血管性血友病因子抗原与白蛋白比值和糖萼素指数评分对乙型肝炎肝硬化食管静脉曲张的预测价值
DOI: 10.3969/j.issn.1001-5256.2022.12.013
Value of von Willebrand factor antigen-to-albumin ratio and glycocalicin index in predicting esophageal varices in hepatitis B cirrhosis
-
摘要:
目的 与血管性血友病因子抗原(vWF-Ag)与血小板比值(VITRO)评分比较,探讨vWF-Ag与白蛋白比值(VAR)和糖萼素指数(GCI)评分在预测食管静脉曲张(EV)发生及分级中的临床价值。 方法 回顾性分析2020年4月—2021年12月于延边大学附属医院住院的乙型肝炎肝硬化患者146例,以胃镜检查为标准,对EV进行诊断和分级。计算VITRO、VAR和GCI值,分析其与EV相关性。正态分布计量资料两组间比较采用t检验,多组间比较采用单因素方差分析;非正态性分布计量资料两组间比较采用Mann-Whitney U检验。计数资料组间比较采用χ2检验。采用Logistic回归模型分析EV的预测因素。使用受试者工作特征曲线(ROC曲线)评估各指标的诊断准确性。 结果 胃镜检查显示无EV患者54例,轻、中、重度EV患者分别为30、33和29例。EV患者的VAR和GCI评分显著高于无EV患者,差异有统计学意义(t值分别为-5.819、-3.449,P值均<0.001)。线性回归显示VAR和GCI均随EV分级而升高(P值分别为0.002、0.005)。多变量回归分析确定VAR(风险比=1.46,95%CI:1.21~ 1.75,P<0.001)和GCI(风险比=1.84,95%CI:1.22~2.77,P=0.003)与EV独立相关。VITRO评分诊断EV和重度EV的ROC曲线下面积(AUC)分别为0.718和0.863;临界值分别为2.77和5.37。VAR和GCI诊断EV的AUC分别为0.745和0.710,临界值分别为8.88和1.70;两者诊断重度EV的AUC分别为0.755和0.787,临界值分别为9.81和2.00。VAR联合GCI对EV诊断效能明显优于VITRO(P=0.009),其AUC为0.808,敏感度为55.43%,特异度为94.44%;而联合模型对重度EV的AUC为0.869,与VITRO比较差异无统计学意义(P=0.421)。 结论 VAR和GCI评分是预测乙型肝炎肝硬化患者EV和风险分层的潜在非侵入性标志物。 Abstract:Objective To investigate the clinical value of von Willebrand factor antigen-to-albumin ratio (VAR) score and glycocalicin index (GCI) score in predicting the development and classification of esophageal varices in comparison with von Willebrand factor antigen-to-platelet ratio (VITRO) score. Methods A retrospective analysis was performed for 146 patients with hepatitis B cirrhosis who were hospitalized from April 2020 to December 2021, and esophageal varices (EV) was diagnosed and graded with the results of gastroscopy as the standard. VITRO, VAR, and GCI were calculated, and their association with EV was analyzed. The t-test was used for comparison of normally distributed continuous data between two groups, and a one-way analysis of variance was used for comparison between multiple groups; the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups. The chi-square test was used for comparison of categorical data between groups. A logistic regression model analysis was used to identify the predictive factors for EV, and the receiver operating characteristic (ROC) curve was used to evaluate the diagnostic accuracy of each index. Results Gastroscopy showed 54 patients without EV, 30 with mild EV, 33 with moderate EV, and 29 with severe EV. The patients with EV had significantly higher VAR and GCI scores than those without EV (t=-5.819 and -3.449, both P < 0.001). The linear regression analysis showed that VAR and GCI increased with the increase in EV grade (P=0.002 and 0.005). The multivariate logistic regression analysis showed that VAR (odds ratio [OR]=1.46, 95% confidence interval [CI]: 1.21-1.75, P < 0.001) and GCI (OR=1.84, 95%CI: 1.22-2.77, P=0.003) were independently associated with EV. VITRO score had an area under the ROC curve (AUC) of 0.718 in diagnosing EV and 0.863 in diagnosing severe EV, with the optimal cut-off values of 2.77 and 5.37, respectively. VAR and GCI had an AUC of 0.745 and 0.710, respectively, in diagnosing EV, with the optimal cut-off values of 8.88 and 1.70, respectively; VAR and GCI had an AUC of 0.755 and 0.787, respectively, in diagnosing severe EV, with the optimal cut-off values of 9.81 and 2.00, respectively. VAR combined with GCI had significantly better efficacy than VITRO in diagnosing EV (P=0.009), with an AUC of 0.808, a sensitivity of 55.43%, and a specificity of 94.44%; VAR combined with GCI had an AUC of 0.869 in diagnosing severe EV, which was similar to VITRO (P=0.421). Conclusion VAR and GCI scores are potential noninvasive markers for the prediction and risk stratification of EV in patients with hepatitis B cirrhosis. -
Key words:
- Hepatitis B /
- Liver Cirrhosis /
- Esophageal and Gastric Varices
-
食管静脉曲张(EV)是门静脉高压的主要临床症状,EV破裂出血是肝硬化患者的常见致死因素[1]。早期评估EV风险对肝硬化患者临床结局具有重要意义。
肝窦内皮细胞参与调节肝内微循环稳态[2],其功能障碍引起肝血管结构异常和血管张力增加,是门静脉高压病理生理学的重要因素[3]。研究报道血管性血友病因子(von Willebrand factor,vWF)是内皮细胞活化标志物[4],其升高程度与肝纤维化程度相关[5]。而白蛋白(Alb) 具有多种生物学效应,可通过免疫调节和抗氧化功能稳定内皮细胞[6]。因此,由vWF与Alb构建的数字模型可能是门静脉高压的间接指标。此外,肝硬化患者血小板(PLT)减少被认为与门静脉高压相关[7],糖萼素指数(glycocalicin index, GCI)反映PLT周转及更新状况[8],研究[9]报道肝硬化患者的GCI水平显著升高,且与肝病严重程度相关。课题组假设VAR和GCI与EV之间可能存在联系。国外研究[10]报道VITRO(vWF与PLT比值)评分与肝静脉压力梯度相关,是诊断门静脉高压的潜在标志物。因此,本研究目的评估VAR和GCI对乙型肝炎肝硬化EV存在及程度的诊断效能;与VITRO评分比较,评估VAR联合GCI是否会提高EV诊断准确性。
1. 资料与方法
1.1 研究对象
回顾性分析2020年4月—2021年12月本院收治的乙型肝炎肝硬化住院患者的临床资料。纳入标准:入院接受内窥镜检查,既往未接受内镜下套扎、经颈静脉肝内门体分流术等静脉曲张治疗术的患者。排除标准:(1)其他病毒性肝炎、酒精性肝炎、脂肪肝和自身免疫性疾病;(2)非肝硬化性门静脉高压、肝内外恶性肿瘤;(3)心血管及周围血管病,非肝硬化性PLT减少疾病,接受抗凝和抗PLT治疗的患者;(4)有任何感染源或正在接受抗感染治疗的患者。
1.2 研究分组
参照食管静脉曲张诊断和分级标准[1],将患者分为EV组和无EV组,并根据食管静脉曲张形态及出血危险程度将EV患者分为轻度、中度、重度三级。轻度(G1):食管静脉曲张呈直线形或略有迂曲,无红色征;中度(G2):曲张静脉呈直线形或略有迂曲,有红色征或呈蛇形迂曲隆起但无红色征;重度(G3):曲张静脉呈蛇形迂曲隆起,且有红色征或呈串珠状、结节状或瘤样(不论是否有红色征)。根据Child-Pugh评分将肝硬化患者分为A、B、C级。
1.3 资料收集
患者的性别、年龄、入院时间和HBV DNA载量,以及血常规、肝肾功能、超声检查结果,均从病历中获得。使用全自动STA分析仪(法国,Stago公司)测量血浆vWF抗原(vWF-Ag)水平,采用ELISA试剂盒(美国,NOVUS公司) 检测血清糖萼素(GC)水平。VAR=[vWF-Ag(%)]/Alb(g/L);VITRO=[vWF-Ag(%)]/ PLT(109/L)[10];GCI=[GC(ng/mL)×250]/PLT(109/L)[8]。式中Alb为血清Alb。
1.4 统计学方法
采用SPSS 17.0和GraphPad prism 7.0软件进行数据分析。符合正态分布的计量资料用x±s表示,两组间比较采用独立样本t检验,多组间比较采用单因素方差分析。非正态分布的计量资料采用M (P25~P75) 表示,两组间比较采用Mann-Whitney U检验。计数资料组间比较采用χ2检验。趋势检验采用线性回归分析。采用Logistic回归模型分析EV的危险因素。计算受试者工作特征曲线(ROC曲线)下面积(AUC),评估研究参数对判断EV存在及程度的准确性。使用MedCalc软件比较各指标ROC曲线的诊断性能。P<0.05为差异有统计学意义。
2. 结果
2.1 一般情况
研究共纳入HBV相关肝硬化患者146例,男96例,女50例,平均57岁。Child-Pugh肝功能分级中A级59例,B级62例,C级25例。根据胃镜检查结果,将患者分为EV组和无EV组。相较于无EV组,EV组患者的脾脏厚度、MELD评分、Child-Pugh评分、vWF-Ag、INR、VAR、VITRO和GCI水平明显升高,PLT和Alb则显著降低,差异均存在统计学意义(P值均<0.05)(表 1)。
表 1 EV组和无EV组患者基本特征Table 1. Comparisons of characteristics between EV and non-EV in patients with cirrhosis指标 无EV组(n=54) EV组(n=92) 统计值 P值 年龄(岁) 56.97±12.97 57.55±12.26 t=-0.276 0.345 男/女(例) 37/17 59/33 χ2=0.291 0.590 HBV DNA(log10 IU/mL) 3.6(3.0~5.1) 3.7(3.0~4.9) Z=-0.409 0.682 脾脏厚度(mm) 35.90±6.46 38.67±8.84 t=-2.183 0.031 PLT(109/L) 132.58±72.91 93.76±42.89 t=3.579 0.001 vWF-Ag(%) 277.43±65.45 316.80±87.30 t=-2.634 0.009 Alb(g/L) 35.52±5.61 31.83±5.20 t=4.026 <0.001 TBil(μmol/L) 41.8(27.8~61.5) 48.3(37.9~70.6) Z=-0.461 0.645 Cre(μmol/L) 72.3(60.5~100.6) 78.0(65.4~120.4) Z=-1.098 0.272 PT-INR 1.26(1.07~1.42) 1.47(1.19~1.79) Z=-3.083 0.002 GC(ng/mL) 0.92±0.50 0.89±0.27 t=0.441 0.661 MELD评分 11.17±4.75 15.66±6.09 t=-4.659 <0.001 Child-Pugh评分 6.31±1.43 8.16±1.86 t=-6.740 <0.001 Child-Pugh分级(A/B/C, 例数) 36/18/0 23/44/25 χ2=30.980 <0.001 GCI 2.00±1.16 2.89±1.67 t=-3.449 <0.001 VITRO 2.58±1.25 4.43±3.25 t=-4.871 <0.001 VAR 7.89±1.98 10.36±3.13 t=-5.819 <0.001 2.2 VAR与EV的相关性
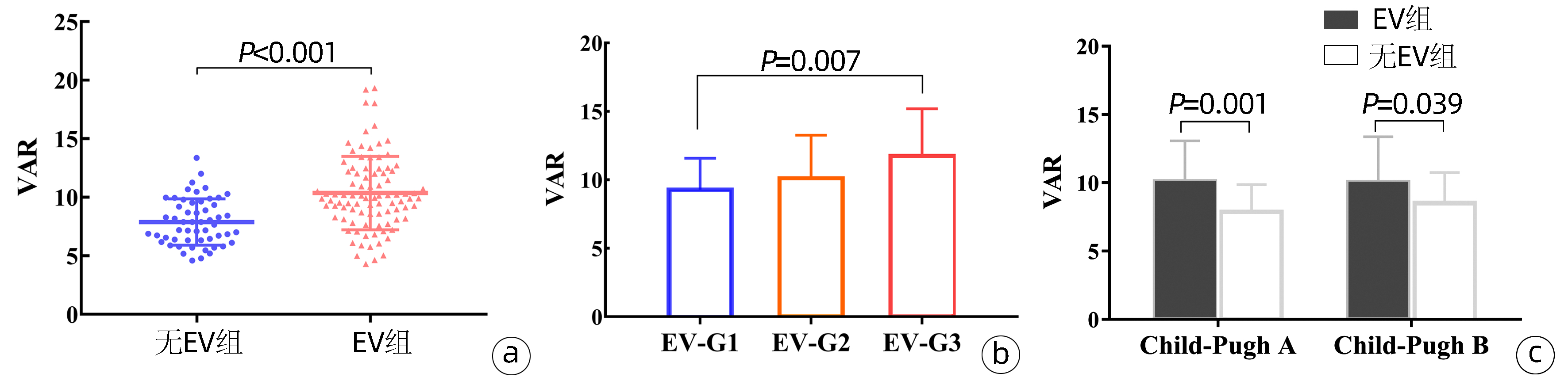
与无EV患者(7.89±1.98)相比,EV患者(10.36±3.13)的VAR水平显著升高(P<0.001)(图 1a)。根据EV严重程度分级G1级30例,G2级33例,G3级29例,线性回归显示EV患者的VAR随EV严重程度而升高(P=0.002),方差分析Bonferroni法显示G3患者的VAR水平显著高于G1患者(P=0.007)(图 1b)。
在Child-Pugh阶段内,有和没有EV的肝硬化患者之间,VAR存在显著差异(P<0.001)。在Child-Pugh A级中,无EV患者的平均VAR为(7.89±1.98),而EV患者为(10.13±2.94)(P=0.001);在Child-Pugh B级中,无EV患者的平均VAR为(8.56±2.19),而EV患者为(10.08±3.29)(P=0.039);在Child-Pugh C级中,所有患者都发生EV(图 1c)。
2.3 GCI与EV的相关性
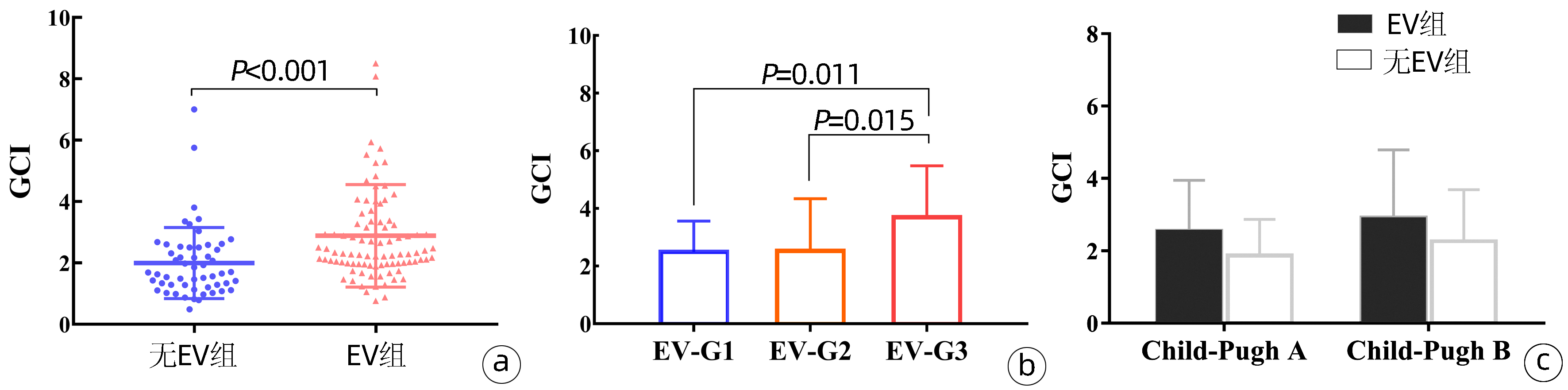
与无EV患者(2.00±1.16) 相比,EV患者(2.89±1.67)的GCI水平显著升高(P<0.001)(图 2a)。线性回归显示EV患者的GCI随EV严重程度而升高(P=0.005),方差分析Bonferroni法显示G3患者的GCI水平显著高于G1和G2患者(P值分别为0.011、0.015)(图 2b)。在Child-Pugh A级中,无EV患者的平均GCI为(1.87±1.00),EV患者为(2.55±1.40)(P=0.054);在Child-Pugh B级中,无EV患者的平均GCI为(2.26±1.43),EV患者为(2.92±1.87)(P=0.179),组间差异均无统计学意义(图 2c)。
2.4 食管静脉曲张危险因素分析
采用二元Logistic回归分析确定EV危险因素。通过单因素分析,PLT、vWF-Ag、Alb、INR、MELD评分、VAR、GCI和VITRO均是EV的影响因素(P值均<0.05)。Pearson相关分析显示,GCI和VAR与VITRO评分之间存在较强的正相关性(r分别为0.758、0.489,P值均<0.001),不能同时放入Logistic回归模型中,通过剔除变量法,将共线性的自变量剔除。多因素分析采用向前逐步回归法对比值比(OR)值调整后,显示GCI和VAR为EV的独立危险因素(P值均<0.05)(表 2)。
表 2 EV危险因素的Logistic回归分析Table 2. Univariable and multivariable analysis of risk factors for esophageal varices指标 单因素分析 多因素分析(模型1) 多因素分析 OR(95%CI) P值 OR(95%CI) P值 OR(95%CI) P值 PLT(109/L) 0.99(0.98~0.99) 0.001 vWF-Ag 1.01(1.00~1.01) 0.001 Alb(g/L) 0.88(0.82~0.94) <0.001 PT-INR 4.18(1.61~10.82) 0.003 MELD评分 1.21(1.10~1.32) <0.001 1.16 (1.05~1.27) 0.003 1.17 (1.07~1.29) 0.001 VAR 1.46(1.24~1.73) <0.001 1.46 (1.21~1.75) <0.001 GCI 1.85(1.27~2.59) <0.001 1.84 (1.22~2.77) 0.003 VITRO 1.73(1.31~2.28) <0.001 1.24 (1.68~2.28) 0.001 2.5 GCI、VAR和VITRO对EV诊断效能比较
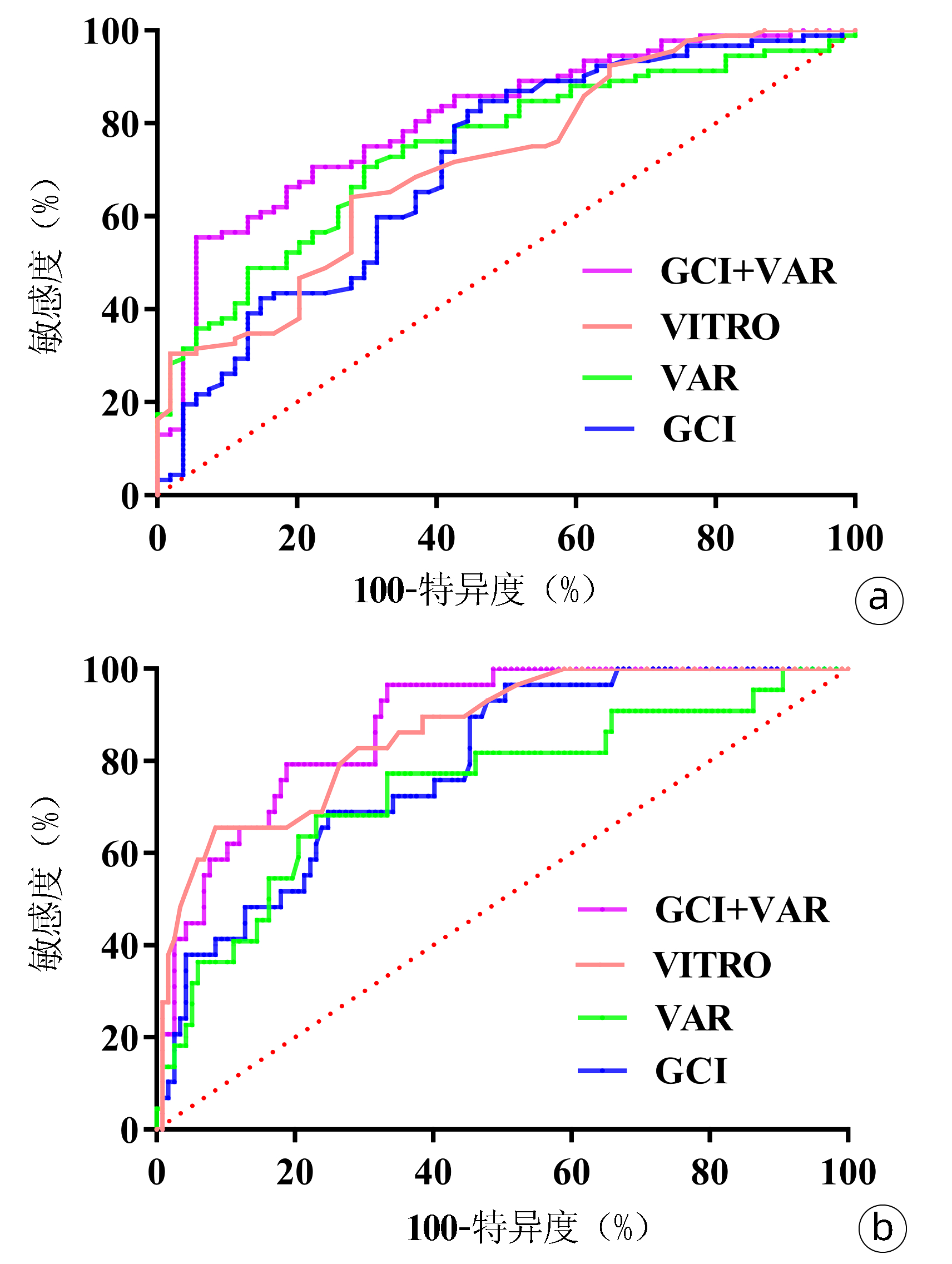
ROC曲线分析GCI、VAR和VITRO对EV的诊断性能。GCI评分的AUC为0.710,敏感度为84.78%,特异度为53.70%;VAR的AUC为0.745,敏感度为70.65%,特异度为70.37%;VITRO的AUC为0.718,敏感度为65.22%,特异度为72.22%。GCI联合VAR对EV诊断性能明显优于VITRO评分(P=0.009),此时的敏感度为55.43%,特异度为94.44%(图 3a,表 3)。
表 3 研究参数对预测EV的诊断性能比较Table 3. Comparison of the diagnostic performance of study variables for esophageal varices组别 AUC(95%CI) cut-off值 敏感度(%) 特异度(%) P值 GCI 0.710(0.629~0.782) 1.70 84.78 53.70 <0.001 VAR 0.745(0.666~0.813) 8.88 70.65 70.37 <0.001 VITRO 0.718(0.638~0.789) 2.77 65.22 72.22 <0.001 GCI+VAR 0.808(0.735~0.869) 55.43 94.44 <0.001 2.6 GCI、VAR和VITRO对重度EV诊断效能比较
ROC曲线分析GCI、VAR和VITRO对重度EV的诊断性能。GCI和VAR的AUC分别为0.787和0.755。VITRO的AUC为0.863,敏感度为65.52%,特异度为92.31%。GCI联合VAR诊断重度EV的AUC为0.869,与VITRO评分无差异(P=0.421),此时敏感度为89.66%,特异度为72.65%(图 3b,表 4)。
表 4 研究参数对重度EV的诊断性能比较Table 4. Comparison of the diagnostic performance of study variables for severe esophageal varices组别 AUC(95%CI) cut-off值 敏感度(%) 特异度(%) P值 GCI 0.787(0.712~0.850) 2.00 96.55 49.57 <0.001 VAR 0.755(0.677~0.822) 9.81 75.86 66.67 <0.001 VITRO 0.863(0.796~0.914) 5.37 65.52 92.31 <0.001 GCI+VAR 0.869(0.803~0.919) 89.66 72.65 <0.001 3. 讨论
本研究以胃镜检查为金标准,发现VAR和GCI评分与肝硬化EV发生独立相关。VAR和GCI可能是Child-Pugh A级肝硬化患者EV风险分层的潜在标志物。
内皮损伤引起的肝内微循环障碍,是门静脉高压病理生理学的重要因素[2]。本研究中EV患者的vWF水平显著高于无EV患者,这可能与剪切应力诱导vWF合成释放增加[11]以及ADAMTS13清除减少有关[12],但多因素分析vWF与EV之间相关性不强,这可能反映内皮功能障碍并不是门静脉高压的唯一决定因素。Alb具有多种生物学效应,临床前模型发现Alb可以通过其对炎症、氧化和细胞应激的作用来稳定内皮功能[6]。另外,临床研究[13]证实Alb通过增加有效循环血容量,改善血流动力学紊乱和循环功能障碍,减少肝硬化患者并发症发生。本研究中EV患者的血清Alb显著低于无EV患者。因此,结合vWF和Alb的VAR评分可能与门静脉高压风险密切相关。线性回归显示VAR与EV分级之间存在显著相关性,而且在Child-Pugh A和B级患者中,EV患者的VAR评分显著高于无EV患者,提示VAR可能是Child-Pugh A和B级肝硬化患者EV风险分层的潜在标志物。
Alb减少是肝硬化患者的常见症状[7]。GC是Alb膜糖蛋白Ib α链的蛋白水解片段,经Alb计数校正后的GCI被认为是Alb周转标志物[8]。本研究中EV患者的GCI显著升高,提示Alb更新加快,这可能与肝硬化脾功能亢进和血小板生成素生成受损有关[9]。另外,ROC曲线显示GCI对重度EV的诊断敏感度高达96.55%,提示GCI有助于识别EV发展风险较高的患者。
国外研究[10]报道VITRO评分与肝静脉压力梯度相关,是诊断门静脉高压的无创标志物。本研究多变量分析显示VAR和GCI与EV发生独立相关,两者联合诊断EV的AUC为0.808,特异度高达94.44%,与VITRO评分相比(AUC=0.718),差异具有统计学意义(P=0.009),提示相较于VITRO评分,联合模型能提高EV的诊断效能。
综上,本研究以肝硬化门静脉高压相关的内皮功能障碍和Alb动力学变化为基础,证实VAR和GCI对乙型肝炎肝硬化患者EV的预判价值。二者联合可以提高EV的诊断准确性,有利于对肝硬化患者的风险分层和管理。此外,本研究还有一些局限。首先,纳入人群均为乙型肝炎肝硬化患者且数量有限,需要在更大样本人群里中验证这些结果。其次,仅对单个时间点VAR和GCI与EV关系进行评价,而其在肝纤维化进程的变化可能对患者的危险分层可能更有意义。
-
表 1 EV组和无EV组患者基本特征
Table 1. Comparisons of characteristics between EV and non-EV in patients with cirrhosis
指标 无EV组(n=54) EV组(n=92) 统计值 P值 年龄(岁) 56.97±12.97 57.55±12.26 t=-0.276 0.345 男/女(例) 37/17 59/33 χ2=0.291 0.590 HBV DNA(log10 IU/mL) 3.6(3.0~5.1) 3.7(3.0~4.9) Z=-0.409 0.682 脾脏厚度(mm) 35.90±6.46 38.67±8.84 t=-2.183 0.031 PLT(109/L) 132.58±72.91 93.76±42.89 t=3.579 0.001 vWF-Ag(%) 277.43±65.45 316.80±87.30 t=-2.634 0.009 Alb(g/L) 35.52±5.61 31.83±5.20 t=4.026 <0.001 TBil(μmol/L) 41.8(27.8~61.5) 48.3(37.9~70.6) Z=-0.461 0.645 Cre(μmol/L) 72.3(60.5~100.6) 78.0(65.4~120.4) Z=-1.098 0.272 PT-INR 1.26(1.07~1.42) 1.47(1.19~1.79) Z=-3.083 0.002 GC(ng/mL) 0.92±0.50 0.89±0.27 t=0.441 0.661 MELD评分 11.17±4.75 15.66±6.09 t=-4.659 <0.001 Child-Pugh评分 6.31±1.43 8.16±1.86 t=-6.740 <0.001 Child-Pugh分级(A/B/C, 例数) 36/18/0 23/44/25 χ2=30.980 <0.001 GCI 2.00±1.16 2.89±1.67 t=-3.449 <0.001 VITRO 2.58±1.25 4.43±3.25 t=-4.871 <0.001 VAR 7.89±1.98 10.36±3.13 t=-5.819 <0.001 表 2 EV危险因素的Logistic回归分析
Table 2. Univariable and multivariable analysis of risk factors for esophageal varices
指标 单因素分析 多因素分析(模型1) 多因素分析 OR(95%CI) P值 OR(95%CI) P值 OR(95%CI) P值 PLT(109/L) 0.99(0.98~0.99) 0.001 vWF-Ag 1.01(1.00~1.01) 0.001 Alb(g/L) 0.88(0.82~0.94) <0.001 PT-INR 4.18(1.61~10.82) 0.003 MELD评分 1.21(1.10~1.32) <0.001 1.16 (1.05~1.27) 0.003 1.17 (1.07~1.29) 0.001 VAR 1.46(1.24~1.73) <0.001 1.46 (1.21~1.75) <0.001 GCI 1.85(1.27~2.59) <0.001 1.84 (1.22~2.77) 0.003 VITRO 1.73(1.31~2.28) <0.001 1.24 (1.68~2.28) 0.001 表 3 研究参数对预测EV的诊断性能比较
Table 3. Comparison of the diagnostic performance of study variables for esophageal varices
组别 AUC(95%CI) cut-off值 敏感度(%) 特异度(%) P值 GCI 0.710(0.629~0.782) 1.70 84.78 53.70 <0.001 VAR 0.745(0.666~0.813) 8.88 70.65 70.37 <0.001 VITRO 0.718(0.638~0.789) 2.77 65.22 72.22 <0.001 GCI+VAR 0.808(0.735~0.869) 55.43 94.44 <0.001 表 4 研究参数对重度EV的诊断性能比较
Table 4. Comparison of the diagnostic performance of study variables for severe esophageal varices
组别 AUC(95%CI) cut-off值 敏感度(%) 特异度(%) P值 GCI 0.787(0.712~0.850) 2.00 96.55 49.57 <0.001 VAR 0.755(0.677~0.822) 9.81 75.86 66.67 <0.001 VITRO 0.863(0.796~0.914) 5.37 65.52 92.31 <0.001 GCI+VAR 0.869(0.803~0.919) 89.66 72.65 <0.001 -
[1] Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery, Chinese Medical Association. Expert consensus on diagnosis and treatment of esophagogastric variceal bleeding in cirrhotic portal hypertension (2015 edition)[J]. Chin J Pract Surg, 2015, 35(10): 1086-1090. DOI: 10.7504/CJPS.ISSN1005-2208.2015.10.16.中华医学会外科学分会门静脉高压症学组. 肝硬化门静脉高压症食管、胃底静脉曲张破裂出血诊治专家共识(2015)[J]. 中国实用外科杂志, 2015, 35(10): 1086-1090. DOI: 10.7504/CJPS.ISSN1005-2208.2015.10.16. [2] POISSON J, LEMOINNE S, BOULANGER C, et al. Liver sinusoidal endothelial cells: Physiology and role in liver diseases[J]. J Hepatol, 2017, 66(1): 212-227. DOI: 10.1016/j.jhep.2016.07.009. [3] IWAKIRI Y, GROSZMANN RJ. Vascular endothelial dysfunction in cirrhosis[J]. J Hepatol, 2007, 46(5): 927-934. DOI: 10.1016/j.jhep.2007.02.006. [4] XU J, LIU Y, LOU WZY, et al. The anti-liver fibrosis effect of Kangxian Ruangan prescription: A cell study based on hepatic sinusoidal capillarization[J]. J Clin Hepatol, 2020, 36(2): 319-323. DOI: 10.3969/j.issn.1001-5256.2020.02.017.许杰, 刘艳, 楼汪洲洋, 等. 基于肝窦毛细血管化探讨抗纤软肝方抗肝纤维化的作用机制[J]. 临床肝胆病杂志, 2020, 36(2): 319-323. DOI: 10.3969/j.issn.1001-5256.2020.02.017. [5] MAIERON A, SALZL P, PECK-RADOSAVLJEVIC M, et al. Von willebrand factor as a new marker for non-invasive assessment of liver fibrosis and cirrhosis in patients with chronic hepatitis C[J]. Aliment Pharmacol Ther, 2014, 39(3): 331-338. DOI: 10.1111/apt.12564. [6] GARCIA-MARTINEZ R, ANDREOLA F, MEHTA G, et al. Immunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failure[J]. J Hepatol, 2015, 62(4): 799-806. DOI: 10.1016/j.jhep.2014.10.031. [7] FENG S, FENG HF, XU J, et al. Value of FibroScan and platelet count to spleen thickness ratio in predicting the degree of esophageal and gastric varices in liver cirrhosis[J]. J Clin Hepatol, 2021, 37(12): 2819-2823. DOI: 10.3969/j.issn.1001-5256.2021.12.018.封爽, 冯慧芬, 徐晶, 等. 肝硬度值和血小板计数/脾脏厚径比率对肝硬化食管胃底静脉曲张程度的预测价值[J]. 临床肝胆病杂志, 2021, 37(12): 2819-2823. DOI: 10.3969/j.issn.1001-5256.2021.12.018. [8] BEER JH, BVCHI L, STEINER B. Glycocalicin: a new assay——the normal plasma levels and its potential usefulness in selected diseases[J]. Blood, 1994, 83(3): 691-702. DOI: 10.1182/blood.V83.3.691.691 [9] WANG X, JIANG W, LI F, et al. Abnormal platelet kinetics are detected before the occurrence of thrombocytopaenia in HBV-related liver disease[J]. Liver Int, 2014, 34(4): 535-543. DOI: 10.1111/liv.12309. [10] HAMETNER S, FERLITSCH A, FERLITSCH M, et al. The VITRO score (Von Willebrand Factor Antigen/Thrombocyte Ratio) as a new marker for clinically significant portal hypertension in comparison to other non-invasive parameters of fibrosis including ELF test[J]. PLoS One, 2016, 11(2): e0149230. DOI: 10.1371/journal.pone.0149230. [11] SIEDLECKI CA, LESTINI BJ, KOTTKE-MARCHANT KK, et al. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor[J]. Blood, 1996, 88(8): 2939-2950. DOI: 10.1182/blood.V88.8.2939.bloodjournal8882939 [12] UEMURA M, FUJIMURA Y, KO S, et al. Pivotal role of ADAMTS13 function in liver diseases[J]. Int J Hematol, 2010, 91(1): 20-29. DOI: 10.1007/s12185-009-0481-4. [13] CARACENI P, TUFONI M, ZACCHERINI G, et al. On-treatment serum albumin level can guide long-term treatment in patients with cirrhosis and uncomplicated ascites[J]. J Hepatol, 2021, 74(2): 340-349. DOI: 10.1016/j.jhep.2020.08.021. 期刊类型引用(1)
1. 李光一. 未成熟血小板分数和糖萼素指数在肝硬化相关血小板减少症中的应用价值. 延边大学医学学报. 2024(02): 148-151 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2647 KB)
PDF下载 ( 2647 KB)

 下载:
下载:




 下载:
下载:

 百度学术
百度学术