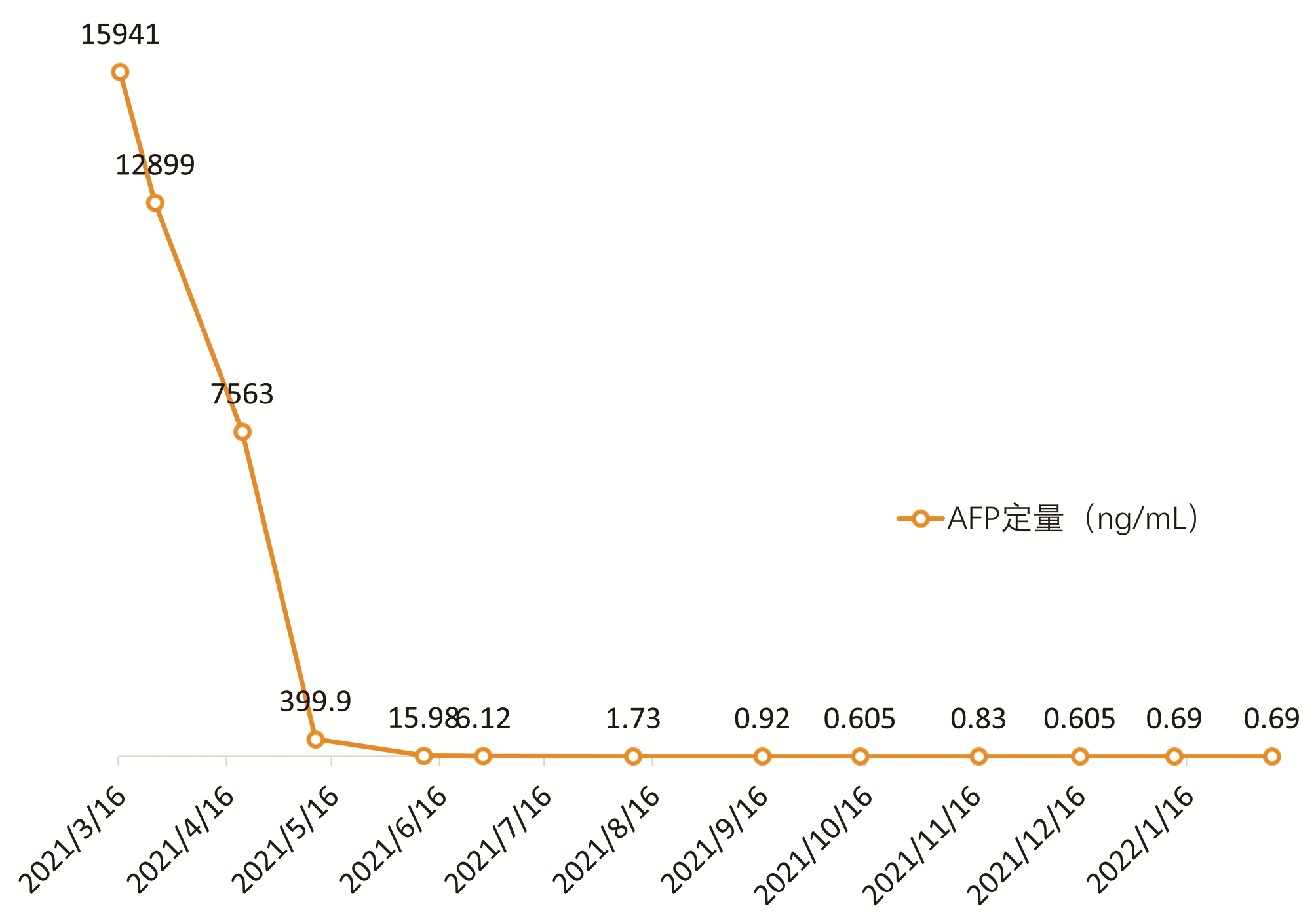
巨块型肝细胞癌转化治疗1例报告
DOI: 10.3969/j.issn.1001-5256.2022.07.028
伦理学声明:本例报告患者已签署知情同意书。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:李思柔负责查找阅读及总结文献,分析资料,撰写论文; 周元龙、刘珂良、梁志波负责收集病例资料以及指导论文撰写; 李靖华负责拟定写作思路,指导撰写文章并最后定稿。
-
-
Key words:
- Carcinoma, Hepatocellular /
- Molecular Targeted Therapy /
- Immunotherapy
-
-
[1] AN L, ZENG HM, ZHENG RS, et al. Liver cancer epidemiology in China, 2015[J]. Chin J Oncol, 2019, 41(10): 721-727. DOI: 10.3760/cma.j.issn.0253-3766.2019.10.001.安澜, 曾红梅, 郑荣寿, 等. 2015年中国肝癌流行情况分析[J]. 中华肿瘤杂志, 2019, 41(10): 721-727. DOI: 10.3760/cma.j.issn.0253-3766.2019.10.001. [2] YANG JH, CHEN GX, ZHOU M, et al. Multidisciplinary treatment of hepatocellular carcinoma with tumor thrombosis in the left portal vein: A case report[J]. J Clin Hepatol, 2021, 37(3): 666-669. DOI:10.3969 /j.issn.1001-5256.2021.03.031.杨季红, 陈国想, 周茉, 等. 肝细胞癌伴门静脉左支癌栓形成多学科综合治疗1例报告[J]. 临床肝胆病杂志, 2021, 37(3) : 666-669. DOI: 10.3969/j.issn.1001-5256.2021.03.031. [3] Professional Committee for Prevention and Control of Hepatobiliary and Pancreatic Diseases of Chinese Preventive Medicine Association, Chinese Society of Liver Cancer, Chinese Anti-Cancer Association, Liver Study Group of Surgery Committee of Beijing Medical Association, et al. Chinese expert consensus on conversion therapy of immune checkpoint inhibitors combined antiangiogenic targeted drugs for advanced hepatocellular carcinoma (2021 Edition)[J/CD]. Electronic J Liver Tumor, 2021, 8(2): 6-15. DOI: 10.3969/j.issn.2095-7815.2021.02.004.中华预防医学会肝胆胰疾病预防与控制专业委员会, 中国抗癌协会肝癌专业委员会, 北京医学会外科学分会肝脏学组, 等. 基于免疫联合靶向方案的晚期肝细胞癌转化治疗中国专家共识(2021版)[J/CD]. 肝癌电子杂志, 2021, 8(2): 6-15. DOI: 10.3969/j.issn.2095-7815.2021.02.004. [4] SONG TQ, LIU YY. Conversion therapy and maintenance therapy for primary liver cancer[J]. Chin J Pract Surg, 2021, 41(3): 269-272. DOI: 10.19538/j.cjps.issn1005-2208.2021.03.06.宋天强, 刘雅月. 原发性肝癌转化治疗及其维持治疗策略[J]. 中国实用外科杂志, 2021, 41(3): 269-272. DOI: 10.19538/j.cjps.issn1005-2208.2021.03.06. [5] Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [6] PARK JW, CHEN M, COLOMBO M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study[J]. Liver Int, 2015, 35(9): 2155-2166. DOI: 10.1111/liv.12818. [7] SUN HC, ZHU XD. Downstaging conversion therapy in patients with initially unresectable advanced hepatocellular carcinoma: an overview[J]. Front Oncol, 2021, 11: 772195. DOI: 10.3389/fonc.2021.772195. [8] Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy in hepatocellular carcinoma(2021 edition)[J]. Chin J Dig Surg, 2021, 20(6): 600-616. DOI: 10.3760/cma.j.cn115610-20210512-00223.中国抗癌协会肝癌专业委员会转化治疗协作组. 肝癌转化治疗中国专家共识(2021版)[J]. 中华消化外科杂志, 2021, 20(6): 600-616. DOI: 10.3760/cma.j.cn115610-20210512-00223. [9] MEHTA N. Hepatocellular carcinoma-how to determine therapeutic options[J]. Hepatol Commun, 2020, 4(3): 342-354. DOI: 10.3389/fonc.2021.772195. [10] MAKARY MS, KHANDPUR U, CLOYD JM, et al. Locoregional therapy approaches for hepatocellular carcinoma: recent advances and management strategies[J]. Cancers (Basel), 2020, 12(7): 1914. DOI: 10.3390/cancers12071914. [11] CHEN D, WANG KB, LI JZ, et al. Present status and challenges of targeted drugs on antiangiogenesis in primary hepatic carcinom[J]. China Cancer, 2017, 26(3): 203-209. DOI: 10.11735/j.issn.1004-0242.2017.03.A008.陈丹, 王凯冰, 李加桩, 等. 原发性肝癌的抗血管生成靶向治疗现状与挑战[J]. 中国肿瘤, 2017, 26(3): 203-209. DOI: 10.11735/j.issn.1004-0242.2017.03.A008. [12] HU H, DUAN Z, LONG X, et al. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study[J]. PLoS One, 2014, 9(5): e96620. DOI: 10.1371/journal.pone.0096620. [13] KUDO M. A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: upfront systemic therapy followed by curative conversion[J]. Liver Cancer, 2021, 10(6): 539-544. DOI: 10.1159/000519749. [14] ZHU DW, YANG SL, LI YF, et al. Efficacy of carrelizumab combined with sorafenib on advanced hepatocellular carcinoma[J]. J Chin Pract Diagn Ther, 2021, 35(10): 1063-1067. DOI: 10.13507/j.issn.1674-3474.2021.10.023.朱帝文, 杨胜利, 李一帆, 等. 卡瑞利珠单抗联合索拉非尼治疗中晚期肝癌疗效分析[J]. 中华实用诊断与治疗杂志, 2021, 35(10): 1063-1067. DOI: 10.13507/j.issn.1674-3474.2021.10.023. [15] HAN Y, HUANG Z, JIANG ZC, et al. Expert consensus on targeted therapy of liver cancer[J/CD]. Electronic J Liver Tumor, 2020, 7(2): 2-11. DOI: 10.19872/j.cnki.2095-7815.2020.02.002.韩玥, 黄振, 姜志超, 等. 肝癌靶向治疗专家共识(草案)[J/CD]. 肝癌电子杂志, 2020, 7(2): 2-11. DOI: 10.19872/j.cnki.2095-7815.2020.02.002. [16] EISENHAUER EA, THERASSE P, BOGAERTS J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-247. DOI: 10.1016/j.ejca.2008.10.026. [17] ZHOU M, ZHANG C, NIE J, et al. Response evaluation and survival prediction following PD-1 inhibitor in patients with advanced hepatocellular carcinoma: comparison of the RECIST 1.1, iRECIST, and mRECIST criteria[J]. Front Oncol, 2021, 11: 764189. DOI: 10.3389/fonc.2021.764189. [18] KASEB AO, VENCE L, BLANDO J, et al. Immunologic correlates of pathologic complete response to preoperative immunotherapy in hepatocellular carcinoma[J]. Cancer Immunol Res, 2019, 7(9): 1390-1395. DOI: 10.1158/2326-6066.CIR-18-0605. [19] ZHAO L, ZHAO H. Conversion surgery for hepatocellular carcinoma in the new era of targeted and immune checkpoint inhibitor therapies[J]. Hepatobiliary Surg Nutr, 2020, 9(6): 809-811. DOI: 10.21037/hbsn-20-693. [20] LYU GY, JIANG C, SUN XD. Conversion therapy strategies for hepatocellular carcinoma[J]. Chin J Dig Surg, 2022, 21(2): 217-223. DOI: 10.3760/cma.j.cn115610-20220125-00058.吕国悦, 蒋超, 孙晓东. 肝癌的转化治疗策略[J]. 中华消化外科杂志, 2022, 21(2): 217-223. DOI: 10.3760/cma.j.cn115610-20220125-00058. [21] ZENG YY, LIN KY, TANG SC. Conversion therapy strategies for hepatocellular carcinoma in the era of targeted therapy combined with immunotherapy[J]. Chin J Dig Surg, 2022, 21(2): 224-230. DOI: 10.3760/cma.j.cn115610-20211130-00606.曾永毅, 林孔英, 唐世川. 靶向联合免疫时代肝细胞癌转化治疗策略[J]. 中华消化外科杂志, 2022, 21(2): 224-230. DOI: 10.3760/cma.j.cn115610-20211130-00606. [22] GUO P, LI B. Application of multidisciplinary team in the conversion therapies of liver cancer[J]. CHSM, 2021, 12(20): 38-40. DOI: 10.3969/j.issn.1674-9316.2021.20.013.郭平, 李滨. 多学科综合诊疗在肝癌转化治疗中的应用[J]. 中国卫生标准管理, 2021, 12(20): 38-40. DOI: 10.3969/j.issn.1674-9316.2021.20.013. [23] ZHAO M, WU JM, SHANG CZ, et al. Advances in the techniques and evaluation of conversion therapy for hepatocellular carcinoma[J]. Chin J Pract Surg, 2021, 41(3): 262-268. DOI: 10.19538/j.cjps.issn1005-2208.2021.03.05.赵明, 伍家鸣, 商昌珍, 等. 肝癌转化治疗相关技术方法及评价[J]. 中国实用外科杂志, 2021, 41(3): 262-268. DOI: 10.19538/j.cjps.issn1005-2208.2021.03.05. -



 PDF下载 ( 2763 KB)
PDF下载 ( 2763 KB)


 下载:
下载: