不同评分系统预测高脂血症性急性胰腺炎严重程度及预后的价值分析
DOI: 10.3969/j.issn.1001-5256.2022.11.022
Value of different scoring systems in predicting the severity and prognosis of hyperlipidemic acute pancreatitis
-
摘要:
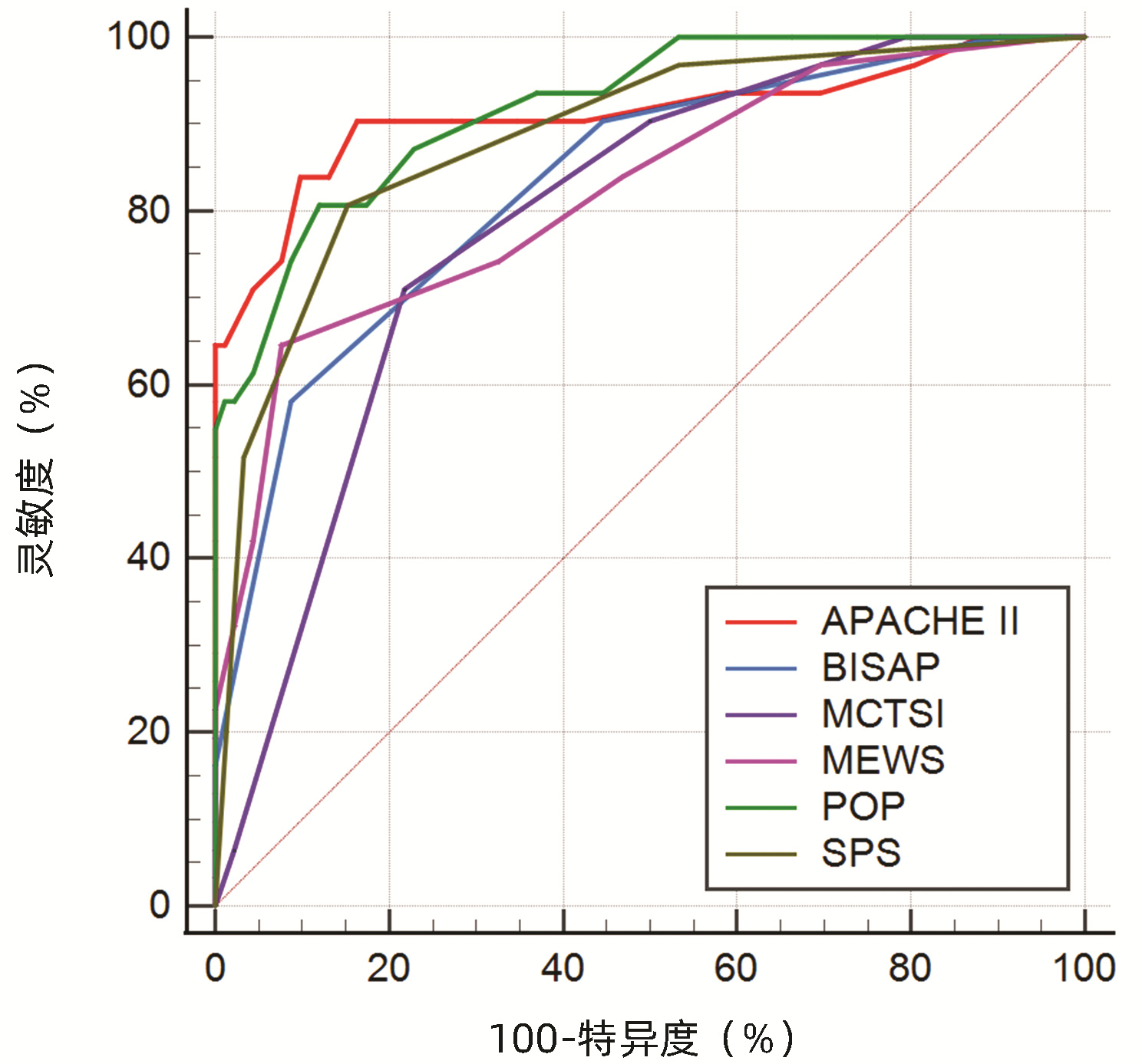
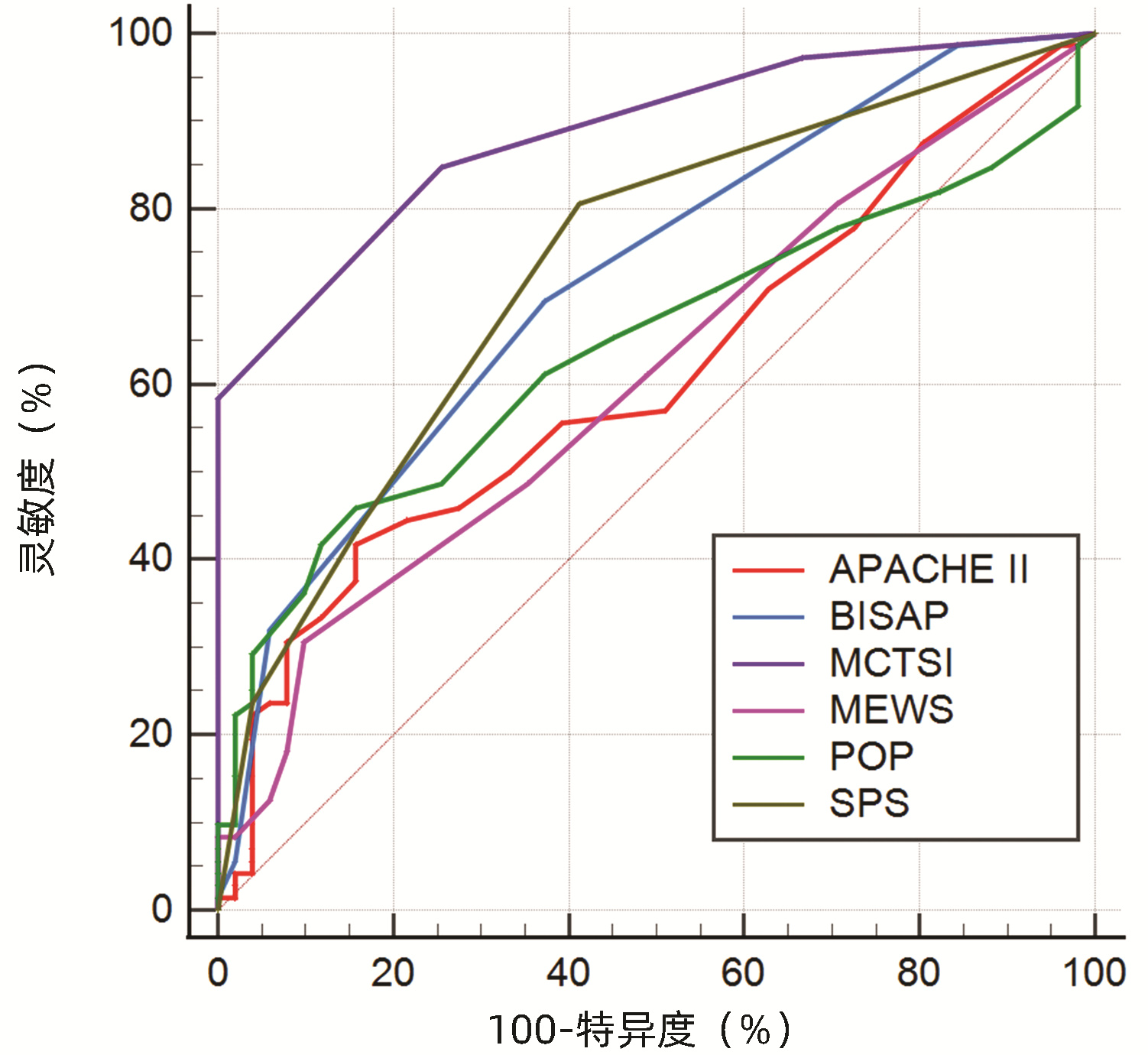
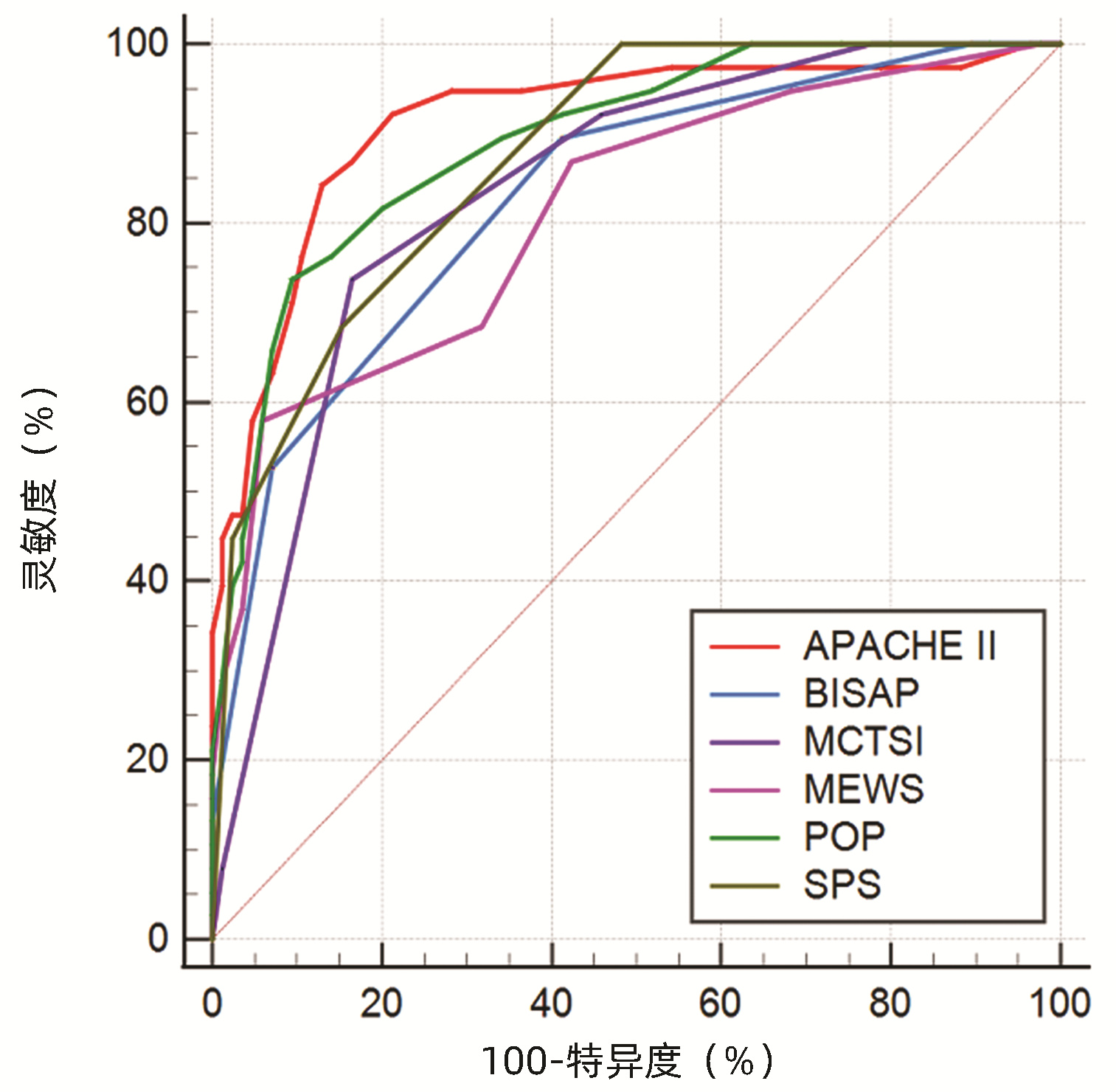
目的 通过对比APACHEⅡ、BISAP、MCTSI、MEWS、POP、SPS、PANC3评分,筛选出预测高脂血症性急性胰腺炎(HLAP)疾病严重程度及预后的最佳评分系统。 方法 回顾性收集昆明医科大学第二附属医院2017年10月—2022年1月住院治疗的123例HLAP患者资料,将患者分为轻症急性胰腺炎(MAP)组(n=24)、中度重症急性胰腺炎(MSAP)组(n=56)和重症急性胰腺炎(SAP)组(n=43),比较3组患者的基本资料和评分系统差异。非正态分布的计量资料多组间比较采用Kruskal-Wallis H检验;计数资料组间比较采用χ2检验。使用MedCalc软件绘制受试者工作特征(ROC)曲线,ROC曲线下面积(AUC)分析比较各评分系统对HLAP患者疾病严重程度、局部及全身并发症的预测价值。 结果 3组患者在糖尿病(χ2=6.880,P<0.05)、住院天数(H=26.494,P<0.001)、局部并发症、急性肾损伤(AKI)、急性呼吸窘迫综合征(ARDS)和多器官功能障碍综合征(MODS)之间存在明显差异(χ2值分别为52.211、38.247、79.201、45.032,P值均<0.001)。评分系统比较,3组患者的APACHEⅡ、BISAP、MCTSI、MEWS、POP、SPS、PANC3评分均有统计学差异(H值分别为47.525、42.662、53.545、31.664、49.233、48.543、9.443,P值均<0.05)。预测SAP发生,APACHEⅡ评分的预测价值高于MEWS评分(Z=2.090,P<0.05),余评分预测价值相同,其中POP评分的AUC最高为0.883。MCTSI评分对局部并发症的预测价值最高(AUC=0.886),当cut-off值为5分时,灵敏度为84.7%、特异度为74.5%。预测AKI发生,APACHEⅡ、POP评分AUC分别为0.911(95%CI:0.835~0.986,P<0.001)、0.920(95%CI:0.866~0.974,P<0.001),预测价值APACHEⅡ评分>MCTSI、MEWS评分,POP评分>MCTSI、MEWS、BISAP评分,SPS评分>MCTSI评分;预测ARDS发生,APACHEⅡ评分的AUC为0.914(95%CI: 0.854~0.973,P<0.001),预测价值大于BISAP、MEWS评分(Z值分别为2.152、3.015, P值均<0.05);预测MODS发生,APACHEⅡ、POP评分的AUC分别为0.969(95%CI:0.941~0.996,P<0.001)、0.932(95%CI:0.880~0.984,P<0.001),预测价值APACHEⅡ评分>SPS、BISAP、MEWS、MCTSI评分。 结论 POP评分预测SAP价值最高,但与除MEWS评分以外的各评分系统预测能力相当;MCTSI评分预测局部并发症能力良好;预测全身并发症,APACHEⅡ、POP评分是值得关注的,两者预测AKI、MODS具有高度准确性,APACHEⅡ评分预测ARDS具有高度准确性。 Abstract:Objective To investigate the best scoring systems for predicting the severity and prognosis of hyperlipidemic acute pancreatitis (HLAP) by comparing APACHEII, BISAP, MCTSI, MEWS, POP, SPS, and PANC3 scores. Methods A retrospective analysis was performed for the data of 123 patients with HLAP who were hospitalized and treated in The Second Affiliated Hospital of Kunming Medical University from October 2017 to January 2022. The patients were divided into mild acute pancreatitis (MAP) group with 24 patients, moderate- severe acute pancreatitis (MSAP) group with 56 patients, and severe acute pancreatitis (SAP) group with 43 patients, and the three groups were compared in terms of basic data and scores of the above scoring systems. The Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups; the chi-square test was used for comparison of categorical data between groups. MedCalc software was used to plot the receiver operating characteristic (ROC) curve, and the area under the ROC curve (AUC) was used to compare the value of these scoring systems in predicting disease severity and local and systemic complications in HLAP patients. Results There were significant differences between the three groups in diabetes mellitus (χ2=6.880, P < 0.05), length of hospital stay (H=26.494, P < 0.001), local complications (χ2=52.211, P < 0.001), acute kidney injury (AKI) (χ2=38.247, P < 0.001), acute respiratory distress syndrome (ARDS) (χ2=79.201, P < 0.001), and multiple organ dysfunction syndrome (MODS) (χ2=45.032, P < 0.001). As for the scores of the above scoring systems, there were significant differences between the three groups in APACHE Ⅱ, BISAP, MCTSI, MEWS, POP, SPS, and PANC3 (H=47.525, 42.662, 53.545, 31.664, 49.233, 48.543, and 9.443, all P < 0.05). APACHE Ⅱ score had a significantly higher value than MEWS score in predicting SAP (Z=2.090, P < 0.05), and the other scores had a similar value, among which POP score had the largest AUC of 0.883. MCTSI score had the highest value in predicting local complications (AUC=0.886), with a sensitivity of 84.7% and a specificity of 74.5% at the cut-off value of 5. APACHE Ⅱ and POP scores had an AUC of 0.911 (95% confidence interval [CI]: 0.835-0.986, P < 0.001) and 0.920 (95%CI: 0.866-0.974, P < 0.001), respectively, in predicting AKI; APACHE Ⅱ score had a higher predictive value than MCTSI and MEWS scores, POP score had a higher predictive value than MCTSI, MEWS, and BISAP scores, and SPS score had a higher predictive value than MCTSI score. APACHE Ⅱ score had an AUC of 0.914 (95%CI: 0.854-0.973, P < 0.001) in predicting ARDS and had a higher predictive value than BISAP and MEWS (Z=2.152 and 3.015, both P < 0.05). APACHE Ⅱ and POP scores had an AUC of 0.969 (95%CI: 0.941-0.996, P < 0.001) and 0.932 (95%CI: 0.880-0.984, P < 0.001), respectively, in predicting MODS, and APACHE Ⅱ score had a higher predictive value than SPS, BISAP, MEWS, and MCTSI. Conclusion POP score has the highest value in predicting SAP, with a comparable predictive ability to all the other scoring systems except MEWS. APACHEII and POP scores have a good value in predicting systemic complications and show a high accuracy in predicting AKI and MODS, and APACHEII score is highly accurate in predicting ARDS. -
Key words:
- Pancreatitis /
- Hyperlipidemias /
- Scoring Systems /
- Prognosis
-
表 1 3组患者一般资料比较
Table 1. Comparison of general data between the three groups
指标 MAP组(n=24) MSAP组(n=56) SAP组(n=43) 统计值 P值 男[例(%)] 17(70.80) 39(69.64) 30(69.77) χ2=0.012 0.994 年龄(岁) 39 (33.00~52.50) 38(32.00~44.00) 43(32.00~50.00) H=2.267 0.322 BMI(kg/m2) 26.44(23.54~30.22) 26.36(24.23~28.41) 27.41(24.97~30.44) H=1.945 0.378 糖尿病[例(%)] 20(83.33) 33(58.92) 22(51.16) χ2=6.880 0.032 高血压[例(%)] 6(25.00) 15(26.78) 10(23.26) χ2=0.161 0.922 糖尿病酮症[例(%)] 14(58.33) 32(57.14) 20(46.51) χ2=1.368 0.505 脂肪肝[例(%)] 19(79.17) 53(94.64) 39(90.70) χ2=4.220 0.113 住院天数(d) 9(7~11) 12(8~15) 19(14~32) H=26.494 <0.001 局部并发症[例(%)] 0 33(58.93) 39(90.70) χ2=52.211 <0.001 AKI[例(%)] 1(4.20) 5(8.93) 25(58.14) χ2=38.247 <0.001 ARDS[例(%)] 0 3(5.37) 35(81.40) χ2=79.201 <0.001 MODS[例(%)] 0 2(3.57) 23(53.49) χ2=45.032 <0.001 死亡[例(%)] 0 1(1.79) 2(4.65) χ2=1.227 0.590 APACHEⅡ评分 5.50(3.00~8.00) 6.00(4.00~7.75) 15.00(11.00~23.00) H=47.525 <0.001 BISAP评分 1(0~2) 1(1~2) 2(2~3) H=42.662 <0.001 MCTSI评分 4(2~4) 6(4~6) 8(6~8) H=53.545 <0.001 MEWS评分 2(1~4) 2(1~4) 5(3~6) H=31.664 <0.001 POP评分 6(4~8) 5(2~8) 12(9~17) H=49.233 <0.001 SPS评分 0(0~1) 1(0~1) 2(1~3) H=48.543 <0.001 PANC3评分 1(1~2) 1(1~2) 2(1~2) H=9.443 <0.050 表 2 各评分系统预测SAP、局部及全身并发症价值比较
Table 2. Comparison of the value of scoring systems in predicting SAP, local and systemic complications
项目 AUC(95%CI) SE cut-off 约登指数 灵敏度 特异度 P值 SAP APACHEⅡ 0.875(0.800~0.951) 0.039 8.5 0.696 0.884 0.813 <0.001 BISAP 0.825(0.749~0.901) 0.039 1.5 0.496 0.884 0.613 <0.001 MCTSI 0.849(0.777~0.920) 0.037 7.0 0.619 0.744 0.875 <0.001 MEWS 0.802(0.720~0.884) 0.042 4.5 0.449 0.512 0.938 <0.001 POP 0.883(0.822~0.943) 0.031 10.5 0.623 0.698 0.925 <0.001 SPS 0.860(0.794~0.925) 0.033 0.5 0.514 0.977 0.538 <0.001 局部并发症 APACHEⅡ 0.612(0.512~0.711) 0.051 11.5 0.260 0.417 0.843 0.036 BISAP 0.718(0.628~0.809) 0.046 1.5 0.322 0.694 0.627 <0.001 MCTSI 0.886(0.829~0.942) 0.029 5.0 0.592 0.847 0.745 <0.001 MEWS 0.608(0.508~0.707) 0.051 4.5 0.208 0.306 0.902 0.042 POP 0.642(0.545~0.739) 0.049 9.5 0.301 0.458 0.843 0.008 SPS 0.732(0.643~0.822) 0.046 0.5 0.394 0.806 0.588 <0.001 AKI APACHEⅡ 0.911(0.835~0.986) 0.039 12.5 0.741 0.839 0.902 <0.001 BISAP 0.831(0.747~0.915) 0.043 2.5 0.494 0.581 0.913 <0.001 MCTSI 0.790(0.705~0.875) 0.043 7.0 0.492 0.710 0.783 <0.001 MEWS 0.822(0.732~0.913) 0.046 4.5 0.569 0.645 0.924 <0.001 POP 0.920(0.866~0.974) 0.028 10.5 0.687 0.806 0.880 <0.001 SPS 0.885(0.815~0.955) 0.036 1.5 0.654 0.806 0.848 <0.001 ARDS APACHEⅡ 0.914(0.854~0.973) 0.030 10.5 0.713 0.842 0.871 <0.001 BISAP 0.828(0.750~0.907) 0.040 1.5 0.483 0.895 0.588 <0.001 MCTSI 0.835(0.762~0.909) 0.038 7.0 0.572 0.737 0.835 <0.001 MEWS 0.812(0.727~0.897) 0.043 4.5 0.520 0.579 0.941 <0.001 POP 0.892(0.832~0.952) 0.031 10.5 0.643 0.737 0.906 <0.001 SPS 0.874(0.812~0.935) 0.031 1.5 0.531 0.684 0.847 <0.001 MODS APACHEⅡ 0.969(0.941~0.996) 0.014 11.5 0.817 0.960 0.857 <0.001 BISAP 0.878(0.813~0.943) 0.033 1.5 0.551 1.000 0.551 <0.001 MCTSI 0.853(0.785~0.921) 0.035 7.0 0.626 0.840 0.786 <0.001 MEWS 0.854(0.771~0.938) 0.043 4.5 0.628 0.720 0.908 <0.001 POP 0.932(0.880~0.984) 0.027 10.5 0.787 0.920 0.867 <0.001 SPS 0.886(0.822~0.950) 0.033 1.5 0.656 0.840 0.816 <0.001 -
[1] YU H, HUANG Y, CHEN L, et al. Assessment of computed tomography-defined muscle and adipose tissue features in relation to length of hospital stay and recurrence of hypertriglyceridemic pancreatitis[J]. Int J Gen Med, 2021, 14: 1709-1717. DOI: 10.2147/IJGM.S311118. [2] JIN M, BAI X, CHEN X, et al. A 16-year trend of etiology in acute pancreatitis: The increasing proportion of hypertriglyceridemia-associated acute pancreatitis and its adverse effect on prognosis[J]. J Clin Lipidol, 2019, 13(6): 947-953. e1. DOI: 10.1016/j.jacl.2019.09.005. [3] WANG Y, CAO LK, WEI Y, et al. The value of modified renal rim grade in predicting acute kidney injury following severe acute pancreatitis[J]. J Comput Assist Tomogr, 2018, 42(5): 680-687. DOI: 10.1097/RCT.0000000000000749. [4] IYER H, ELHENCE A, MITTAL S, et al. Pulmonary complications of acute pancreatitis[J]. Expert Rev Respir Med, 2020, 14(2): 209-217. DOI: 10.1080/17476348.2020.1698951. [5] AN WH, HE XC, YANG J, et al. Value of early admission scoring systems in predicting the severity and prognosis of acute pancreatitis[J]. J Clin Hepatol, 2020, 36(6): 1342-1346. DOI: 10.3969/j.issn.1001-5256.2020.06.030.安文慧, 何旭昶, 杨婧, 等. 入院早期评分系统对急性胰腺炎严重程度及预后的预测价值[J]. 临床肝胆病杂志, 2020, 36(6): 1342-1346. DOI: 10.3969/j.issn.1001-5256.2020.06.030. [6] Pancreas Study Group, Chinese Society of Gastroenterology, Chinese Medical Association; Editorial Board of Chinese Journal of Pancreatology; Editorial Board of Chinese Journal of Digestion. Chinese guidelines for the management of acute pancreatitis (Shenyang, 2019)[J]. J Clin Hepatol, 2019, 35(12): 2706-2711. DOI: 10.3969/j.issn.1001-5256.2019.12.013.中华医学会消化病学分会胰腺疾病学组, 《中华胰腺病杂志》编委会, 《中华消化杂志》编委会. 中国急性胰腺炎诊治指南(2019年, 沈阳)[J]. 临床肝胆病杂志, 2019, 35(12): 2706-2711. DOI: 10.3969/j.issn.1001-5256.2019.12.013. [7] BANKS PA, BOLLEN TL, DERVENIS C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus[J]. Gut, 2013, 62(1): 102-111. DOI: 10.1136/gutjnl-2012-302779. [8] SCHEPERS NJ, BAKKER OJ, BESSELINK MG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis[J]. Gut, 2019, 68(6): 1044-1051. DOI: 10.1136/gutjnl-2017-314657. [9] LI X, KE L, DONG J, et al. Significantly different clinical features between hypertriglyceridemia and biliary acute pancreatitis: a retrospective study of 730 patients from a tertiary center[J]. BMC Gastroenterol, 2018, 18(1): 89. DOI: 10.1186/s12876-018-0821-z. [10] LARVIN M, MCMAHON MJ. APACHE-Ⅱ score for assessment and monitoring of acute pancreatitis[J]. Lancet, 1989, 2(8656): 201-205. DOI: 10.1016/s0140-6736(89)90381-4. [11] HAGJER S, KUMAR N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis - A prospective observational study[J]. Int J Surg, 2018, 54(Pt A): 76-81. DOI: 10.1016/j.ijsu.2018.04.026. [12] YANG L, LIU J, XING Y, et al. Comparison of BISAP, Ranson, MCTSI, and APACHE Ⅱ in predicting severity and prognoses of hyperlipidemic acute pancreatitis in Chinese patients[J]. Gastroenterol Res Pract, 2016, 2016: 1834256. DOI: 10.1155/2016/1834256. [13] PANDO E, ALBERTI P, MATA R, et al. Early changes in Blood Urea Nitrogen (BUN) can predict mortality in acute pancreatitis: comparative study between BISAP score, APACHE-Ⅱ, and other laboratory markers-a prospective observational study[J]. Can J Gastroenterol Hepatol, 2021, 2021: 6643595. DOI: 10.1155/2021/6643595. [14] WU Q, WANG J, QIN M, et al. Accuracy of conventional and novel scoring systems in predicting severity and outcomes of acute pancreatitis: a retrospective study[J]. Lipids Health Dis, 2021, 20(1): 41. DOI: 10.1186/s12944-021-01470-4. [15] ZENG QX, JIANG KL, WU ZH, et al. Pleural effusion is associated with severe renal dysfunction in patients with acute pancreatitis[J]. Med Sci Monit, 2021, 27: e928118. DOI: 10.12659/MSM.928118. [16] ALBERTI P, PANDO E, MATA R, et al. Evaluation of the modified computed tomography severity index (MCTSI) and computed tomography severity index (CTSI) in predicting severity and clinical outcomes in acute pancreatitis[J]. J Dig Dis, 2021, 22(1): 41-48. DOI: 10.1111/1751-2980.12961. [17] ÇAKAR İ, KEVEN A, ESERO ǦLU E, et al. Role of extrapancreatic necrosis volume in determining early prognosis in patients with acute pancreatitis[J]. Abdom Radiol (NY), 2020, 45(5): 1507-1516. DOI: 10.1007/s00261-019-02188-9. [18] BHATNAGAR M, SIROHI N, DUBEY AB. Prediction of hospital outcome in emergency medical admissions using modified early warning score (MEWS): Indian experience[J]. J Family Med Prim Care, 2021, 10(1): 192-198. DOI: 10.4103/jfmpc.jfmpc_1426_20. [19] AYGUN H, ERAYBAR S. The role of emergency department triage early warning score (TREWS) and modified early warning score (MEWS) to predict in-hospital mortality in COVID-19 patients[J]. Ir J Med Sci, 2022, 191(3): 997-1003. DOI: 10.1007/s11845-021-02696-y. [20] HARRISON DA, D'AMICO G, SINGER M. The Pancreatitis Outcome Prediction (POP) Score: a new prognostic index for patients with severe acute pancreatitis[J]. Crit Care Med, 2007, 35(7): 1703-1708. DOI: 10.1097/01.CCM.0000269031.13283.C8. [21] ESCOBAR-ARELLANO R, GURAIEB-BARRAGáN E, MANSANARES-HERNÁNDEZ A, et al. Sensitivity, specificity and reliability of the POP score vs. APACHE Ⅱ score as predictors of severe acute biliary pancreatitis[J]. Cir Cir, 2019, 87(4): 402-409. DOI: 10.24875/CIRU.18000662. [22] MOUNZER R, LANGMEAD CJ, WU BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis[J]. Gastroenterology, 2012, 142(7): 1476-1482; quiz e15-16. DOI: 10.1053/j.gastro.2012.03.005. [23] UEDA T, TAKEYAMA Y, YASUDA T, et al. Simple scoring system for the prediction of the prognosis of severe acute pancreatitis[J]. Surgery, 2007, 141(1): 51-58. DOI: 10.1016/j.surg.2006.05.008. [24] RATHNAKAR SK, VISHNU VH, MUNIYAPPA S, et al. Accuracy and predictability of PANC-3 scoring system over APACHE Ⅱ in acute pancreatitis: a prospective study[J]. J Clin Diagn Res, 2017, 11(2): PC10-PC13. DOI: 10.7860/JCDR/2017/23168.9375. -



 PDF下载 ( 3145 KB)
PDF下载 ( 3145 KB)


 下载:
下载: