者HREGV无创指标的logistic分析
Table 2. Logistic analysis of HREGV non-invasive indicators in patients with compensated cirrhosis
指标 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 年龄(岁) 0.97(0.927~1.023) 0.292 性别 男 1.00 女 0.58(0.19~1.77) 0.342 病因 乙型肝炎 1.00 丙型肝炎 2.18(0.41~11.45) 0.359 0.34(0.03~3.55) 0.366 ALD 11.60(1.16~116.42) 0.037 5.65(0.41~77.50) 0.200 其他 7.25(1.21~43.44) 0.030 4.11(0.39~42.88) 0.240 CTP分级 A 1.00 B 3.50(0.30~41.07) 0.319 ALT(U/L) 1.01(0.98~1.03) 0.701 AST(U/L) 1.01(0.99~1.03) 0.229 TBil(μmol/L) 1.03(0.99~1.08) 0.174 SWE(kPa) 1.02(0.91~1.13) 0.789 SWD(m·s-1·kHz-1) 1.64(1.19~2.26) 0.003 1.67(1.17~2.39) 0.005 脾脏直径(mm) 1.03(1.00~1.06) 0.025 1.03(0.99~1.07) 0.101 门静脉直径(mm) 1.34(0.99~1.80) 0.057 1.18(0.82~1.71) 0.376 表 3 肝脏SWD/SWE诊断代偿期肝硬化患者HREGV的ROC分析
Table 3. ROC analysis of liver SWD/SWE diagnoses HREGV in patients with compensated cirrhosis
指标 AUC 95%CI 阈值 特异度(%) 敏感度(%) 阳性预测值(%) 阴性预测值(%) SWE(kPa) 0.596 0.447~0.745 9.25 52.78 68.18 59.10 62.41 SWD(m·s-1·kHz-1) 0.786 0.655~0.917 15.35 80.56 81.82 80.83 81.58 -
[1] de FRANCHIS R, Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension[J]. J Hepatol, 2015, 63(3): 743-752. DOI: 10.1016/j.jhep.2015.05.022. [2] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Gastroenterology, Chinese Medical Association, Chinese Society of Endoscopy, Chinese Medical Association. Guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding in cirrhotic portal hypertension[J]. J Clin Hepatol, 2016, 32(2): 203-219. DOI: 10.3969/j.issn.1001-5256.2016.02.002.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会内镜学分会. 肝硬化门静脉高压食管胃静脉曲张出血的防治指南[J]. 临床肝胆病杂志, 2016, 32(2): 203-219. DOI: 10.3969/j.issn.1001-5256.2016.02.002. [3] GARCIA-TSAO G, ABRALDES JG, BERZIGOTTI A, et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases[J]. Hepatology, 2017, 65(1): 310-335. DOI: 10.1002/hep.28906. [4] SHIHA G, IBRAHIM A, HELMY A, et al. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: A 2016 update[J]. Hepatol Int, 2017, 11(1): 1-30. DOI: 10.1007/s12072-016-9760-3. [5] MANATSATHIT W, SAMANT H, KAPUR S, et al. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis[J]. J Gastroenterol Hepatol, 2018, 33(10): 1696-1706. DOI: 10.1111/jgh.14271. [6] SUGIMOTO K, MORIYASU F, OSHIRO H, et al. Viscoelasticity measurement in rat livers using shear-wave US elastography[J]. Ultrasound Med Biol, 2018, 44(9): 2018-2024. DOI: 10.1016/j.ultrasmedbio.2018.05.008. [7] LEE DH, CHO EJ, BAE JS, et al. Accuracy of two-dimensional shear wave elastography and attenuation imaging for evaluation of patients with nonalcoholic steatohepatitis[J]. Clin Gastroenterol Hepatol, 2021, 19(4): 797-805. e7. DOI: 10.1016/j.cgh.2020.05.034. [8] TANG Y, KONG W, ZHAO J, et al. Can viscoelasticity measurements obtained through shear-wave US elastography be used to monitor hepatic ischemia-reperfusion injury and treatment response? An animal study[J]. Ultrasound Med Biol, 2020, 46(9): 2464-2471. DOI: 10.1016/j.ultrasmedbio.2020.04.021. [9] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [10] JIANG SL, XIONG JB, LOU X, et al. Influence of endoscopic dense ligation combined with sclerosing agent on hepatic synthesis function in treatment of severe esophageal and gastric varices in liver cirrhosis[J]. Clin J Med Offic, 2020, 48(9): 1097-1098. DOI: 10.16680/j.1671-3826.2020.09.38.蒋树林, 熊加彬, 娄晓, 等. 内镜下密集套扎结合硬化剂治疗肝硬化食管胃重度静脉曲张对患者肝合成功能影响[J]. 临床军医杂志, 2020, 48(9): 1097-1098. DOI: 10.16680/j.1671-3826.2020.09.38. [11] SHI XJ, SHEN JH, NIU HM, et al. Clinical effect and prognostic value of endoscopic ultrasound-guided treatment of esophageal varices in liver cirrhosis[J]. Chin J Gerontol, 2021, 41(8): 1634-1638. DOI: 10.3969/j.issn.1005-9202.2021.08.021.石小静, 申军华, 牛红梅, 等. 超声内镜指导下对肝硬化并发食管静脉曲张内镜下治疗的临床疗效及预后价值[J]. 中国老年学杂志, 2021, 41(8): 1634-1638. DOI: 10.3969/j.issn.1005-9202.2021.08.021. [12] QI X, BERZIGOTTI A, CARDENAS A, et al. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension[J]. Lancet Gastroenterol Hepatol, 2018, 3(10): 708-719. DOI: 10.1016/S2468-1253(18)30232-2. [13] WANG L, FENG Y, MA X, et al. Diagnostic efficacy of noninvasive liver fibrosis indexes in predicting portal hypertension in patients with cirrhosis[J]. PLoS One, 2017, 12(8): e0182969. DOI: 10.1371/journal.pone.0182969. [14] DENG H, QI X, ZHANG T, et al. Supersonic shear imaging for the diagnosis of liver fibrosis and portal hypertension in liver diseases: A meta-analysis[J]. Expert Rev Gastroenterol Hepatol, 2018, 12(1): 91-98. DOI: 10.1080/17474124.2018.1412257. [15] LI Q, WANG R, GUO X, et al. Contrast-enhanced CT may be a diagnostic alternative for gastroesophageal varices in cirrhosis with and without previous endoscopic variceal therapy[J]. Gastroenterol Res Pract, 2019, 2019: 6704673. DOI: 10.1155/2019/6704673. [16] QI X, AN W, LIU F, et al. Virtual hepatic venous pressure gradient with CT angiography (CHESS 1601): A prospective multicenter study for the noninvasive diagnosis of portal hypertension[J]. Radiology, 2019, 290(2): 370-377. DOI: 10.1148/radiol.2018180425. [17] ABRALDES JG, BUREAU C, STEFANESCU H, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The "Anticipate" study[J]. Hepatology, 2016, 64(6): 2173-2184. DOI: 10.1002/hep.28824. [18] Chinese Foundation for Hepatitis Prevention and Control, Chinese Society of Infectious Disease, Chinese Society of Hepatology and Chinese Medical Association Liver Disease Committee of Chinese Research Hospital Association. Consensus on clinical application of transient alastography detecting liver fibrosis: A 2018 update[J]. Chin J Hepatol, 2019, 27(3): 182-191. DOI: 10.3760/cma.j.issn.1007-3418.2019.03.004.中国肝炎防治基金会, 中华医学会感染病学分会, 中华医学会肝病学分会和中国研究性医院学会肝病专业委员会. 瞬时弹性成像技术诊断肝纤维化专家共识(2018年更新版)[J]. 中华肝脏病杂志, 2019, 27(3): 182-191. DOI: 10.3760/cma.j.issn.1007-3418.2019.03.004 [19] XIE LT, YAN CH, ZHAO QY, et al. Quantitative and noninvasive assessment of chronic liver diseases using two-dimensional shear wave elastography[J]. World J Gastroenterol, 2018, 24(9): 957-970. DOI: 10.3748/wjg.v24.i9.957.l. [20] Panel of Elastography Assessment of Liver Fibrosis, Study Group of Interventional Ultrasound, Society of Ultrasound in Medicine of Chinese Medical Association. Guidelines for clinical application of two-dimensional shear wave elastography in assessment of liver fibrosis in chronic hepatitis B[J]. J Clin Hepatol, 2018, 34(2): 255-261. DOI: 10.3969/j.issn.1001-5256.2018.02.008.中华医学会超声医学分会介入超声学组弹性成像评估肝纤维化专家组. 二维剪切波弹性成像评估慢性乙型肝炎肝纤维化临床应用指南[J]. 临床肝胆病杂志, 2018, 34(2): 255-261. DOI: 10.3969/j.issn.1001-5256.2018.02.008. [21] CHEN S, URBAN MW, PISLARU C, et al. Liver elasticity and viscosity quantification using shearwave dispersion ultrasound vibrometry (SDUV)[J]. Annu Int Conf IEEE Eng Med Biol Soc, 2009, 2009: 2252-2255. DOI: 10.1109/IEMBS.2009.5334992. [22] LEE DH, CHO EJ, BAE JS, et al. Accuracy of two-dimensional shear wave elastography and attenuation imaging for evaluation of patients with nonalcoholic steatohepatitis[J]. Clin Gastroenterol Hepatol, 2021, 19(4): 797-805. e7. DOI: 10.1016/j.cgh.2020.05.034. [23] LEE DH, LEE JY, BAE JS, et al. Shear-wave dispersion slope from US shear-wave elastography: Detection of allograft damage after liver transplantation[J]. Radiology, 2019, 293(2): 327-333. DOI: 10.1148/radiol.2019190064. [24] NIGHTINGALE KR, ROUZE NC, ROSENZWEIG SJ, et al. Derivation and analysis of viscoelastic properties in human liver: Impact of frequency on fibrosis and steatosis staging[J]. IEEE Trans Ultrason Ferroelectr Freq Control, 2015, 62(1): 165-175. DOI: 10.1109/TUFFC.2014.006653. [25] DEFFIEUX T, GENNISSON JL, BOUSQUET L, et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography[J]. J Hepatol, 2015, 62(2): 317-324. DOI: 10.1016/j.jhep.2014.09.020. [26] VIZZUTTI F, ARENA U, ROMANELLI RG, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis[J]. Hepatology, 2007, 45(5): 1290-1297. DOI: 10.1002/hep.21665. [27] JEON SK, LEE JM, JOO I, et al. Two-dimensional shear wave elastography with propagation maps for the assessment of liver fibrosis and clinically significant portal hypertension in patients with chronic liver disease: A prospective study[J]. Acad Radiol, 2020, 27(6): 798-806. DOI: 10.1016/j.acra.2019.08.006. [28] YOU MW, KIM KW, PYO J, et al. A Meta-analysis for the diagnostic performance of transient elastography for clinically significant portal hypertension[J]. Ultrasound Med Biol, 2017, 43(1): 59-68. DOI: 10.1016/j.ultrasmedbio.2016.07.025. [29] GILLIGAN LA, TROUT AT, BENNETT P, et al. Repeatability and agreement of shear wave speed measurements in phantoms and human livers across 6 ultrasound 2-dimensional shear wave elastography systems[J]. Invest Radiol, 2020, 55(4): 191-199. DOI: 10.1097/RLI.0000000000000627. [30] DENG H, QI X, ZHANG T, et al. Supersonic shear imaging for the diagnosis of liver fibrosis and portal hypertension in liver diseases: A meta-analysis[J]. Expert Rev Gastroenterol Hepatol, 2018, 12(1): 91-98. DOI: 10.1080/17474124.2018.1412257. [31] BERZIGOTTI A. Non-invasive evaluation of portal hypertension using ultrasound elastography[J]. J Hepatol, 2017, 67(2): 399-411. DOI: 10.1016/j.jhep.2017.02.003. [32] SHARMA P, KIRNAKE V, TYAGI P, et al. Spleen stiffness in patients with cirrhosis in predicting esophageal varices[J]. Am J Gastroenterol, 2013, 108(7): 1101-1107. DOI: 10.1038/ajg.2013.119. -



 PDF下载 ( 2530 KB)
PDF下载 ( 2530 KB)

 下载:
下载: