非酒精性脂肪性肝病的药物治疗进展
DOI: 10.3969/j.issn.1001-5256.2022.07.033
-
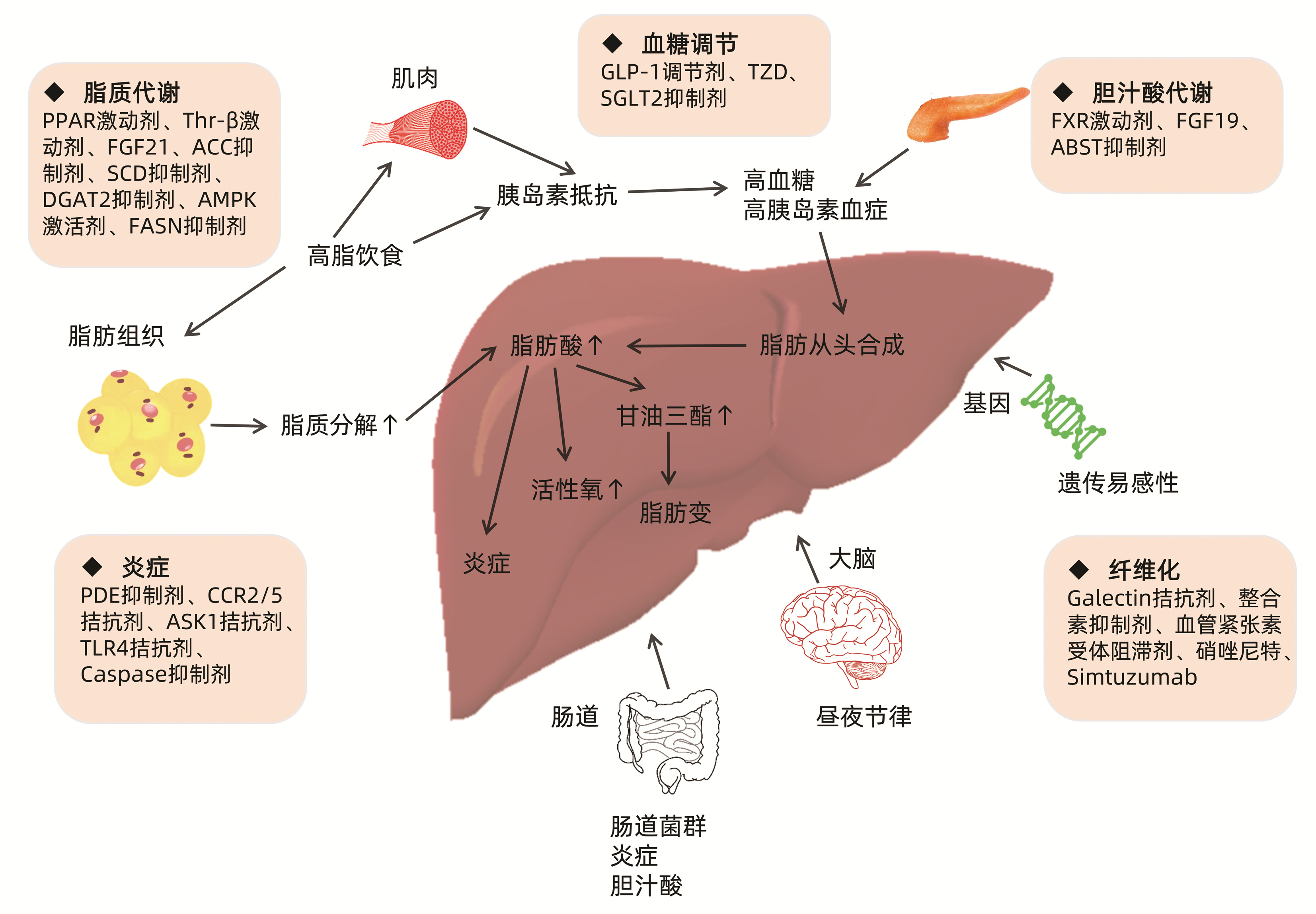
摘要: 非酒精性脂肪性肝病(NAFLD)发病率逐年上升,正逐渐成为全球成人和儿童最常见的慢性肝病,其疾病谱中的非酒精性脂肪肝炎(NASH)可导致肝硬化和肝细胞癌。然而因NAFLD发病机制复杂,目前尚无有效的治疗药物。因此,NASH的新药研发是近年来研究的热点。本文总结了NASH发病机制重点阐明NASH潜在的治疗靶点及药物研究进展。Abstract: The incidence rate of nonalcoholic fatty liver disease (NAFLD) is increasing year by year, and it gradually becomes the most common chronic liver disease in adults and children around the world. Nonalcoholic steatohepatitis (NASH) in its disease spectrum can lead to liver cirrhosis and hepatocellular carcinoma. However, due to the complex pathogenesis of NAFLD, there are currently no effective drugs for treatment. Therefore, the research and development of new drugs for NASH have been a research hotspot in recent years. This article summarizes the pathogenesis of NASH and elaborates on the research advances in potential therapeutic targets and drugs for NASH.
-
表 1 有治疗潜力的NASH临床试验Ⅱ期药物
Table 1. Pharmacologic agents in phase Ⅱ studies for NASH
药物 作用机制 研究人群 主要结果 NCT代码 Cilofexor (GS-9674) 非甾体FXR激动剂 NASH (F1~F3) 耐受性良好,可显著降低肝脏脂肪变、血清胆汁酸与肝脏生化指标,不改变血清脂质 NCT02854605 Cilofexor+ firsocostat FXR激动剂+ ACC抑制剂 NASH (F3~F4) 耐受性良好,可改善NASH活性,并可能具有抗纤维化作用 NCT03449446 培马贝特(K-877) 选择性PPARα调节剂 NASH 不会降低LFC,但可以显著降低MRE测得的肝脏硬度 NCT03350165 VK2809 Thr-β激动剂 NAFLD 改善LDL-C和降低肝脏脂肪 NCT02927184 Efruxifermin Fc-FGF21融合蛋白 NASH (F1~F3) 肝脏脂肪显著减少,纤维化获得逆转 NCT03976401 BIO89-100 FGF21类似物 NASH 安全性及耐受性良好,肝脏脂肪和关键脂质标志物明显降低 NCT04929483 PF-05221304 ACC1/2抑制剂 NAFLD 显著减少肝脏脂肪,并改善了一些NASH相关的生物标志物,可能会引起高脂血症进而增加心脏代谢疾病的风险 NCT03248882 PF-05221304+ PF-06865571 ACC1/2抑制剂+ DGAT2抑制剂 NAFLD 显著减少肝脏脂肪,有效缓解ACC抑制剂介导的TG升高 NCT03776175 PXL770 AMPK激活剂 NAFLD 未达到肝脏脂肪改善的主要结果,但治疗后代谢特征得到改善; 治疗耐受性良好 NCT03763877 TVB-2640 (ASC40) FASN抑制剂 NASH 以剂量依赖的方式显著降低LFC并改善生化、炎症和纤维化生物标志物 NCT03938246 Tipelukast PDE抑制剂 NAFLD 显著降低平均血清TG水平 NCT02681055 Namodenoson A3AR激动剂 NAFLD/NASH 改善ALT、AST和脂联素等各种肝脏参数; 安全性良好 NCT02927314 表 2 NASH临床试验Ⅲ期在研药物
Table 2. The ongoing phase Ⅲ clinical trials for NASH
药物 作用机制 研究人群 主要终点 NCT代码 奥贝胆酸 FXR激动剂 NASH F2/3期 (1)肝纤维化改善≥1级且无NASH病情恶化,或(2)在无肝纤维化恶化的情况下,NASH病情缓解 NCT02548351 Saroglitazar+ Vitamin E 联合用药 NASH NAFLD肝纤维化评分在8、16和24周的变化 NCT04193982 Lanifibranor PPAR泛激动剂 NASH F2/3期 (1)通过肝组织学评估Lanifibranor对脂肪性肝炎缓解和肝纤维化改善的影响; (2)通过复合终点测量评估Lanifibranor对延缓NASH疾病进展的效果 NCT04849728 Resmetirom THR-β激动剂 NASH F2/3期 (1)NASH消退(NAS降低≥2分且无肝纤维化恶化); (2)对长期复合终点的影响 NCT03900429 Aramchol SCD1抑制剂 NASH 开放性标签研究:在24、48和72周治疗期间与NASH和纤维化相关的组织学结局指标和测量几种无创检查的动力学; 双盲部分:(1)对肝组织学的影响; (2)对长期复合终点的影响 NCT04104321 Semaglutide GLP-1受体激动剂 NASH (1)从随机化(第0周)至第72周,脂肪性肝炎缓解且肝纤维化无恶化(是/否); (2)从随机化(第0周)至第72周,肝纤维化改善且脂肪性肝炎无恶化(是/否); (3)从随机化(第0周)至第240周,至首例肝脏相关临床事件的时间(复合终点) NCT04822181 MSDC-0602K MPC 糖尿病前期或糖尿病合并NAFLD或NASH (1)从基线到第26周糖化血红蛋白(HbA1c) 的变化; (2)从基线到第26周标准化AST、CK-18和HbA1c值的加权平均值(标准偏差)的变化 NCT03970031 GR-MD-02 Galectin-3拮抗剂 NASH 治疗组中在治疗78周时出现新的食管静脉曲张的患者比例 NCT04365868 -
[1] HUANG DQ, EL-SERAG HB, LOOMBA R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(4): 223-238. DOI: 10.1038/s41575-020-00381-6. [2] Recommendations of the Polish Group of Experts for Non-Alcoholic Fatty Liver Disease (PGE-NAFLD), TOMASIEWICZ K, FLISIAK R, et al. Recommendations for the management of non-alcoholic fatty liver disease (NAFLD)[J]. Clin Exp Hepatol, 2018, 4(3): 153-157. DOI: 10.5114/ceh.2018.78118. [3] BERARDO C, di PASQUA LG, CAGNA M, et al. Nonalcoholic fatty liver disease and non-alcoholic steatohepatitis: current issues and future perspectives in preclinical and clinical research[J]. Int J Mol Sci, 2020, 21(24). DOI: 10.3390/ijms21249646. [4] PAN M, CEDERBAUM AI, ZHANG YL, et al. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production[J]. J Clin Invest, 2004, 113(9): 1277-1287. DOI: 10.1172/JCI19197. [5] BUZZETTI E, PINZANI M, TSOCHATZIS EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD)[J]. Metabolism, 2016, 65(8): 1038-1048. DOI: 10.1016/j.metabol.2015.12.012. [6] HEBBARD L, GEORGE J. Animal models of nonalcoholic fatty liver disease[J]. Nat Rev Gastroenterol Hepatol, 2011, 8(1): 35-44. DOI: 10.1038/nrgastro.2010.191. [7] CAI J, ZHANG XJ, LI H. Progress and challenges in the prevention and control of nonalcoholic fatty liver disease[J]. Med Res Rev, 2019, 39(1): 328-348. DOI: 10.1002/med.21515. [8] POWELL EE, WONG VW, RINELLA M. Non-alcoholic fatty liver disease[J]. Lancet, 2021, 397(10290): 2212-2224. DOI: 10.1016/S0140-6736(20)32511-3. [9] SUMIDA Y, OKANOUE T, NAKAJIMA A, et al. Phase 3 drug pipelines in the treatment of non-alcoholic steatohepatitis[J]. Hepatol Res, 2019, 49(11): 1256-1262. DOI: 10.1111/hepr.13425. [10] YOUNOSSI ZM, RATZIU V, LOOMBA R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial[J]. Lancet, 2019, 394(10215): 2184-2196. DOI: 10.1016/S0140-6736(19)33041-7. [11] POCKROS PJ, FUCHS M, FREILICH B, et al. CONTROL: A randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients[J]. Liver Int, 2019, 39(11): 2082-2093. DOI: 10.1111/liv.14209. [12] SUMIDA Y, YONEDA M, OGAWA Y, et al. Current and new pharmacotherapy options for non-alcoholic steatohepatitis[J]. Expert Opin Pharmacother, 2020, 21(8): 953-967. DOI: 10.1080/14656566.2020.1744564. [13] FIORUCCI S, BIAGIOLI M, SEPE V, et al. Bile acid modulators for the treatment of nonalcoholic steatohepatitis (NASH)[J]. Expert Opin Investig Drugs, 2020, 29(6): 623-632. DOI: 10.1080/13543784.2020.1763302. [14] HARRISON SA, ROSSI SJ, PAREDES AH, et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis[J]. Hepatology, 2020, 71(4): 1198-1212. DOI: 10.1002/hep.30590. [15] FRANCQUE S, SZABO G, ABDELMALEK MF, et al. Nonalcoholic steatohepatitis: The role of peroxisome proliferator-activated receptors[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(1): 24-39. DOI: 10.1038/s41575-020-00366-5. [16] GAWRIEH S, NOUREDDIN M, LOO N, et al. Saroglitazar, a PPAR-α/γ agonist, for treatment of NAFLD: A randomized controlled double-blind phase 2 trial[J]. Hepatology, 2021, 74(4): 1809-1824. DOI: 10.1002/hep.31843. [17] STAELS B, RUBENSTRUNK A, NOEL B, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis[J]. Hepatology, 2013, 58(6): 1941-1952. DOI: 10.1002/hep.26461. [18] HARRISON S, RATZIU V, ANSTEE QM, et al. Baseline levels of NIS4TM and other fibrosis biomarkers and prediction of histological progression to advanced fibrosis in NASH[J]. Hepatology 72(S1): 896A. [19] LEFERE S, PUENGEL T, HUNDERTMARK J, et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages[J]. J Hepatol, 2020, 73(4): 757-770. DOI: 10.1016/j.jhep.2020.04.025. [20] VATNER DF, WEISMANN D, BEDDOW SA, et al. Thyroid hormone receptor-β agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways[J]. Am J Physiol Endocrinol Metab, 2013, 305(1): E89- E100. DOI: 10.1152/ajpendo.00573.2012. [21] HARRISON SA, BASHIR MR, GUY CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial[J]. Lancet, 2019, 394(10213): 2012-2024. DOI: 10.1016/S0140-6736(19)32517-6. [22] KHARITONENKOV A, ADAMS AC. Inventing new medicines: The FGF21 story[J]. Mol Metab, 2014, 3(3): 221-229. DOI: 10.1016/j.molmet.2013.12.003. [23] SANYAL A, CHARLES ED, NEUSCHWANDER-TETRI BA, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial[J]. Lancet, 2019, 392(10165): 2705-2717. DOI: 10.1016/S0140-6736(18)31785-9. [24] HARRISON SA, RUANE PJ, FREILICH BL, et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial[J]. Nat Med, 2021, 27(7): 1262-1271. DOI: 10.1038/s41591-021-01425-3. [25] DOBRZYN P, DOBRZYN A, MIYAZAKI M, et al. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver[J]. Proc Natl Acad Sci U S A, 2004, 101(17): 6409-6414. DOI: 10.1073/pnas.0401627101. [26] LOOMBA R, MOHSENI R, LUCAS KJ, et al. TVB-2640 (FASN Inhibitor) for the treatment of nonalcoholic steatohepatitis: FASCINATE-1, a randomized, placebo-controlled phase 2a trial[J]. Gastroenterology, 2021, 161(5): 1475-1486. DOI: 10.1053/j.gastro.2021.07.025. [27] KIM CW, ADDY C, KUSUNOKI J, et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation[J]. Cell Metab, 2017, 26(3): 576. DOI: 10.1016/j.cmet.2017.08.011. [28] CALLE RA, AMIN NB, CARVAJAL-GONZALEZ S, et al. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: Two parallel, placebo-controlled, randomized phase 2a trials[J]. Nat Med, 2021, 27(10): 1836-1848. DOI: 10.1038/s41591-021-01489-1. [29] LOOMBA R, MORGAN E, WATTS L, et al. Novel antisense inhibition of diacylglycerol O-acyltransferase 2 for treatment of non-alcoholic fatty liver disease: a multicentre, double-blind, randomised, placebo-controlled phase 2 trial[J]. Lancet Gastroenterol Hepatol, 2020, 5(9): 829-838. DOI: 10.1016/S2468-1253(20)30186-2. [30] CUSI K, ALKHOURI N, HARRISON SA, et al. Efficacy and safety of PXL770, a direct AMP kinase activator, for the treatment of non-alcoholic fatty liver disease (STAMP-NAFLD): a randomised, double-blind, placebo-controlled, phase 2a study[J]. Lancet Gastroenterol Hepatol, 2021, 6(11): 889-902. DOI: 10.1016/S2468-1253(21)00300-9. [31] ARMSTRONG MJ, GAUNT P, AITHAL GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study[J]. Lancet, 2016, 387(10019): 679-690. DOI: 10.1016/S0140-6736(15)00803-X. [32] HARTMAN ML, SANYAL AJ, LOOMBA R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes[J]. Diabetes Care, 2020, 43(6): 1352-1355. DOI: 10.2337/dc19-1892. [33] CUI J, PHILO L, NGUYEN P, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial[J]. J Hepatol, 2016, 65(2): 369-376. DOI: 10.1016/j.jhep.2016.04.021. [34] COLCA JR, MCDONALD WG, ADAMS WJ. MSDC-0602K, a metabolic modulator directed at the core pathology of non-alcoholic steatohepatitis[J]. Expert Opin Investig Drugs, 2018, 27(7): 631-636. DOI: 10.1080/13543784.2018.1494153. [35] HARRISON SA, ALKHOURI N, DAVISON BA, et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase Ⅱb study[J]. J Hepatol, 2020, 72(4): 613-626. DOI: 10.1016/j.jhep.2019.10.023. [36] SAFADI R, BRAUN M, FRANCIS A, et al. Randomised clinical trial: A phase 2 double-blind study of namodenoson in non-alcoholic fatty liver disease and steatohepatitis[J]. Aliment Pharmacol Ther, 2021, 54(11-12): 1405-1415. DOI: 10.1111/apt.16664. [37] FRIEDMAN SL, RATZIU V, HARRISON SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis[J]. Hepatology, 2018, 67(5): 1754-1767. DOI: 10.1002/hep.29477. [38] RATZIU V, SANYAL A, HARRISON SA, et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study[J]. Hepatology, 2020, 72(3): 892-905. DOI: 10.1002/hep.31108. [39] ANSTEE QM, NEUSCHWANDER-TETRI BA, WONG VW, et al. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: AURORA Phase 3 study design[J]. Contemp Clin Trials, 2020, 89: 105922. DOI: 10.1016/j.cct.2019.105922. [40] BRENNER C, GALLUZZI L, KEPP O, et al. Decoding cell death signals in liver inflammation[J]. J Hepatol, 2013, 59(3): 583-594. DOI: 10.1016/j.jhep.2013.03.033. [41] HSU MC, LIU SH, WANG CW, et al. JKB-122 is effective, alone or in combination with prednisolone in Con A-induced hepatitis[J]. Eur J Pharmacol, 2017, 812: 113-120. DOI: 10.1016/j.ejphar.2017.07.012. [42] HENDERSON NC, SETHI T. The regulation of inflammation by galectin-3[J]. Immunol Rev, 2009, 230(1): 160-171. DOI: 10.1111/j.1600-065X.2009.00794.x. [43] HARRISON SA, MARRI SR, CHALASANI N, et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis[J]. Aliment Pharmacol Ther, 2016, 44(11-12): 1183-1198. DOI: 10.1111/apt.13816. [44] CHALASANI N, ABDELMALEK MF, GARCIA-TSAO G, et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension[J]. Gastroenterology, 2020, 158(5): 1334-1345. e5. DOI: 10.1053/j.gastro.2019.11.296. [45] HARRISON SA, ABDELMALEK MF, CALDWELL S, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis[J]. Gastroenterology, 2018, 155(4): 1140-1153. DOI: 10.1053/j.gastro.2018.07.006. [46] MCPHERSON S, WILKINSON N, TINIAKOS D, et al. A randomised controlled trial of losartan as an anti-fibrotic agent in non-alcoholic steatohepatitis[J]. PLoS One, 2017, 12(4): e0175717. DOI: 10.1371/journal.pone.0175717. -



 PDF下载 ( 2729 KB)
PDF下载 ( 2729 KB)

 下载:
下载:


