KRAS基因突变对经肝动脉化疗栓塞术治疗的中晚期原发性肝癌患者预后的预测价值
DOI: 10.3969/j.issn.1001-5256.2022.11.015
Prognostic value of KRAS mutations in patients with advanced primary liver cancer treated with transcatheter arterial chemoembolization
-
摘要:
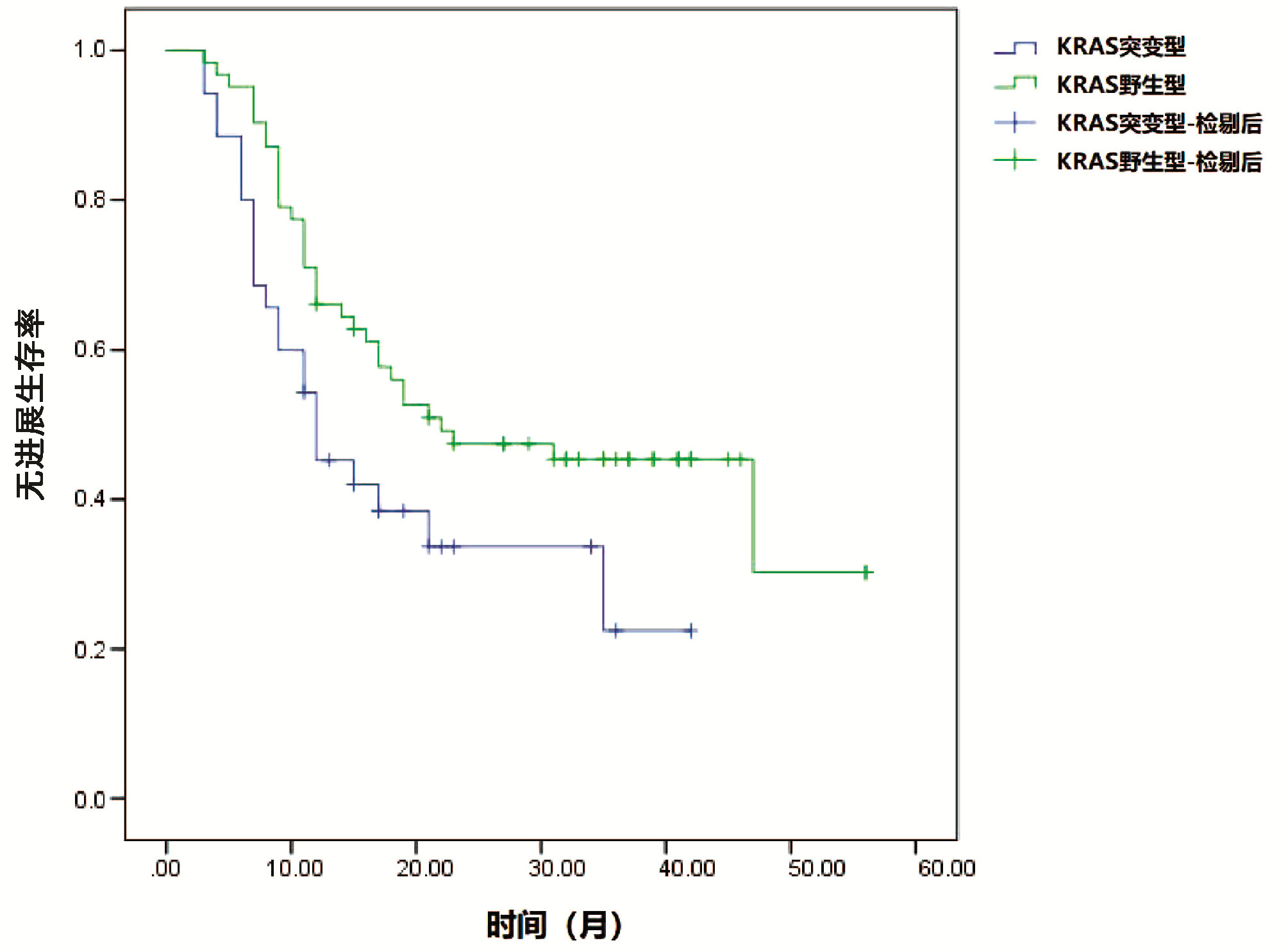
目的 探讨KRAS基因状态对经肝动脉化疗栓塞(TACE)治疗中晚期肝癌患者预后的预测价值。 方法 选择2017年4月—2020年5月在海南省第三人民医院接受TACE治疗的中晚期肝癌患者97例为研究对象。检测患者KRAS基因突变状态,并分析KRAS基因突变状态与患者TACE治疗预后的关系。计量资料两组间比较采用t检验,计数资料两组间比较采用χ2检验,生存分析绘制Kaplan-Meier生存曲线,生存曲线比较采用Log-rank检验,对可能影响患者预后的各因素进行Cox回归分析。 结果 97例患者中共检出KRAS基因突变患者34例(35.05%),其中检出12号密码子突变患者21例(61.76%),13号密码子突变患者13例(38.24%)。KRAS基因突变与患者肝硬化、肝内转移、肿瘤数目均显著相关(χ2值分别为0.035、3.965、6.593,P值均<0.05)。Kaplan-Meier生存分析结果显示,KRAS基因野生型患者无进展生存期及总体生存时间均显著优于KRAS突变型(χ2值分别为4.465、4.280,P值均<0.05)。Cox分析结果显示,KRAS基因状态、肝内转移、肿瘤数目、BCLC分期进入回归模型为影响患者总体生存预后的重要因素(P值均<0.05)。 结论 KRAS基因突变在肝癌患者中较为常见,KRAS基因突变与患者TACE术后不良预后密切相关,可成为患者临床预后监测的潜在指标。 Abstract:Objective To assess the prognostic value of KRAS mutation in patients with advanced primary liver cancer treated with transcatheter arterial chemoembolization (TACE). Methods Ninety-seven patients with advanced primary liver cancer who received TACE treatment in The Third People's hospital from April 2017 to May 2020 were included. The mutation status of KRAS was detected, and its relationship with the prognosis of TACE was investigated. The t-test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. Survival analysis was performed using Kaplan-Meier survival curve and compared using Log-rank test. Cox regression analysis was performed to identify the prognostic factors. Results Among 97 patients with advanced liver cancer, KRAS mutations were detected in 34 patients (35.05%), including 21 patients with codon 12 mutation (61.76%) and 13 patients with codon 13 mutation (38.24%). KRAS mutation was associated with liver cirrhosis, intrahepatic metastasis and the number of tumors (χ2=0.035, 3.965, and 6.593, all P < 0.05). Survival analysis showed that the progression free survival and overall survival were significantly longer in KRAS wild-type patients than in KRAS mutant patients (χ2=4.465 and 4.280, all P < 0.05). Multivariate Cox analysis revealed that KRAS mutation, intrahepatic metastasis, number of tumors and BCLC stage were important factors affecting the overall survival and prognosis of patients (all P < 0.05). Conclusion KRAS mutation is common in patients with advanced primary liver cancer and is closely associated with a poor prognosis after TACE. It may become a potential indicator of clinical prognosis. -
Key words:
- Liver Neoplasms /
- Genes /
- Chemoembolization, Therapeutic /
- Prognosis
-
全球有超过2.4亿慢性HBV感染者,若不及时进行有效、规范的治疗,15%~40%的患者会进展为肝硬化,最终导致肝衰竭和肝细胞癌(HCC)[1]。虽然乙型肝炎疫苗的接种使HBV感染的患病率逐年下降,但多数亚洲地区仍归为中至高流行区。我国HBsAg流行率为5%~6%,但因为人口基数大,所以仍存在许多的慢性HBV感染者,其中需要治疗的慢性乙型肝炎(CHB)患者约有2000万~3000万[2-3];因此慢性HBV感染依然是我国的重大公共卫生问题。当前用于抗病毒治疗的核苷(酸)类似物(nucleos(t)ide analogues, NAs)虽可有效抑制病毒复制,延缓疾病进展,但对肝细胞核内的共价闭合环状DNA(covalently closed circular DNA,cccDNA)均无明确作用,使得病毒无法完全清除。cccDNA作为HBV复制的原始模板,检测肝内cccDNA水平是评价抗病毒疗效及停止治疗的重要指标[4-5],由于肝活检为侵入性操作,cccDNA在肝组织内分布不均,松弛环状双链DNA(relaxed circularDNA,rcDNA)的存在影响cccDNA含量等因素导致cccDNA检测难以在临床广泛开展[6-7]。因此需要寻找能反映肝内cccDNA活性且方便操作的临床替代指标。近年来HBV RNA作为新的血清学标志物被广泛提出,因为其只能来自肝内cccDNA, 所以能更好的反映HBV转录活性,本研究主要探讨血清HBV RNA在HBeAg阳性CHB患者不同时期的表达水平及检测价值。
1. 资料与方法
1.1 研究对象
收集2019年8月—2020年12月在杭州市西溪医院肝病科门诊及住院部诊治的CHB患者,纳入标准:(1)诊断符合《慢性乙型肝炎防治指南(2019年版)》[8];(2)准备接受或已接受NAs抗病毒治疗。排除标准:(1)合并HAV、HCV、巨细胞病毒等其他嗜肝病毒感染;(2)合并HIV感染;(3)HCC患者;(4)代谢性肝病、自身免疫性肝病及近期使用肝损伤性药物者;(5)合并其他系统恶性肿瘤或严重疾病者;(6)研究者认为不适合入组的其他情况。
1.2 实验室检测
本研究使用的HBV RNA定量检测试剂盒由湖南圣湘科技有限公司提供,检测原理通过逆转录HBV核酸中的pgRNA(经DNA酶消化),利用针对HBV pgRNA序列设计的一组特异性引物与荧光探针,配以PCR反应液,在荧光定量PCR仪上,应用一步法RT实时荧光定量PCR检测技术,通过荧光信号的变化实现HBV pgRNA的定量检测,HBV RNA检测下限为50拷贝/mL。应用化学发光免疫分析法在美国雅培Alinity i全自动化学发光免疫分析仪上行HBV血清学标志物检测;用HBV DNA定量检测试剂盒,在ABI7500荧光定量PCR仪上行HBV DNA检测;HBV DNA检测下限为30 IU/mL;用Beckman Coulter AU5831全自动生化分析仪检测ALT(正常范围9~50 U/L)、AST(正常范围15~40 U/L)。
1.3 伦理学审查
本研究经杭州市西溪医院伦理委员会批准, 批号:2019年(科)伦审第21号,所有患者均签署知情同意书。
1.4 统计学方法
采用SPSS 25.0进行统计学处理,正态分布的计量资料用x ±s表示,2组间比较采用独立样本t检验;非正态分布的计量资料用M(P25~P75)表示,2组间比较采用Mann-Whitney U检验;计数资料组间比较采用χ2检验;采用Pearson或Spearman相关分析描述两变量间的相关性。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
本研究共纳入61例CHB患者,平均年龄(39.95±9.88)岁。按HBeAg及HBV DNA状态分为3组:HBeAg阳性CHB[HBeAg(+)、HBV DNA(+)]未治患者,HBeAg血清学转换前[HBeAg(+)、HBV DNA(-)]经治患者,HBeAg血清学转换后[HBeAg(-)、HBV DNA(-)]经治患者(表 1)。
表 1 纳入患者分组情况及基线资料组别 例数 男 女 年龄(岁) HBeAg(+)、HBV DNA(+) 22 13 9 35.36±7.55 HBeAg(+)、HBV DNA(-) 17 11 6 39.35±7.98 HBeAg(-)、HBV DNA(-) 22 13 9 45.00±11.16 2.2 不同时期血清HBV RNA的表达水平
HBeAg阳性CHB未治患者血清HBV RNA阳性率100%(22/22),HBV RNA载量最大值为9 log10拷贝/mL,平均为7 log10拷贝/mL;HBeAg血清学转换前经治患者血清HBV RNA阳性率88.2%(15/17),HBV RNA载量最大值为5 log10拷贝/mL,平均4 log10拷贝/mL;HBeAg血清学转换后经治患者血清HBV RNA阳性率22.7%(6/22),HBV RNA载量最大值为4 log10拷贝/mL。
2.3 经治HBeAg阳性组与HBeAg阴性组血清学指标比较
经治HBeAg阳性组的HBV RNA阳性率显著高于HBeAg阴性组,差异有统计学意义(P<0.001), 2组间HBV RNA、HBsAg水平比较,差异均有统计学意义(P值均<0.05)(表 2)。
表 2 经治患者HBeAg阳性组与HBeAg阴性组临床资料比较项目 HBeAg阳性组(n=17) HBeAg阴性组(n=22) 统计值 P值 男/女(例) 11/6 13/9 0.753 年龄(岁) 39.35±7.98 45.00±11.16 t=-1.77 0.086 HBV RNA阳性[例(%)] 15(88.2) 6(27.3) <0.001 HBV RNA(log10拷贝/mL) 4.05(3.01~5.18) 1.40(1.40~1.83) Z=-4.44 <0.001 HBsAg(log10IU/mL) 3.24(2.86~3.50) 2.76(2.07~3.35) Z=-2.41 0.016 ALT(U/L) 22.24±10.83 25.09±11.76 t=-0.78 0.442 AST(U/L) 23.00±4.48 23.9±14.23 t=-0.65 0.521 2.4 HBV DNA阳性组与HBV DNA阴性组血清学指标比较
CHB患者NAs治疗实现HBV DNA阴转时,肝功能复常,ALT、AST水平降低,HBV RNA、HBsAg水平也均有所降低(P值均<0.001)(表 3)。对于治疗后HBV DNA阴转的CHB患者,Spearman相关分析示,血清HBV RNA与血清HBsAg无相关性(P=0.091)。
表 3 HBV DNA阳性组与HBV DNA阴性组临床资料比较项目 HBV DNA阳性组(n=22) HBV DNA阴性组(n=39) 统计值 P值 男/女(例) 13/9 24/15 χ2=0.035 0.851 年龄(岁) 35.36±7.55 42.54±10.18 t=-2.88 0.005 HBV RNA(log10拷贝/mL) 7.62(6.51~8.55) 1.94(1.40~4.04) Z=-6.16 <0.001 HBsAg(log10IU/mL) 3.94(3.26~4.23) 2.90(2.61~3.44) Z=-4.07 <0.001 ALT(U/L) 249.50(114.25~664.50) 20.00(16.00~31.00) Z=-6.45 <0.001 AST(U/L) 106.00(63.75~418.75) 23.00(21.00~27.00) Z=-6.45 <0.001 HBV DNA(log10IU/ml) 7.21±1.23 - 2.5 不同时期HBV RNA与HBsAg、HBV DNA的相关性
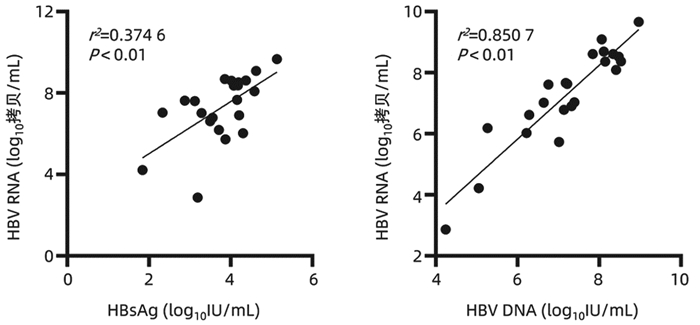
在HBeAg阳性CHB未治患者中,HBV RNA与HBsAg(r=0.612,P<0.01)、HBV DNA(r=0.922, P<0.01)均具有较强的相关性(图 1);在HBeAg血清学转换前和HBeAg血清学转换后的经治患者中,HBV RNA与HBsAg无相关性(P>0.05);3组患者HBV RNA与年龄、ALT、AST均无相关性(P值均>0.05)。
3. 讨论
CHB是导致肝硬化、肝衰竭、HCC的主要危险因素,每年约有80万人死于HBV感染相关性疾病[9],因此如何有效的管理CHB患者仍是当前临床工作的热点和难点。随着对HBV RNA的研究深入,已知HBV感染患者血清中的HBV RNA就是前基因组RNA(pgRNA),即3.5 kb mRNA;pgRNA是HBV复制的中间产物,利用cccDNA作为模板在病毒核衣壳内转录,最后释放完整的子代病毒,因此血清HBV RNA与HBsAg不同,其只能来自于肝内cccDNA;而NAs是通过取代HBV复制过程中聚合酶区的核苷来抑制病毒复制,并不能影响cccDNA转录生成mRNA,由此可以推断HBV RNA与肝内cccDNA相关,检测外周血HBV RNA水平可以反映肝内cccDNA的转录活性。因此相比于HBsAg、HBV DNA等传统的血清学指标,血清HBV RNA水平可以更好的反映肝内病毒复制水平,其能作为CHB患者管理的新型标志物[10-11]。
HBeAg阳性CHB患者在接受抗病毒药物治疗后会逐步实现HBV DNA阴转、HBeAg血清学转换等目标,本研究通过观察HBeAg阳性的CHB患者不同时期的HBV RNA表达水平,发现未治疗的22例CHB患者血清中均能检测出较高的血清HBV RNA水平;39例治疗后HBV DNA阴转的CHB患者中仍有21例可以检测出HBV RNA,这表明在评估病毒复制方面HBV RNA比HBV DNA更灵敏,即使是在HBV DNA持续低于检测下限且发生HBeAg血清学转换也有部分患者血清HBV RNA阳性,意味HBeAg的消失只能表示病毒复制减弱,肝内cccDNA仍可能存在低水平的转录。
对HBeAg阳性CHB患者而言,发生HBeAg血清学转换是比较满意的终点,是发生HBsAg阴转的基本条件。本文通过比较治疗后HBV DNA阴转的HBeAg阳性组与HBeAg阴性组,发现HBeAg阳性组HBV RNA阳性率显著高于HBeAg阴性组(88.2% vs 27.3%),两组间HBV RNA、HBsAg水平差异也具有统计学意义,因HBV RNA只能来自于肝内cccDNA, 提示HBeAg阳性CHB患者肝内cccDNA转录活性更高。因此临床上对HBeAg阳性的CHB患者要努力实现HBeAg血清学转换。
通过分析HBV DNA阳性组与HBV DNA阴性组患者,发现接受NAs治疗后HBV DNA、HBV RNA均会发生下降,这是因为NAs类药物抑制pgRNA的逆转录,致使DNA合成受阻,而长时间接受NAs治疗使rcDNA的形成受到抑制,影响cccDNA池的回补,被感染的肝细胞数量减少,进一步导致HBV RNA的生成也减少;另外接受NAs治疗的CHB人群在病毒清除的过程中可能会促进机体免疫应答的能力从而减少cccDNA池。但是在临床上发现即使CHB患者经过长期NAs抗病毒治疗,能实现完全治愈的患者极为罕见,可能是只需要极少部分逆转录酶存在活性就能对cccDNA池进行补充,因此对大部分CHB患者而言需要长期口服NAs抗病毒治疗。
本研究发现,未接受治疗的CHB患者HBV RNA与HBsAg(r=0.612,P<0.01)、HBV DNA(r=0.922, P<0.01)均具有较强的正相关,这种血清学间良好的相关性有助于基层医院或者医疗资源匮乏地区,使用HBsAg定量水平反映HBeAg阳性CHB患者体内病毒复制水平,而另外两组HBV DNA阴性的CHB患者HBV RNA与HBsAg无相关性,这与Mak等[12]的研究结果一致,随着治疗时间的延长,HBV RNA与HBsAg相关性逐渐下降,从一定程度反映了治疗后的HBsAg水平不能准确反映肝内cccDNA转录活性。
综上所述,对未接受抗病毒治疗的CHB患者,血清HBV RNA与其他血清学标志物有一定的相关性,对NAs治疗后HBV DNA阴转的患者有望使用HBV RNA继续监测肝内病毒复制活性,为CHB患者长期抗病毒治疗提供依据。但本研究样本量较少,相关结论有待进一步证实,后续可以在未治疗组继续纳入合适患者并进行前瞻性队列研究,观察HBeAg阳性CHB患者在NAs治疗过程中HBV RNA的动态变化。
-
表 1 KRAS基因突变状态与患者临床特征的关系
Table 1. The relationship between KRAS gene mutation status and clinical characteristics of patients
临床资料 KRAS突变型
(n=34)KRAS野生型
(n=63)统计值 P值 性别(例) χ2=0.370 0.543 男 20 41 女 14 22 年龄(岁) 55.12±14.21 56.93±10.04 t=0.730 0.468 乙型肝炎病史(例) χ2=0.057 0.811 阴性 30 56 阳性 4 7 ALT(U/L) 49.85±14.20 51.24±15.22 t=0.439 0.662 凝血酶原时间(s) 12.83±1.84 13.02±2.10 t=0.443 0.659 总胆红素(mmol/L) 17.94±3.73 18.05±3.16 t=0.153 0.878 白蛋白(g/L) 40.27±6.49 40.53±5.98 t=0.198 0.843 AFP(ng/L) 438.95±109.32 409.64±97.58 t=1.353 0.179 肿瘤大小(cm) 7.39±2.30 7.02±2.15 t=0.789 0.432 肝硬化(例) χ2=0.035 0.852 有 27 49 无 7 14 肝内转移(例) χ2=3.965 0.047 有 16 17 无 18 46 肿瘤数目(例) χ2=6.593 0.010 单个 23 56 多个 11 7 腹水(例) χ2=0.057 0.811 无 31 55 有 3 8 Child-Pugh分级(例) χ2=0.056 0.812 A 24 43 B 10 20 BCLC分期(例) χ2=0.074 0.785 B 22 39 C 12 24 表 2 影响患者预后的Cox分析
Table 2. Cox analysis affecting patient outcomes
自变量 RR 95%CI P值 性别(男性=0,女性=1) 2.734 0.983~7.935 0.283 年龄(≥60岁=0,<60岁=1) 3.038 0.627~10.293 0.276 KRAS基因状态(突变型=0,野生型=1) 18.273 5.584~98.305 0.001 乙型肝炎病史(有=0,无=1) 5.484 0.719~9.380 0.193 ALT(≥40 U/L=0,<40 U/L=1) 2.079 0.417~12.953 0.389 凝血酶原时间(≥12 s=0,<12 s=1) 1.092 0.271~9.182 0.849 总胆红素(≥18 nmol/L=0,<18
nmol/L=1)1.684 0.495~7.293 0.711 白蛋白(≥40 g/L=0,<40 g/L=1) 2.087 0.408~11.235 0.419 AFP(≥400 ng/L=0,<400 ng/L=1) 2.193 0.602~11.293 0.619 肿瘤大小(≥7 cm=0,<7 cm=1) 2.183 0.485~7.304 0.280 肝硬化(有=0,无=1) 1.804 0.198~10.237 0.695 肝内转移(有=0,无=1) 11.475 3.029~56.490 0.018 肿瘤数目(单发=0,多发=1) 10.038 2.973~19.328 0.021 腹水(有=0,无=1) 2.013 0.421~8.106 0.174 Child-Pugh分级(A=0,B=1) 2.189 0.569~7.491 0.209 BCLC分期(B=0,C=1) 12.384 2.385~29.305 0.011 -
[1] LI X, RAMADORI P, PFISTER D, et al. The immunological and metabolic landscape in primary and metastatic liver cancer[J]. Nat Rev Cancer, 2021, 21(9): 541-557. DOI: 10.1038/s41568-021-00383-9. [2] YU SX, ZHOU WP. Progress and hot spots of comprehensive treatment for primary liver cancer[J]. Chin J Dig Surg, 2021, 20(2): 163-170. DOI: 10.3760/cma.j.cn115610-20201211-00776.袁声贤, 周伟平. 原发性肝癌综合治疗的进展和热点[J]. 中华消化外科杂志, 2021, 20(2): 163-170. DOI: 10.3760/cma.j.cn115610-20201211-00776. [3] LIU CX, CHANG K, NA WL, et al. Role of differential expression and regulatory mechanism of miR-152-3 p target proteins in the recurrence of hepa-tocellular carcinoma[J]. J Clin Hepatol, 2021, 37(2): 364-369. DOI: 10.3969/j.issn.1001-5256.2021.02.023.刘晨霞, 常凯, 那琬琳, 等. miR-152-3p靶蛋白差异表达及调控机制在肝癌复发中的作用[J]. 临床肝胆病杂志, 2021, 37(2): 364-369. DOI: 10.3969/j.issn.1001-5256.2021.02.023. [4] BLACKBURN H, WEST S. Management of postembolization syndrome following hepatic transarterial chemoembolization for primary or metastatic liver cancer[J]. Cancer Nurs, 2016, 39(5): E1-E18. DOI: 10.1097/NCC.0000000000000302. [5] CHENG YR, YAN D, YANG JD, et al. Effect of hepatic artery chemoembolization combined with sorafenib in the treatment of primary liver cancer and its influence on patients' immune function[J]. Clin J Med Offic, 2021, 49(3): 290-291. DOI: 10.16680/j.1671-3826.2021.03.16.程瑜蓉, 严冬, 杨建东, 等. 肝动脉栓塞化疗联合索拉非尼在原发性肝癌治疗中应用效果及对患者免疫功能影响[J]. 临床军医杂志, 2021, 49(3): 290-291. DOI: 10.16680/j.1671-3826.2021.03.16. [6] RECK M, MOK T, NISHIO M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial[J]. Lancet Respir Med, 2019, 7(5): 387-401. DOI: 10.1016/S2213-2600(19)30084-0. [7] CUI FQ, LI T, WANG Z, et al. A pilot study on cetuximab and KRAS gene mutation in treatment of patients with primary liver can-cer[J]. J Prac Hepatol, 2019, 22(4): 565-568. DOI: 10.3969/j.issn.1672-5069.2019.04.029.崔发强, 李涛, 王铮, 等. 原发性肝癌患者外周血KRAS基因水平及其对西妥昔单克隆抗体治疗疗效的影响[J]. 实用肝脏病杂志, 2019, 22(4): 565-568. DOI: 10.3969/j.issn.1672-5069.2019.04.029. [8] PAN CF, ZHAO SK, LI Y, et al. Mutation study of related genes in non-small cell lung cancer drug targeting sites[J]. Hainan Med J, 2018, 29(19): 2696-2698. DOI: 10.3969/j.issn.1003-6350.2018.19.009.潘长芳, 赵胜科, 李洋, 等. 非小细胞肺癌药物靶向位点的相关基因突变研究[J]. 海南医学, 2018, 29(19): 2696-2698. DOI: 10.3969/j.issn.1003-6350.2018.19.009. [9] WU ST. Application of contrast-enhanced CT Scan in curative effect evaluation of primary hepatocellular carcinoma after TACE[J]. Chin J CT and MRI, 2022, 20(3): 91-93. DOI: 10.3969/j.issn.1672-5131.2022.03.030.吴水天. CT增强扫描在评估原发性肝细胞肝癌TACE术后疗效中的应用[J]. 中国CT和MRI杂志, 2022, 20(3): 91-93. DOI: 10.3969/j.issn.1672-5131.2022.03.030. [10] KIM D, LEE JH, MOON H, et al. Development and evaluation of an ultrasound-triggered microbubble combined transarterial chemoembolization (TACE) formulation on rabbit VX2 liver cancer model[J]. Theranostics, 2021, 11(1): 79-92. DOI: 10.7150/thno.45348. [11] CHEN XW, JIANG JW, LIN FH. Longdan xiegan decoction in the treatment of embolism syndrome after interventional therapy on primary hepatocellular carcinoma[J]. J Nangjing Univ Tradit Chin Med, 2016, 32(3): 224-228. DOI: 10.14148/j.issn.1672-0482.2016.0224.陈学武, 姜靖雯, 林福煌. 龙胆泻肝汤治疗原发性肝癌TACE术后栓塞综合征的疗效观察[J]. 南京中医药大学学报, 2016, 32(3): 224-228. DOI: 10.14148/j.issn.1672-0482.2016.0224. [12] YOU GM, JING BL, PAN Q, et al. Compression hemostasis with Shunlin arterial hemostatic dressing for patients with hepatocellular carcinoma after transcatheter arterial chemoembolization: its clinical application and efficacy[J]. J Intervent Radiol, 2021, 30(5): 519-522. DOI: 10.3969/j.issn.1008-794X.2021.05.021.尤国美, 经碧玲, 潘琴, 等. 瞬灵动脉止血敷料压迫止血在肝癌TACE患者的临床应用与效果研究[J]. 介入放射学杂志, 2021, 30(5): 519-522. DOI: 10.3969/j.issn.1008-794X.2021.05.021. [13] LUO Y, JIANG Y. Comparison of efficiency of TACE plus HIFU and TACE alone on patients with primary liver cancer[J]. J Coll Physicians Surg Pak, 2019, 29(5): 414-417. DOI: 10.29271/jcpsp.2019.05.414. [14] VOGL TJ, MARKO C, LANGENBACH MC, et al. Transarterial chemoembolization of colorectal cancer liver metastasis: improved tumor response by DSM-TACE versus conventional TACE, a prospective, randomized, single-center trial[J]. Eur Radiol, 2021, 31(4): 2242-2251. DOI: 10.1007/s00330-020-07253-2. [15] ZHANG X, ZHOU J, ZHU DD, et al. CalliSpheres® drug-eluting beads (DEB) transarterial chemoembolization (TACE) is equally efficient and safe in liver cancer patients with different times of previous conventional TACE treatments: a result from CTILC study[J]. Clin Transl Oncol, 2019, 21(2): 167-177. DOI: 10.1007/s12094-018-1902-8. [16] AARTS BM, MUÑOZ F, WILDIERS H, et al. Intra-arterial therapies for liver metastatic breast cancer: a systematic review and meta-analysis[J]. Cardiovasc Intervent Radiol, 2021, 44(12): 1868-1882. DOI: 10.1007/s00270-021-02906-1. [17] SUN ZQ, JIANG CY, LI BP, et al. Clinical efficacy and adverse reactions of TACE combined with different doses of apatinib in the treatment of advanced liver cancer[J]. Chin J Gerontol, 2022, 42(3): 557-560. DOI: 10.3969/j.issn.1005-9202.2022.03.014.孙志强, 姜成毅, 李佰萍, 等. TACE联合不同剂量阿帕替尼治疗中晚期肝癌的临床疗效及不良反应[J]. 中国老年学杂志, 2022, 42(3): 557-560. DOI: 10.3969/j.issn.1005-9202.2022.03.014. [18] HUANG CS, YU W, WANG Q, et al. Clinical efficacy of sorafenib and TACE for primary liver cancer and its effect on bFGF and VEGF level[J]. Pract J Cancer, 2017, 32(6): 943-945. DOI: 10.3969/j.issn.1001-5930.2017.06.021黄长山, 余伟, 王谦, 等. 索拉菲尼与TACE联合治疗原发性肝癌的临床效果及对bFGF、VEGF水平的影响[J]. 实用癌症杂志, 2017, 32(6): 943-945. DOI: 10.3969/j.issn.1001-5930.2017.06.021. [19] YE PL, JIA HY, PENG L. Mechanism of action of GP73 in the regulation of liver cancer: An analysis based on transcriptome sequencing[J]. J Clin Hepatol, 2021, 37(8): 1861-1866. DOI: 10.3969/j.issn.1001-5256.2021.08.022.叶佩灵, 嘉红云, 彭亮. 转录组测序分析高尔基体蛋白73参与调控肝癌的作用机制[J]. 临床肝胆病杂志, 2021, 37(8): 1861-1866. DOI: 10.3969/j.issn.1001-5256.2021.08.022. [20] QIN L, ZHAN Z, WEI C, et al. Hsa-circRNA-G004213 promotes cisplatin sensitivity by regulating miR-513b-5p/PRPF39 in liver cancer[J]. Mol Med Rep, 2021, 23(6): 421. DOI: 10.3892/mmr.2021.12060. [21] YIN XL, XU Q. The clinical significance of KRAS and BRAF oncogene mutations in hepatocellular carcinoma[J]. J Modern Oncology, 2016, 24(15): 2419-2422. DOI: 10.3969/j.issn.1672-4992.2016.15.022.尹小兰, 许青. 原发性肝癌中KRAS及BRAF基因突变及其临床意义[J]. 现代肿瘤医学, 2016, 24(15): 2419-2422. DOI: 10.3969/j.issn.1672-4992.2016.15.022. -




 PDF下载 ( 2594 KB)
PDF下载 ( 2594 KB)

 下载:
下载:

 下载:
下载: