第三代头孢菌素治疗社区获得性自发性细菌性腹膜炎的疗效预测模型
DOI: 10.3969/j.issn.1001-5256.2022.11.012
Establishment of a model for predicting the efficacy of third-generation cephalosporin in treatment of community-acquired spontaneous bacterial peritonitis
-
摘要:
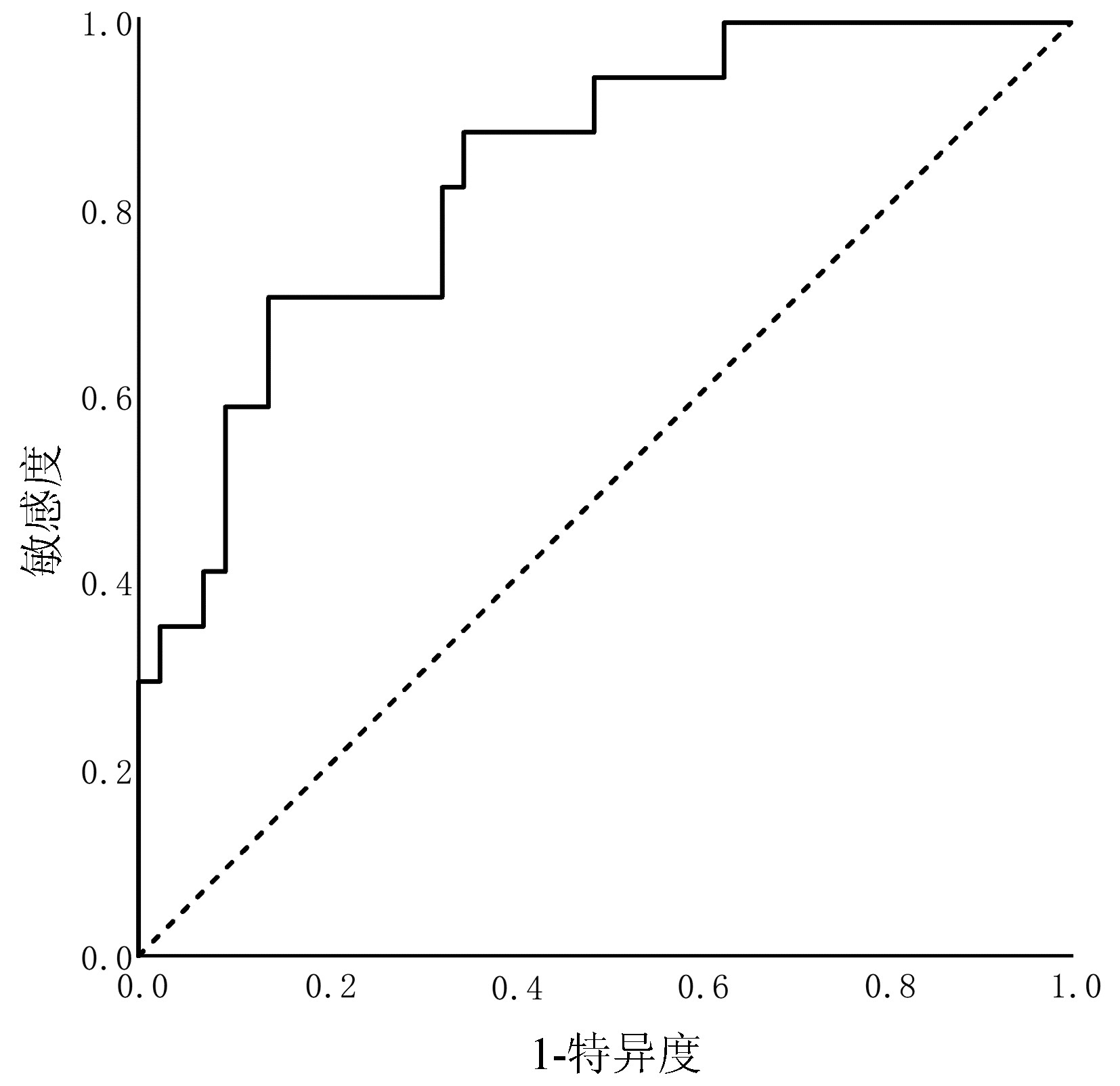
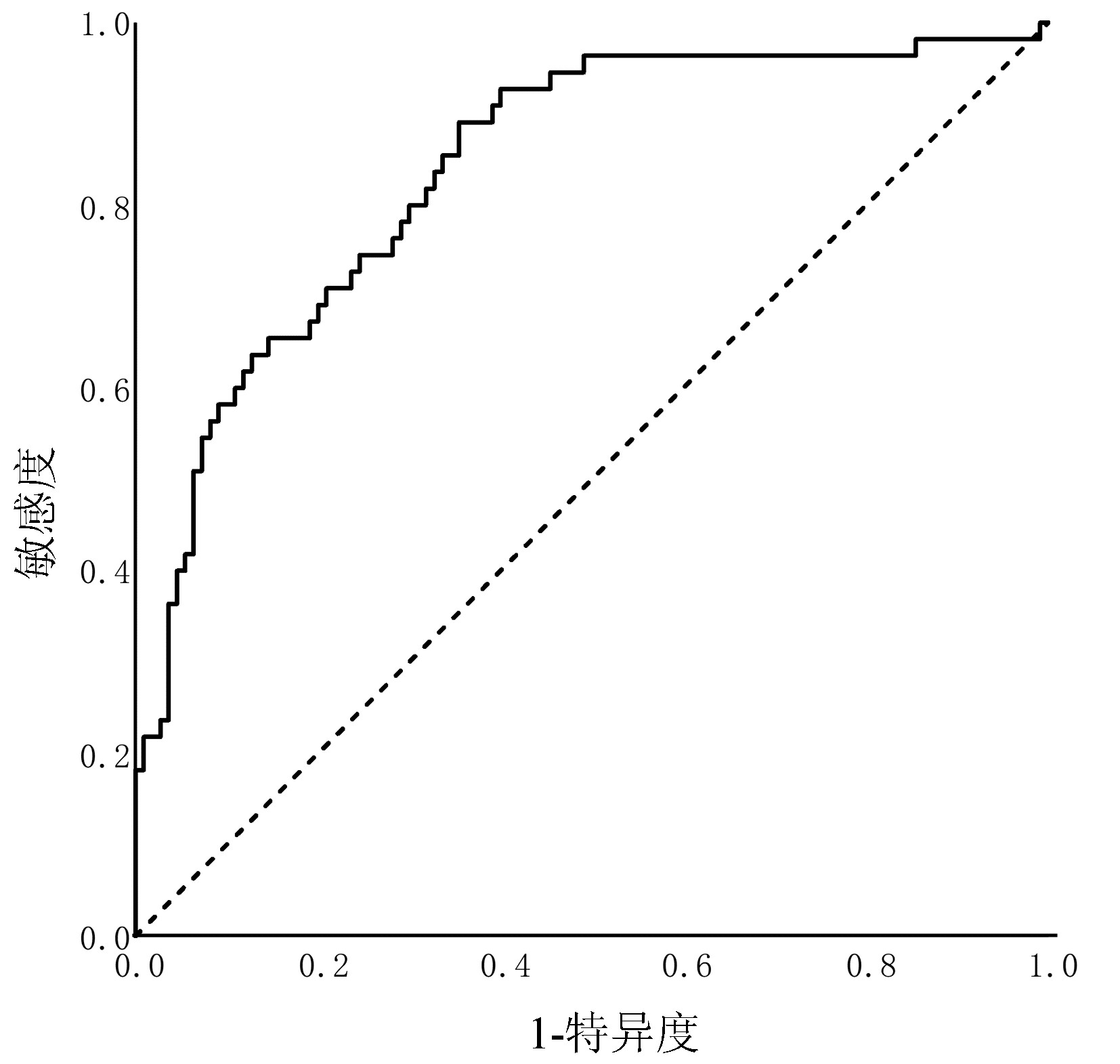
目的 探讨第三代头孢菌素(3rd GC)治疗社区获得性自发性细菌性腹膜炎(CASBP)疗效的预测因素,构建并验证3rd GC治疗CASBP的疗效预测模型。 方法 回顾性收集南昌市第九医院的肝硬化伴CASBP患者,均单用3rd GC进行初始治疗,依据疗效分为无效组与有效组。2013年—2018年住院的纳入建模库(无效组55例和有效组110例),2019年—2020年住院的纳入验证库(无效组17例和有效组43例)。在建模库中比较两组的一般资料、基础病、既往史、临床表现和实验室检查等指标,采用单因素分析(符合正态分布的计量资料组间比较采用t检验;不符合正态分布的计量资料组间比较采用Mann-Whitney U检验, 计数资料组间比较采用χ2检验或Fisher精确概率法)和二元Logistic回归分析识别疗效预测因素,根据Logistic回归方程构建疗效预测模型。采用受试者工作特征(ROC)曲线分别在建模库和验证库中对模型行内部验证和外部验证。 结果 全组以男性为主(占80.0%),平均(51.6±12.0)岁,肝硬化病因以乙型肝炎为主(66.7%),3rd GC总有效率为68.0%。建模库中,无效组的SBP首次发病占比、腹水多形核细胞(PMN)计数及腹水白细胞计数明显低于有效组(P值均<0.05),而无效组的广谱抗生素暴露占比与血小板均显著高于有效组(P值均<0.05);Logistic回归分析显示,SBP首次发病[比值比(OR)=0.158,95%置信区间(CI):0.064~0.392,P<0.001]、腹水PMN计数(OR=0.728,95%CI:0.530~0.998,P=0.046)、广谱抗生素暴露(OR=9.152,95%CI:1.513~55.351,P=0.016)及血小板(OR=1.012,95%CI:1.006~1.019,P<0.001)是3rd GC治疗无效的独立预测因素;疗效预测模型的ROC曲线下面积为0.840,依据约登指数最大原则,预测评分≥0.207是预测治疗无效的最佳界值,对应的约登指数为0.536,敏感度89.1%,特异度63.6%,阳性预测值55.1%,阴性预测值92.1%。验证库中,该模型的ROC曲线下面积为0.837。 结论 SBP首次发病和更高的腹水PMN计数是3rd GC治疗CASBP无效的保护因素,广谱抗生素暴露和更高的血小板是其危险因素,以此建立的3rd GC治疗CASBP的疗效预测模型具有良好的预测效能,具备临床应用潜能。 -
关键词:
- 肝硬化 /
- 腹膜炎 /
- 社区获得性感染 /
- 头孢菌素类 /
- Logistic模型
Abstract:Objective To investigate the factors for predicting the efficacy of third-generation cephalosporin (3rd GC) in the treatment of community-acquired spontaneous bacterial peritonitis (CASBP), and to establish and validate an efficacy predictive model for 3rd GC in the treatment of CASBP. Methods A retrospective analysis was performed for the clinical data of the patients with liver cirrhosis and CASBP who received 3rd GC monotherapy for initial treatment in The Ninth Hospital of Nanchang, and according to their treatment outcome, they were divided into non-response group and response group. The patients hospitalized from 2013 to 2018 were included in the modeling cohort (55 patients the non-response group and 110 in the response group), and those hospitalized from 2019 to 2020 were included in the validation cohort (17 patients in the non-response group and 43 in the response group). In the modeling cohort, the two groups were compared in terms of the indices including general information, underlying diseases, past history, clinical manifestation, and laboratory test results. Univariate analyses (the t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the chi-square test or the Fisher's exact test was used for comparison of categorical data between groups) and a binary Logistic regression analysis were used to identify efficacy predictors, and an efficacy predictive model was established based on the logistic regression equation. The receiver operating characteristic (ROC) curve was plotted to perform internal and external validations of the model in the modeling cohort and the validation cohort, respectively. Results The study population had a mean age of 51.6±12.0 years, and male patients accounted for 80.0%; hepatitis B was the main cause of liver cirrhosis (66.7%), and 3rd GC had an overall response rate of 68.0%. In the modeling cohort, compared with the response group, the non-response group had significantly lower proportion of patients with the first onset of SBP, polymorphonuclear (PMN) count in ascites, and leukocyte count in ascites (all P < 0.05), as well as significantly higher proportion of patients with exposure to broad-spectrum antibiotic and platelet count (both P < 0.05). The Logistic regression analysis showed that the first onset of SBP (odds ratio [OR]=0.158, 95% confidence interval [CI]: 0.064-0.392, P < 0.001), ascites PMN count (OR=0.728, 95%CI: 0.530-0.998, P=0.046), exposure to broad-spectrum antibiotic (OR=9.152, 95%CI: 1.513-55.351, P=0.016), and platelet count (OR=1.012, 95%CI: 1.006-1.019, P < 0.001) were independent predictive factors for non-response to 3rd GC. The efficacy predictive model had an area under the ROC curve (AUC) of 0.840, and based on the maximum Youden index, predictive score ≥ 0.207 was the optimal cut-off value for predicting non-response, with a corresponding Youden index of 0.536, a sensitivity of 89.1%, a specificity of 63.6%, a positive predictive value of 55.1%, and a negative predictive value of 92.1%. This model had an AUC of 0.837 in the validation cohort. Conclusion The first onset of SBP and higher ascites PMN count are the protective factors against non-response to 3rd GC for the treatment of CASBP, and exposure to broad-spectrum antibiotic and higher platelet count are the risk factors for such non-response. The model established for predicting the efficacy of 3rd GC in the treatment of CASBP has good predictive performance and thus holds promise for clinical application. -
Key words:
- Liver Cirrhosis /
- Peritonitis /
- Community-Acquired Infections /
- Cephalosporins /
- Logistic Models
-
表 1 建模库与验证库患者的临床特征
Table 1. Clinical characteristics of modeling cohort and validation cohort
指标 建模库(n=165) 验证库(n=60) 统计值 P值 男[例(%)] 133(80.6) 47(78.3) χ2=0.142 0.706 年龄(岁) 51.2±11.9 52.5±12.4 t=-0.615 0.539 2型糖尿病[例(%)] 21(12.7) 8(13.3) χ2=0.014 0.905 肝癌[例(%)] 43(26.1) 15(25.0) χ2=0.026 0.872 既往脾切除术[例(%)] 19(11.5) 5(8.3) χ2=0.467 0.494 广谱抗生素暴露[例(%)] 16(9.7) 6(10.0) χ2=0.005 0.946 SBP首次发病[例(%)] 127(77.0) 49(81.7) χ2=0.570 0.450 诺氟沙星预防SBP[例(%)] 4(2.4) 2(3.3) χ2=0.009 0.925 Child-Pugh评分 10.3±1.9 10.5±1.9 t=-0.675 0.500 MELD评分 14.1±7.2 13.9±6.7 t=0.202 0.840 肝性脑病[例(%)] 11(6.7) 7(11.7) χ2=1.495 0.222 消化道出血[例(%)] 1(0.6) 0(0) >0.05 中大量腹水[例(%)] 124(75.2) 48(80.0) χ2=0.574 0.449 腹痛[例(%)] 16(9.7) 5(8.3) χ2=0.097 0.756 腹部压痛[例(%)] 66(40.0) 25(41.7) χ2=0.051 0.822 腹部反跳痛[例(%)] 66(40.0) 26(43.3) χ2=0.202 0.653 体温(℃) 36.9±0.7 36.8±0.6 t=-0.324 0.746 血白细胞(×109/L) 5.9(4.2~8.0) 6.3(4.1~7.8) Z=-0.102 0.919 血红蛋白(g/L) 104.7±20.7 101.4±20.3 t=1.084 0.280 血小板(×1012/L) 89.0(53.5~135.0) 65.0(46.3~146.8) Z=-1.041 0.298 ALT(U/L) 38.0(23.0~80.5) 35.0(18.4~71.8) Z=-0.393 0.695 AST(U/L) 66.1(36.0~129.5) 79.0(36.4~132.0) Z=-0.354 0.723 TBil(μmol/L) 54.2(26.9~72.4) 56.3(29.7~70.6) Z=-0.058 0.954 Alb(g/L) 28.3±5.4 28.5±5.3 t=-0.269 0.788 血尿素氮(mmol/L) 5.3(3.6~7.4) 5.0(3.9~7.0) Z=-0.036 0.971 血肌酐(μmol/L) 72.0(58.5~89.0) 64.5(54.0~88.5) Z=-1.232 0.218 血钠(mmol/L) 135.5±5.4 135.6±5.4 t=-0.223 0.824 血碳酸氢盐(mmol/L) 22.5±3.8 23.0±3.6 t=-0.810 0.419 凝血酶原时间(s) 20.0±6.7 19.9±6.9 t=0.064 0.949 国际标准化比值 1.7±0.6 1.6±0.6 t=0.119 0.905 腹水PMN计数(×109/L) 0.400(0.290~1.000) 0.398(0.293~0.885) Z=-0.119 0.905 腹水白细胞计数(×109/L) 0.940(0.600~1.940) 0.945(0.603~1.873) Z=-0.161 0.872 表 2 建模库单因素分析的阳性结果
Table 2. Positive results of univariate analysis in modeling cohort
指标 无效组(n=55) 有效组(n=110) 统计值 P值 广谱抗生素暴露[例(%)] 11(20.0) 5(4.5) χ2=10.001 0.002 SBP首次发病[例(%)] 30(54.5) 97(88.2) χ2=23.403 <0.001 血小板(×1012/L) 125.0(81.0~178.0) 72.0(49.0~119.0) Z=-4.328 <0.001 腹水PMN计数(×109/L) 0.340(0.270~0.540) 0.475(0.300~1.583) Z=-3.465 0.001 腹水白细胞计数(×109/L) 0.750(0.570~1.290) 1.140(0.608~2.428) Z=-2.235 0.025 表 3 建模库的二元Logistic回归分析结果
Table 3. Binary Logistic regression analysis in modeling cohort
指标 回归系数 标准误 Wald值 P值 OR(95%CI) SBP首次发病(是=1,否=0) -1.844 0.463 15.843 <0.001 0.158(0.064~0.392) 腹水PMN计数(×109/L) -0.318 0.162 3.853 0.046 0.728(0.530~0.998) 广谱抗生素暴露(是=1,否=0) 2.214 0.918 5.813 0.016 9.152(1.513~55.351) 血小板(×1012/L) 0.012 0.003 14.822 <0.001 1.012(1.006~1.019) 常数项 -0.543 0.519 1.093 0.029 0.581(NA) -
[1] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of ascites and complications in cirrhosis[J]. J Chin Hepatol, 2017, 33(10): 1847-1863. DOI: 10.3969/j.issn.1001-5256.2017.10.003.中华医学会肝病学分会. 肝硬化腹水及相关并发症的诊疗指南[J]. 临床肝胆病杂志, 2017, 33(10): 1847-1863. DOI: 10.3969/j.issn.1001-5256.2017.10.003. [2] BIGGINS SW, ANGELI P, GARCIA-TSAO G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases[J]. Hepatology, 2021, 74(2): 1014-1048. DOI: 10.1002/hep.31884. [3] FIORE M, GENTILE I, MARAOLO AE, et al. Are third-generation cephalosporins still the empirical antibiotic treatment of community-acquired spontaneous bacterial peritonitis? A systematic review and meta-analysis[J]. Eur J Gastroenterol Hepatol, 2018, 30(3): 329-336. DOI: 10.1097/MEG.0000000000001057. [4] PUGH RN, MURRAY-LYON IM, DAWSON JL, et al. Transection of the oesophagus for bleeding oesophageal varices[J]. Br J Surg, 1973, 60(8): 646-649. DOI: 10.1002/bjs.1800600817. [5] KAMATH PS, WIESNER RH, MALINCHOC M, et al. A model to predict survival in patients with end-stage liver disease[J]. Hepatology, 2001, 33(2): 464-470. DOI: 10.1053/jhep.2001.22172. [6] SUNJAYA DB, LENNON RJ, SHAH VH, et al. Prevalence and predictors of third- generation cephalosporin resistance in the empirical treatment of spontaneous bacterial peritonitis[J]. Mayo Clin Proc, 2019, 94(8): 1499-1508. DOI: 10.1016/j.mayocp.2018.12.036.. [7] PEDUZZI P, CONCATO J, KEMPER E, et al. A simulation study of the number of events per variable in Logistic regression analysis[J]. J Clin Epidemiol, 1996, 49(12): 1373-1379. DOI: 10.1016/s0895-4356(96)00236-3. [8] TAY P, XIAO J, TAN D, et al. An epidemiological meta-analysis on the worldwide prevalence, resistance, and outcomes of spontaneous bacterial peritonitis in cirrhosis[J]. Front Med (Lausanne), 2021, 8: 693652. DOI: 10.3389/fmed.2021.693652. [9] SARWAR S, TARIQUE S, WARIS U, et al. Cephalosporin resistance in community acquired spontaneous bacterial peritonitis[J]. Pak J Med Sci, 2019, 35(1): 4-9. DOI: 10.12669/pjms.35.1.17. [10] Ministry of Health of the People's Republic of China. Regulations for clinical application of antibacterial agents[J]. Chin J Clin Infect Dis, 2012, 5(4): 193-196. DOI:10.3760/cma.j.issn. 1674-2397.2012.04.001.中华人民共和国卫生部. 抗菌药物临床应用管理办法[J]. 中华临床感染病杂志, 2012, 5(4): 193-196. DOI: 10.3760/cma.j.issn.1674-2397.2012.04.001. [11] ZHU LC, GAN DK, HU QQ, et al. Predictive factors for third-generation cephalosporin- resistant spontaneous bacterial peritonitis[J]. J Chin Hepatol, 2019, 35(7): 1501-1504. DOI: 10.3969/j.issn.1001-5256.2019.07.016.朱龙川, 甘达凯, 胡青青, 等. 第三代头孢菌素耐药的自发性细菌性腹膜炎发生的预测因素[J]. 临床肝胆病杂志, 2019, 35(7): 1501-1504. DOI: 10.3969/j.issn.1001-5256.2019.07.016. [12] ARIZA X, LORA-TAMAYO J, CASTELLOTE J, et al. Polymorphonuclear counts in ascitic fluid and microorganisms producing spontaneous bacterial peritonitis: an under-recognized relationship[J]. Scand J Gastroenterol, 2013, 48(10): 1213-1221. DOI: 10.3109/00365521.2013.832367. [13] ZHANG GY, TIAN CY, HU H. Recent progress in immune dysfunction of neutrophils in patients with cirrhosis[J]. Chin Gen Pract, 2021, 24(21): 2734-2743. DOI: 10.12114/j.issn. 1007-9572.2021.00.457.张国远, 田彩云, 胡晗. 肝硬化患者中性粒细胞免疫功能障碍的研究进展[J]. 中国全科医学, 2021, 24(21): 2734-2743. DOI: 10.12114/j.issn.1007-9572.2021.00.457. [14] MISHRA D, DAS AK, CHAPAGAIN RH, et al. Thrombocytosis as a predictor of serious bacterial infection in febrile infants[J]. J Nepal Health Res Counc, 2019, 16(41): 401-404. DOI: 10.33314/jnhrc.v16i41.1673. [15] YUAN SN, ZHAO JJ, HAN XY, et al. Influence of bacterial and fungal infections on platelets[J]. Chin J Nosocomiol, 2016, 26(22): 5083-5086. DOI: 10.11816/cn.ni.2016-154312.袁胜男, 赵俊暕, 韩晓燕, 等. 细菌与真菌感染对血小板的影响研究[J]. 中华医院感染学杂志, 2016, 26(22): 5083-5086. DOI: 10.11816/cn.ni.2016-154312. -



 PDF下载 ( 2187 KB)
PDF下载 ( 2187 KB)

 下载:
下载: