1型肝肾综合征患者90天预后危险因素分析及预测模型构建
DOI: 10.3969/j.issn.1001-5256.2022.07.019
Risk factors for the 90-day prognosis of patients with type I hepatorenal syndrome and establishment of a predictive model
-
摘要:
目的 探讨影响失代偿期肝硬化合并1型肝肾综合征(HRS)患者90 d预后的相关危险因素。 方法 回顾性收集北京地坛医院2008年10月—2018年10月299例失代偿期肝硬化合并1型HRS的住院患者的临床资料,按照患者90 d的预后情况分为生存组(n=135)和死亡组(n=164)。符合正态分布的计量资料两组间比较用t检验,非正态分布的计量资料两组间比较采用Mann-Whitney U检验;计数资料两组间比较用χ2检验。采用多因素二元logistic回归分析影响HRS患者90 d预后的因素,绘制不同因素受试者工作特征曲线(ROC曲线)。 结果 单因素分析显示,两组间终末期肝病模型(MELD)评分、Child-Turcotte-Pugh (CTP)评分、肝性脑病、血清钠(Na)、血肌酐(SCr)、血尿素氮(BUN)、尿酸(UA)、ALT、AST、TBil、Alb、胆碱酯酶(ChE)、WBC、中性粒细胞(NE)、中性粒细胞与淋巴细胞比值(NLR)、红细胞(RBC)、红细胞分布宽度(RDW)、PLT、国际标准化比值(INR)比较,差异均有统计学意义(P值均<0.05)。logistic多因素分析发现,Na、BUN、RDW、INR是失代偿期肝硬化合并HRS患者90 d预后不良的独立影响因素[OR(95%CI)分别为0.918(0.880~0.957)、1.077(1.029~1.127)、1.019(1.005~1.032)、3.478(2.096~5.771),P值均<0.05]。针对以上4个因素建立预测模型HRS-D,绘制各个因素的ROC曲线并计算曲线下面积,HRS-D的ROC曲线下面积为0.813(95%CI:0.762~0.864),诊断界值为-1.264,灵敏度为76.50%,特异度为72.50%。HRS-D和MELD相比,差异有统计学意义(Z=3.804,P<0.001),HRS-D评分与CTP评分、CTP评分与MELD评分相比,差异均无统计学意义(P值均>0.05)。 结论 Na、BUN、RDW、INR是失代偿期肝硬化合并1型HRS患者90 d预后不良的独立影响因素,据此建立的预测模型可以较好预测患者的90 d预后情况。 Abstract:Objective To investigate the risk factors for the 90-day prognosis of patients with decompensated liver cirrhosis and type I hepatorenal syndrome (HRS). Methods A retrospective analysis was performed for the clinical data of 299 patients with decompensated liver cirrhosis and type I HRS who were hospitalized in Beijing Ditan Hospital from October 2008 to October 2018, and according to the 90-day prognosis, they were divided into survival group with 135 patients and death group with 164 patients. The t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical data between groups. A multivariate binary logistic regression analysis was used to investigate the influencing factors for 90-day prognosis, and the receiver operating characteristic (ROC) curve was plotted for each factor. Results The univariate analysis showed that there were significant differences between the two groups in Model for End-Stage Liver Disease (MELD) score, Child-Turcotte-Pugh (CTP) score, hepatic encephalopathy, serum Na, serum creatinine (Cr), blood urea nitrogen (BUN), uric acid (UA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), albumin (Alb), cholinesterase (CHE), white blood cell count (WBC), neutrophil (NE), neutrophil-to-lymphocyte ratio (NLR), red blood cell count (RBC), red blood cell distribution width (RDW), platelet count (PLT), and international normalized ratio (INR) (P < 0.05). The multivariate logistic regression analysis showed that Na (odds ratio [OR]=0.918, 95% confidence interval [CI]: 0.880-0.957, P < 0.05), BUN (OR=1.077, 95% CI: 1.029-1.127, P < 0.05), RDW (OR=1.019, 95% CI: 1.005-1.032, P < 0.05), and INR (OR=3.478, 95% CI: 2.096-5.771, P < 0.05) were independent influencing factors for poor 90-day prognosis in patients with decompensated liver cirrhosis and type I HRS. A predictive model, HRS-D, was established using the four factors above, and the ROC curves were plotted for each factor to calculate the area under the ROC curve (AUC), which showed that HRS-D had an AUC of 0.813 (95% CI: 0.762-0.864), with a sensitivity of 76.50% and a specificity of 72.50% at the diagnostic cut-off value of -1.264. There was a significant difference between HRS-D score and MELD score (Z=3.804, P < 0.001), while there was no significant difference between HRS-D score and CTP score and between CTP score and MELD score (both P > 0.05). Conclusion Na, BUN, RDW, and INR are independent influencing factors for poor 90-day prognosis in patients with decompensated liver cirrhosis and type I HRS, and the predictive model based on these indices can better predict the 90-day prognosis of HRS patients. -
Key words:
- Hepatorenal Syndrome /
- Liver Cirrhosis /
- Prognosis /
- Risk Factors
-
肝肾综合征(hepatorenal syndrome,HRS)是发生在肝硬化患者中的一种特殊类型的肾衰竭,是晚期合并腹水的肝硬化患者中最常见的并发症,临床以少尿、无尿、氮质血症和电解质紊乱等肾损伤为主要表现。在合并腹水的肝硬化患者中,1年和5年内HRS的发生率分别为18%和39%[1]。一项前瞻性研究[2]观察到肝硬化患者中HRS的发生率为29%,90 d的病死率约为58%。早期准确预测HRS患者的预后,对指导患者治疗、延长患者生存时间具有重要意义。本研究旨在发现HRS患者90 d预后的临床影响因素,建立临床应用方便的预测模型,对患者的临床90 d预后情况评估提供参考。
1. 资料与方法
1.1 研究对象
选取2008年10月—2018年10月首都医科大学附属北京地坛医院收治的失代偿期肝硬化合并HRS的住院患者,随访时间90 d,根据患者的预后分为生存组和死亡组。
1.2 纳入与排除标准
纳入标准:失代偿期肝硬化的诊断根据《慢性乙型肝炎防治指南(2015年更新版) 》[3]中的肝硬化诊断标准;HRS诊断标准按照2017年《肝硬化腹水及相关并发症的诊疗指南》[4]中HRS的诊断标准。排除标准:(1)肝脏或其他部位恶性肿瘤;(2)妊娠或哺乳期妇女;(3)合并高血压、糖尿病及其他肾脏疾病;(4)肝、肾移植,经颈静脉肝内门体分流术后患者;(5)基线信息不全者。
1.3 诊断标准
1.3.1 肝硬化的诊断标准[3]
基于临床诊断:具有典型肝硬化表现,伴有肝功能失代偿及门静脉高压表现,至少两种影像学检查提示具有典型的肝硬化征象(包括超声、CT)。
1.3.2 HRS的诊断标准[4]
在肝硬化合并腹水的基础上,无休克表现;血清肌酐(SCr)升高>基线水平50% 以上或>1.5 mg/dL;至少停用2 d利尿剂(如果使用利尿剂)并且使用人血白蛋白1 g·kg-1·d-1,直至最大100 g/d,扩容后肾功能无持续性改善(SCr<133 μmol/L);近期无肾毒性药物使用史(非甾体类抗炎药、氨基糖甙类抗菌药物、造影剂等);无肾实质疾病。其中在48 h内血清SCr急性升高并超过基线水平的50%,并最终≥1.5 mg/dL(133 μmol/L)为急性肾损伤(AKI)型HRS。本研究中的HRS类型即为AKI型,即1型HRS。
1.4 研究方法
回顾性收集两组患者的临床资料,主要包括年龄、性别、肝硬化的主要病因、并发症。除此之外,记录患者入院诊断为HRS时的各项实验室检查结果,包括:血常规、肝肾功能离子、凝血功能等。
1.5 统计学方法
应用SPSS 23.0统计学软件进行数据分析。正态分布的计量资料以x±s表示,两组间比较采用t检验;非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。采用多因素二元logistic回归分析影响HRS患者90 d预后的临床因素,构建肝肾综合征死亡评分(HRS-D)预测模型;绘制Child-Turcotte-Pugh (CTP)评分、终末期肝病模型(MELD)评分、HRS-D受试者工作特征曲线(ROC曲线),3种评分的ROC曲线下面积(AUC)比较采用Z检验。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
本研究共纳入299例患者,其中生存组135例,男105例,女30例,平均(54±12)岁;死亡组164例,其中男127例,女37例,平均(54±13)岁。死亡组血SCr、血尿素氮(BUN)、ALT、AST、TBil、WBC、中性粒细胞(NE)、中性粒细胞与淋巴细胞比值(NLR)、红细胞分布宽度(RDW)、国际标准化比值(INR)等水平明显高于生存组,死亡组血清钠(Na)、Alb、胆碱酯酶(ChE)、尿酸(UA)、RBC、Hb、红细胞压积(HCT)、PLT、凝血酶原时间(PT)等水平明显低于生存组,差异均有统计学意义(P值均<0.05)。死亡组肝性脑病的发病率也明显高于生存组(P<0.05)(表 1)。
表 1 HRS患者的基线特征Table 1. Baseline characteristics of patients with HRS指标 生存组(n=135) 死亡组(n=164) 统计值 P值 年龄(岁) 54±12 54±13 t=0.168 0.867 男/女(例) 105/30 127/37 χ2=0.005 0.944 慢性乙型肝炎[例(%)] 53(39.3) 63(38.4) χ2=0.022 0.881 慢性丙型肝炎[例(%)] 7(5.2) 11(6.7) χ2=0.303 0.582 酒精性肝病[例(%)] 40(29.6) 44(26.8) χ2=0.287 0.607 免疫性肝病[例(%)] 9(6.7) 18(11.0) χ2=1.674 0.196 肝性脑病[例(%)] 16(11.9) 42(25.6) χ2=8.964 0.003 自发性腹膜炎[例(%)] 93(69.4) 117(71.8) χ2=0.200 0.654 消化道出血[例(%)] 25(18.5) 32(19.5) χ2=0.047 0.828 K(mmol/L) 4.34(3.86~4.82) 4.31(3.54~5.28) Z=-0.226 0.821 Na(mmol/L) 134.46±6.43 130.29±7.73 t=-5.085 <0.001 SCr(μmol/L) 143.00(137.50~177.00) 154.80(138.50~188.52) Z=-2.391 0.017 BUN(μmol/L) 13.59±5.36 17.86±8.76 t=5.191 <0.001 UA(mmol/L) 499.00(348.00~606.00) 414.00(243.25~577.78) Z=-3.204 0.001 ALT(U/L) 23.80(14.90~53.10) 43.60(24.18~103.48) Z=-5.731 <0.001 AST(U/L) 36.20(28.00~70.30) 68.80(38.30~150.30) Z=-4.124 <0.001 TBil(mmol/L) 38.00(20.70~106.6) 251.90(80.150~418.15) Z=-7.900 <0.001 Alb(g/L) 30.16±6.22 27.70±5.36 t=-5.074 <0.001 GGT(mmol/L) 41.40(18.20~73.60) 39.30(21.68~66.13) Z=-0.716 0.474 ChE(U/L) 2099(1548~3536) 1563(1064~2224) Z=-6.598 <0.001 WBC(×109/L) 5.96(4.20~8.69) 8.54(5.84~12.34) Z=-4.258 <0.001 NE(×109/L) 3.74(2.80~6.33) 6.45(4.23~10.53) Z=-4.694 <0.001 LY(×109/L) 0.96(0.62~1.57) 0.98(0.61~1.37) Z=-0.826 0.409 NLR 4.83(2.65~9.62) 7.94(4.57~13.98) Z=-4.843 <0.001 RBC(×1012/L) 2.93±0.79 2.53±0.93 t=-3.979 <0.001 Hb(g/L) 96.74±26.75 87.25±30.47 t=-3.198 <0.001 HCT(%) 28.50(24.42~33.90) 19.69(14.26~24.10) Z=-2.870 0.004 RDW(fL) 48.50(17.18~59.70) 56.75(20.39~63.48) Z=-4.139 <0.001 PLT(×109/L) 80.00(48.00~116.00) 67.00(35.28~99.50) Z=-2.802 0.005 INR 4.26(2.75~6.92) 6.68(4.39~13.05) Z=-7.438 <0.001 PT(s) 56.00(43.00~67.00) 34.50(21.90~47.65) Z=-8.226 <0.001 注:K,钾;LY,淋巴细胞计数。 2.2 logistic单因素和多因素回归分析
临床指标进行单因素二项logistic回归,结果如表 2所示,和两组间的基线临床指标比较结果相似。综合考虑基线数据单因素分析结果、避免因素共线性及临床意义等多个方面,将年龄、肝性脑病、Na、BUN、UA、SCr、ALT、AST、TBil、Alb、WBC、RBC、RDW、PLT、NLR、INR这16个指标进行多重共线性分析,结果各指标的容忍度均>0.1,方差膨胀因子均<10,表明各指标间不存在多重共线性关系。再将上述因素纳入多因素logistic二项回归分析,将临床指标为自变量,患者的90 d预后情况为因变量,结果显示Na、BUN、RDW、INR是影响HRS患者90 d预后的独立影响因素(P值均<0.05)(表 3)。根据多因素logistic回归的结果,将B作为因素的系数,得到一个logistic回归方程HRS-D=-0.086×Na+0.074×BUN+0.018×RDW+1.246×INR。
表 2 logistic单因素回归分析Table 2. logistic regression univariate analysis变量 B值 OR(95%CI) P值 年龄 0.034 1.002(0.984~1.020) 0.866 肝性脑病 0.940 0.391(0.208~0.732) 0.035 CTP 0.493 1.637(1.434~1.868) <0.001 MELD 0.098 1.103(1.062~1.146) <0.001 Na(mmol/L) -0.082 1.637(1.434~1.868) <0.001 SCr(μmol/L) 0.008 1.008(1.002~1.014) <0.001 BUN(μmol/L) 0.090 1.094(1.053~1.138) <0.001 UA(mmol/L) -0.002 0.998(0.997~0.999) 0.003 ALT(U/L) 0.005 1.005(1.002~1.009) 0.006 AST(U/L) 0.005 1.005(1.002~1.008) 0.003 TBil(mmol/L) 0.005 1.005(1.004~1.007) <0.001 Alb(g/L) -0.105 0.900(0.862~0.940) <0.001 ChE(U/L) -0.001 0.999(0.999~1.000) <0.001 WBC(×109/L) 0.078 1.081(1.032~1.133) 0.001 NE(×109/L) 0.087 1.091(1.036~1.149) 0.001 NLR 0.047 1.071(1.031~1.111) <0.001 RBC(×1012/L) -0.528 0.590(0.448~0.778) <0.001 RDW(fL) 0.016 1.017(1.006~1.027) 0.002 PLT(×109/L) -0.006 0.994(0.989~0.999) 0.010 INR 1.384 3.989(2.449~6.497) <0.001 表 3 logistic多因素回归分析Table 3. logistic regression multivariate analysis变量 B值 SE Wald P值 OR(95%CI) Na(mmol/L) -0.086 0.021 15.999 <0.001 0.918(0.880~0.957) BUN(μmol/L) 0.074 0.023 10.071 0.002 1.077(1.029~1.127) RDW(fL) 0.018 0.007 7.712 0.005 1.019(1.005~1.032) INR 1.246 0.258 23.263 <0.001 3.478(2.096~5.771) 2.3 各影响因素的ROC曲线分析
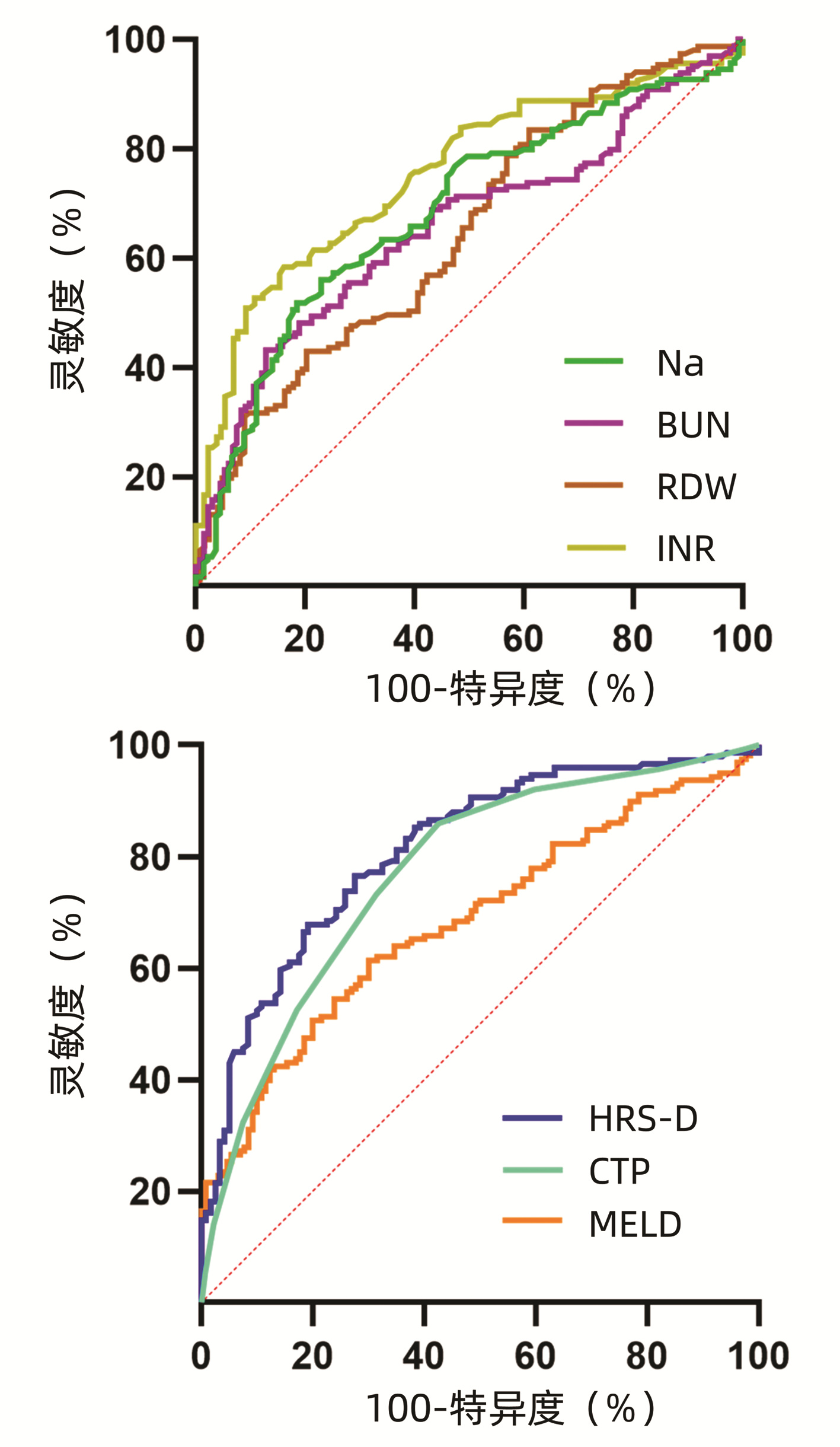
死亡组的3种评分模型得分均明显高于生存组(P值均<0.001)(表 4)。多因素logistic回归结果中各因素和3种评分模型的ROC曲线如图 1所示,Na的AUC为0.685(95%CI:0.624~0.746),BUN为0.654(95% CI:0.592~0.716),RDW为0.647(95%CI:0.581~0.712),INR为0.754(95%CI:0.698~0.809);HRS-D评分和CTP评分AUC分别为0.813(95%CI:0.762~0.864)和0.772(95%CI:0.717~0.827),诊断价值中等;MELD评分AUC为0.685(95%CI:0.623~0.744),诊断价值一般。各评分模型ROC曲线的截断值(cut-off值)、灵敏度和特异度见表 5。进一步用MedCalc软件对3种评分的ROC曲线进行两两比较,发现HRS-D和MELD相比,差异有统计学意义(Z=3.804, P<0.001),HRS-D评分与CTP评分、CTP评分与MELD评分相比,差异均无统计学意义(Z值分别为1.683、1.982,P值分别为0.092、0.052)。
表 4 两组3种评分模型的比较Table 4. Characteristics comparison of three scoring models in two group模型 生存组(n=135) 死亡组(n=164) 统计值 P值 HRS-D -8.43±0.91 -7.38±1.40 t=-7.155 <0.001 MELD 30.67±5.59 35.41±8.07 t=5.864 <0.001 CTP 9(8~11) 12(11~13) Z=-8.119 <0.001 表 5 3种评分模型ROC曲线特点及比较Table 5. Comparison of the characteristics of the ROC curves of three scoring models模型 约登指数 cut-off值 灵敏度 特异度 HRS-D 0.49 -1.264 0.765 0.725 CTP 0.43 9.50 0.860 0.570 MELD 0.31 33.25 0.610 0.700 3. 讨论
HRS是肝硬化患者的一种严重并发症,是肝硬化患者死亡的重要原因之一,既往有研究[5]发现肝硬化患者合并有消化道出血、感染、大量放腹水较易发生HRS,同时CTP评分和MELD评分也和HRS的发生呈正相关,除此之外,PTA、中性粒细胞比率也与HRS发生率相关。本研究也发现患者是否患有肝性脑病、肝功能指标、WBC、NLR也是患者的危险因素。但目前并无肝硬化患者合并1型HRS患者死亡的相关预测模型。由本研究通过纳入299例1型HRS患者,通过logistic回归分析,结果发现Na、BUN、RDW、INR是影响HRS患者90 d预后的独立影响因素,据此4个指标建立的预测模型,可以较好的预测HRS患者的死亡。
对于肝硬化合并腹水的患者,会采用饮食限Na和利尿治疗,而肝硬化患者因体内血管加压素、醛固酮等增多的缘故,常伴有低钠血症,已有研究表明,低钠血症可作为肾素-血管紧张素-醛固酮系统激活的标志,预示疾病的进展。有研究表明低钠血症是HRS发生的危险因素[6],也是乙型肝炎肝硬化患者死亡的独立危险因素[7],乙型肝炎肝硬化合并肾损伤患者的血清钠水平更低[8]。本研究发现,血清Na是HRS患者预后不良的保护因素,生存组患者的血清钠水平较死亡组高(P<0.001)。在临床上,用BUN、SCr和肾小球滤过率联合评估肾功能,有研究[9]发现单纯的BUN升高也能预测肾损伤的发生。本研究发现BUN水平和HRS患者90 d预后呈正相关,生存组患者的BUN水平较死亡组低(P<0.001),死亡组患者的SCr水平更高(P<0.001)。SCr在logistic多因素分析中差异无统计学意义,考虑和本研究纳入的病例均为HRS患者,SCr水平均较高有关。RDW被认为是一种新的氧化应激的标志物,可以反映系统炎性反应情况,有研究[10-11]称RDW与系统红斑狼疮、干燥综合征、心脑血管病和糖尿病有关。有研究表明,RDW水平与脓毒症合并AKI患者存活率呈负相关[12],也与慢性肾脏病患者的预后相关[13]。本研究发现死亡组的RDW水平较生存组更高(P<0.001),与患者的病死率呈正相关,与其他研究结果相符。凝血水平的变化能反应肝硬化患者的肝损伤情况,有研究[14]表明INR是肝硬化患者的再住院和预后不良的危险因素之一。本研究结果表明死亡组患者的凝血功能较生存组患者更差(P<0.001),和既往研究结果一致。
综上所述,本研究探讨了1型HRS患者90 d预后的危险因素,并据此建立了一个预测模型,可以较好的预测本研究纳入的HRS患者的90 d预后,其中包含的4个指标(Na、BUN、RDW、INR),指标在临床容易获取。但本研究为单中心回顾性研究,所纳入的人群数量较少,故所得结论有一定的局限性,若想广泛推广应用,还需扩大样本量,并进行多中心的外部队列验证。
-
表 1 HRS患者的基线特征
Table 1. Baseline characteristics of patients with HRS
指标 生存组(n=135) 死亡组(n=164) 统计值 P值 年龄(岁) 54±12 54±13 t=0.168 0.867 男/女(例) 105/30 127/37 χ2=0.005 0.944 慢性乙型肝炎[例(%)] 53(39.3) 63(38.4) χ2=0.022 0.881 慢性丙型肝炎[例(%)] 7(5.2) 11(6.7) χ2=0.303 0.582 酒精性肝病[例(%)] 40(29.6) 44(26.8) χ2=0.287 0.607 免疫性肝病[例(%)] 9(6.7) 18(11.0) χ2=1.674 0.196 肝性脑病[例(%)] 16(11.9) 42(25.6) χ2=8.964 0.003 自发性腹膜炎[例(%)] 93(69.4) 117(71.8) χ2=0.200 0.654 消化道出血[例(%)] 25(18.5) 32(19.5) χ2=0.047 0.828 K(mmol/L) 4.34(3.86~4.82) 4.31(3.54~5.28) Z=-0.226 0.821 Na(mmol/L) 134.46±6.43 130.29±7.73 t=-5.085 <0.001 SCr(μmol/L) 143.00(137.50~177.00) 154.80(138.50~188.52) Z=-2.391 0.017 BUN(μmol/L) 13.59±5.36 17.86±8.76 t=5.191 <0.001 UA(mmol/L) 499.00(348.00~606.00) 414.00(243.25~577.78) Z=-3.204 0.001 ALT(U/L) 23.80(14.90~53.10) 43.60(24.18~103.48) Z=-5.731 <0.001 AST(U/L) 36.20(28.00~70.30) 68.80(38.30~150.30) Z=-4.124 <0.001 TBil(mmol/L) 38.00(20.70~106.6) 251.90(80.150~418.15) Z=-7.900 <0.001 Alb(g/L) 30.16±6.22 27.70±5.36 t=-5.074 <0.001 GGT(mmol/L) 41.40(18.20~73.60) 39.30(21.68~66.13) Z=-0.716 0.474 ChE(U/L) 2099(1548~3536) 1563(1064~2224) Z=-6.598 <0.001 WBC(×109/L) 5.96(4.20~8.69) 8.54(5.84~12.34) Z=-4.258 <0.001 NE(×109/L) 3.74(2.80~6.33) 6.45(4.23~10.53) Z=-4.694 <0.001 LY(×109/L) 0.96(0.62~1.57) 0.98(0.61~1.37) Z=-0.826 0.409 NLR 4.83(2.65~9.62) 7.94(4.57~13.98) Z=-4.843 <0.001 RBC(×1012/L) 2.93±0.79 2.53±0.93 t=-3.979 <0.001 Hb(g/L) 96.74±26.75 87.25±30.47 t=-3.198 <0.001 HCT(%) 28.50(24.42~33.90) 19.69(14.26~24.10) Z=-2.870 0.004 RDW(fL) 48.50(17.18~59.70) 56.75(20.39~63.48) Z=-4.139 <0.001 PLT(×109/L) 80.00(48.00~116.00) 67.00(35.28~99.50) Z=-2.802 0.005 INR 4.26(2.75~6.92) 6.68(4.39~13.05) Z=-7.438 <0.001 PT(s) 56.00(43.00~67.00) 34.50(21.90~47.65) Z=-8.226 <0.001 注:K,钾;LY,淋巴细胞计数。 表 2 logistic单因素回归分析
Table 2. logistic regression univariate analysis
变量 B值 OR(95%CI) P值 年龄 0.034 1.002(0.984~1.020) 0.866 肝性脑病 0.940 0.391(0.208~0.732) 0.035 CTP 0.493 1.637(1.434~1.868) <0.001 MELD 0.098 1.103(1.062~1.146) <0.001 Na(mmol/L) -0.082 1.637(1.434~1.868) <0.001 SCr(μmol/L) 0.008 1.008(1.002~1.014) <0.001 BUN(μmol/L) 0.090 1.094(1.053~1.138) <0.001 UA(mmol/L) -0.002 0.998(0.997~0.999) 0.003 ALT(U/L) 0.005 1.005(1.002~1.009) 0.006 AST(U/L) 0.005 1.005(1.002~1.008) 0.003 TBil(mmol/L) 0.005 1.005(1.004~1.007) <0.001 Alb(g/L) -0.105 0.900(0.862~0.940) <0.001 ChE(U/L) -0.001 0.999(0.999~1.000) <0.001 WBC(×109/L) 0.078 1.081(1.032~1.133) 0.001 NE(×109/L) 0.087 1.091(1.036~1.149) 0.001 NLR 0.047 1.071(1.031~1.111) <0.001 RBC(×1012/L) -0.528 0.590(0.448~0.778) <0.001 RDW(fL) 0.016 1.017(1.006~1.027) 0.002 PLT(×109/L) -0.006 0.994(0.989~0.999) 0.010 INR 1.384 3.989(2.449~6.497) <0.001 表 3 logistic多因素回归分析
Table 3. logistic regression multivariate analysis
变量 B值 SE Wald P值 OR(95%CI) Na(mmol/L) -0.086 0.021 15.999 <0.001 0.918(0.880~0.957) BUN(μmol/L) 0.074 0.023 10.071 0.002 1.077(1.029~1.127) RDW(fL) 0.018 0.007 7.712 0.005 1.019(1.005~1.032) INR 1.246 0.258 23.263 <0.001 3.478(2.096~5.771) 表 4 两组3种评分模型的比较
Table 4. Characteristics comparison of three scoring models in two group
模型 生存组(n=135) 死亡组(n=164) 统计值 P值 HRS-D -8.43±0.91 -7.38±1.40 t=-7.155 <0.001 MELD 30.67±5.59 35.41±8.07 t=5.864 <0.001 CTP 9(8~11) 12(11~13) Z=-8.119 <0.001 表 5 3种评分模型ROC曲线特点及比较
Table 5. Comparison of the characteristics of the ROC curves of three scoring models
模型 约登指数 cut-off值 灵敏度 特异度 HRS-D 0.49 -1.264 0.765 0.725 CTP 0.43 9.50 0.860 0.570 MELD 0.31 33.25 0.610 0.700 -
[1] WONG LP, BLACKLEY MP, ANDREONI KA, et al. Survival of liver transplant candidates with acute renal failure receiving renal replacement therapy[J]. Kidney Int, 2005, 68(1): 362-370. DOI: 10.1111/j.1523-1755.2005.00408.x. [2] ALLEGRETTI AS, ORTIZ G, WENGER J, et al. Prognosis of acute kidney injury and hepatorenal syndrome in patients with cirrhosis: A prospective cohort study[J]. Int J Nephrol, 2015, 2015: 108139. DOI: 10.1155/2015/108139. [3] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: A 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. [4] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of ascites and complications in cirrhosis Chinese Society of Hepatology[J]. J Clin Hepatol, 2017, 33(10): 1847-1863. DIO: 10.3969/j.issn.1001-5256.2017.10.003. DOI: 10.3969/j.issn.1001-5256.2017.10.003中华医学会肝病学分会. 肝硬化腹水及相关并发症的诊疗指南[J]. 临床肝胆病杂志, 2017, 33(10): 1847-1863. DOI: 10.3969/j.issn.1001-5256.2017.10.003. [5] YE YN, ZHANG ZQ, HE G, et al. Analysis of high-risk factors for hepatorenal syndrome in patients with hepatitis B-related acute-on-chronic liver failure[J]. Hainan Med J, 2019, 30(24): 3145-3149. DOI: 10.3969/j.issn.1003-6350.2019.24.004.叶一农, 张志侨, 何纲, 等. 乙型肝炎相关慢加急性肝衰竭患者发生肝肾综合征的高危因素分析[J]. 海南医学, 2019, 30(24): 3145-3149. DOI: 10.3969/j.issn.1003-6350.2019.24.004. [6] LIU PP, LIU J, YE WF. Relationship between Cys-C, hyponatremia, blood ammonia and hepatorenal syndrome[J]. Contemp Med, 2019, 25(19): 82-84. DOI: 10.3969/j.issn.1009-4393.2019.19.033.刘萍萍, 刘娟, 叶文峰. Cys-C、低钠血症、血氨与肝肾综合征的关系[J]. 当代医学, 2019, 25(19): 82-84. DOI: 10.3969/j.issn.1009-4393.2019.19.033. [7] MA Y, WANG XZ, YAN GW, et al. Effect of hyponatremia on prognosis of patients with decompensated hepatitis B cirrhosis[J]. Med Recapitulate, 2021, 27(4): 796-803, 808. DOI: 10.3969/j.issn.1006-2084.2021.04.032.马燕, 王晓忠, 延国威, 等. 低钠血症对乙肝肝硬化失代偿期患者预后的影响[J]. 医学综述, 2021, 27(4): 796-803, 808. DOI: 10.3969/j.issn.1006-2084.2021.04.032. [8] MA Y, WANG XZ, YAN GW, et al. The relationship between serum sodium, creatinine, cystatin C and the degree of renal damage in patients with hepatitis B liver cirrhosis and hyponatremia[J]. Med Inf, 2020, 33(19): 55-58. DOI: 10.3969/j.issn.1006-1959.2020.19.017.马燕, 王晓忠, 延国威, 等. 乙肝肝硬化低血钠症患者血清钠、肌酐以及胱抑素C与肾功能损害程度的关系[J]. 医学信息, 2020, 33(19): 55-58. DOI: 10.3969/j.issn.1006-1959.2020.19.017. [9] XU XY, DIAO ZL, SU JR. Reduction in estimated glomerular filtration rate in patients with elevated blood urea nitrogen but normal for any other markers of kid- ney damage[J]. J Clin Exp Med, 2017, 16(22): 2262-2264. DOI: 10.3969/j.issn.1671-4695.2017.22.025.徐小用, 刁宗礼, 苏建荣. 单纯血尿素氮升高患者肾小球滤过率下降趋势分析[J]. 临床和实验医学杂志, 2017, 16(22): 2262-2264. DOI: 10.3969/j.issn.1671-4695.2017.22.025. [10] BHUTTO AR, ABBASI A, ABRO AH. Correlation of hemoglobin A1c with red cell width distribution and other parameters of red blood cells in type Ⅱ diabetes mellitus[J]. Cureus, 2019, 11(8): e5533. DOI: 10.7759/cureus.5533. [11] BLASLOV K, KRULJAC I, MIROŠEVIĆ G, et al. The prognostic value of red blood cell characteristics on diabetic retinopathy development and progression in type 2 diabetes mellitus[J]. Clin Hemorheol Microcirc, 2019, 71(4): 475-481. DOI: 10.3233/CH-180422. [12] FAN JQ, FAN YQ, LI ZJ. Value of red blood cell distribution width in prognosis of acute kidney injury associated with sepsis[J]. Hainan Med J, 2014, 25(20): 3012-3014. DOI: 10.3969/j.issn.1003-6350.2014.20.1184.范金强, 范银强, 李振军. 红细胞体积分布宽度对脓毒症急性肾损伤患者预后的评估价值[J]. 海南医学, 2014, 25(20): 3012-3014. DOI: 10.3969/j.issn.1003-6350.2014.20.1184. [13] HSIEH YP, CHANG CC, KOR CT, et al. The predictive role of red cell distribution width in mortality among chronic kidney disease patients[J]. PLoS One, 2016, 11(12): e0162025. DOI: 10.1371/journal.pone.0162025. [14] WANG Z, HOU W, WANG KF, et al. Risk factors of 30 days readmission and 3 years mortality of patients with liver cirrhosis and ascites[J/CD]. Chin J Liver Dis (Electronic Version), 2021, 13(3): 16-24. DOI: 10.3969/j.issn.1674-7380.2021.03.003.王征, 侯维, 王克菲, 等. 肝硬化腹水患者30 d再住院和3年病死的危险因素分析[J/CD]. 中国肝脏病杂志(电子版), 2021, 13(3): 16-24. DOI: 10.3969/j.issn.1674-7380.2021.03.003. 期刊类型引用(1)
1. 张欢,邢腾龙,张盼,尚润润,王明媚,赵钰莹,许万博. 肝硬化自发性门体分流与肝肾综合征的相关性研究. 临床肝胆病杂志. 2023(12): 2824-2830 .  本站查看
本站查看其他类型引用(1)
-




 PDF下载 ( 2217 KB)
PDF下载 ( 2217 KB)

 下载:
下载:


 下载:
下载: