胰腺癌合并新发糖尿病患者的临床特征分析
DOI: 10.3969/j.issn.1001-5256.2022.12.018
Clinical characteristics of patients with pancreatic cancer combined with new onset diabetes mellitus
-
摘要:
目的 探讨胰腺癌(PC)合并新发糖尿病(DM)患者的临床特征,为定义PC高危人群提供依据。 方法 回顾性选取2016年1月—2021年12月山西医科大学第一医院收治的426例PC病例,将其分为新发DM组(病程≤2年, n=74)、长期DM组(病程>2年, n=50)及单纯PC组(无DM, n=302),收集其基本人口学资料、吸烟饮酒史、既往史、家族史、DM用药情况、临床特征(首发症状、肿瘤直径、肿块位置、胰管扩张、手术切除情况)及生化指标(FPG、CA19-9、CA125),同时对PC合并DM行手术切除者的血糖情况进行随访,时间为术后半年。正态分布的计量资料组间比较采用t检验;非正态分布的计量资料组间比较采用Wilcoxon秩和检验。计数资料组间比较采用χ2检验或Fisher精确检验。 结果 426例PC患者中,68.3%为男性,31.7%为女性,PC合并DM人群中,新发DM占59.7%。较长期DM组,新发DM组发病年龄低(t=-2.041,P=0.043),合并高血压(χ2=3.950,P=0.047)、DM家族史(χ2=3.893,P=0.048)比例低,FPG水平低(Z=-2.740,P=0.005),吸烟者比例高(χ2=7.032,P=0.008),体质量变化明显(Z=-2.161,P=0.031),肿瘤直径大(Z=-2.269,P=0.023),伴胰管扩张者比例高(χ2=4.870,P=0.027),两组患者在DM用药方面差异有统计学意义(χ2=1.976,P<0.05)。对目标人群随访半年后,新发DM手术组血糖改善者7例(36.8%),长期DM手术组0例。较单纯PC组,新发DM组发病年龄低(t=-0.273,P=0.039),BMI水平稍高(t=-2.139,P=0.033),体质量变化明显(Z=-2.262,P=0.024),合并高血压比例高(χ2=17.438,P<0.001),FPG水平高(Z=-8.322,P<0.001),胰管扩张者比例高(χ2=3.983,P=0.046)。 结论 PC患者中,合并新发DM者发病年龄相对较低,有吸烟史、无DM家族史、伴体重明显下降、FPG水平难控制、伴胰管扩张的新发DM患者可能是PC高危人群,应注意早期筛查。 Abstract:Objective To investigate the clinical characteristics of patients with pancreatic cancer (PC) complicated with new-onset diabetes mellitus (DM), and to provide a basis for defining a high-risk group for PC. Methods The 426 PC cases admitted to the First Hospital of Shanxi Medical University from January 2016 to December 2021 were retrospectively selected and divided into new DM group (disease duration ≤2 years, n=74), long-term DM group (disease duration > 2 years, n=50) and simple PC group (no DM, n=302). We collected their basic demographic information, smoking and drinking history, disease history, family history, DM medication, clinical characteristics (first symptoms, tumor diameter, mass location, pancreatic duct dilatation, surgical resection) and biochemical indexes (FPG, CA19-9, CA125). The glycemic status of those who underwent surgical resection was monitored for six months after surgery. The t-test was used for comparison of normally distributed continuous data between two groups, and the Wilcoxon rank-sum test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test and the Fisher's exact test were used for comparison of categorical data between two groups. Results Of the 426 PC patients, 68.3% were male and 31.7% were female. New-onset DM accounted for 59.7% of the PC patients with DM. Compared with the long-term DM group, the new-onset DM group had a lower age of onset (t=-2.041, P=0.043), a lower proportion of combined hypertension (χ2=3.950, P=0.047), a lower family history of DM (χ2=3.893, P=0.048), a lower FPG level (Z=-2.740, P=0.005), a higher proportion of smokers (χ2=7.032, P=0.008), significant weight change (Z=-2.161, P=0.031), larger tumor diameter (Z=-2.269 P=0.023), high proportion of those with pancreatic duct dilatation (χ2=4.870, P=0.027), and significant differences in DM medication (χ2=1.976, P < 0.05). At six months of follow-up, 7 patients (36.8%) in the new-onset DM surgery group had glycemic improvement, but none in the long-term DM surgery group. Compared with the PC-only group, the new-onset DM group had a lower age of onset (t=-0.273, P=0.039), a slightly higher BMI level (t=-2.139, P=0.033), a significant weight change (Z=-2.262, P=0.024), a higher proportion of complicated hypertension (χ2=17.438, P < 0.001), a higher FPG level (Z=-8.322, P < 0.001), and a high proportion of dilated pancreatic ducts (χ2=3.983, P=0.046). Conclusion Among PC patients, the onset age is relatively young in those complicated with new-onset DM. Patients with new-onset DM who smoke, have no family history of DM, have significant weight loss, have difficulty in controlling FPG levels, and pancreatic duct dilatation may be at high-risk for PC and should be screened early. -
Key words:
- Pancreatic Neoplasms /
- Diabetes Mellitus /
- Pathology, Clinical
-
急性胰腺炎(AP)是临床常见急症之一,病死率较高,近年来,高脂血症已成为引起AP的第二大常见病因[1-2],与胆源性胰腺炎相比,高甘油三酯血症性急性胰腺炎(hypertriglyceridemia acute pancreatitis,HTGAP)患者趋向年轻化,重症化倾向更明显,并发症多并且病死率高[3]。为了更好地判断AP的严重程度及预后,国内外提出了许多评分系统,但少有研究对HTGAP的严重程度进行相对全面的比较。本研究回顾性分析HTGAP患者的临床资料,探讨五种评分系统对HTGAP病情和预后的预测价值。
1. 资料与方法
1.1 研究对象
本研究收集了宁夏医科大学总医院2016年1月— 2022年1月收治的HTGAP患者。纳入标准:(1)AP诊断标准符合2019年发布的中国急性胰腺炎诊治指南[4];(2) HTGAP诊断标准为血甘油三酯(TG)>11.3 mmol/L,或血TG水平在5.56~11.3 mmol/L且血清呈乳糜状。排除其他如酒精性、自身免疫性、胆源性、药物性等病因所致的AP。
1.2 研究方法
收集患者的临床病历资料,记录如下信息。(1)一般资料:年龄、性别、BMI等;(2)既往史、个人史、合并症、药物服用史等;(3)入院24 h实验室检查结果;(4)根据患者入院24 h内的病历资料进行HAPS评分、BISAP评分和APACHE-Ⅱ评分,根据48 h内的病历资料进行Ranson评分,根据患者临床资料进行PASS评分,进而根据患者病历资料评估病情严重程度及预后[5],每项指标均选择最为异常的数据进行评分。
1.3 统计学方法
采用SPSS 24.0软件对数据进行统计学分析。符合正态分布的计量资料以x±s表示,组间比较采用单因素方差分析;偏态资料以M(P25~P75)表示,三组间比较采用Kruskal-Wallis H秩和检验。计数资料组间比较采用χ2检验。并绘制受试者工作特征曲线(ROC曲线),计算敏感度、特异度、约登指数、95%CI、标准误,以最大约登指数对应的值为临界值(cut-off值),比较指标的ROC曲线下面积(AUC)。P<0.05为差异有统计学意义。
2. 结果
2.1 一般情况
共收集300例患者,其中男247例,女53例,年龄19~70岁,平均(36.8±8.7)岁。根据患者病情程度分为轻症急性胰腺炎(MAP)、中度重症急性胰腺炎(MSAP)和重症急性胰腺炎(SAP),三组患者分别占比为66.3%(199例)、16.1%(48例)和17.6%(53例),经比较三组间性别、年龄、BMI等基线资料差异均无统计学意义,对入院患者的生命体征进行比较,结果提示SAP组和MSAP患者呼吸频率明显高于MAP组,差异均有统计学意义(P值均<0.01),但三组患者心率差异无统计学意义;其次,本研究针对三组患者的个人史(吸烟史、饮酒史)、既往药物服用史、合并症(脂肪肝、代谢综合征及腹腔积液)进行比较,差异均无统计学意义,但经比较,SAP组患者合并呼吸衰竭、SIRS、代谢性酸中毒、脓毒症、MODS及胸腔积液的比例明显升高,且差异均有统计学意义(P值均<0.01)(表 1)。
表 1 3组患者一般情况比较Table 1. Comparison of the general information among the three groups指标 MAP(n=199) MSAP(n=48) SAP(n=53) 统计值 P值 年龄(岁) 35.0(30.0~40.0) 37.0(29.5~45.0) 36.0(31.3~42.8) χ2=2.518 0.284 BMI(kg/m2) 26.29(23.86~29.27) 27.34(25.47~28.52) 26.74(24.13~29.42) χ2=2.180 0.336 呼吸(次/min) 20(20~21) 21(20~21) 21(20~21) χ2=15.989 <0.001 心率(次/min) 26.74±4.48 26.50±4.46 36.21±8.61 F=0.608 0.545 性别[例(%)] χ2=1.263 0.540 男 167(83.9) 36(75.0) 44(83.0) 女 32(16.1) 12(25.0) 9(17.0) 吸烟[例(%)] 93(46.7) 23(47.9) 20(37.7) χ2=2.906 0.241 饮酒[例(%)] 80(40.2) 23(47.9) 21(39.6) χ2=2.180 0.349 药物服用史[例(%)] 39(19.6) 5(10.4) 13(24.5) χ2=2.531 0.286 合并脂肪肝[例(%)] 145(72.9) 38(79.2) 38(71.7) χ2=3.701 0.160 合并糖尿病[例(%)] 57(28.6) 16(33.3) 27(50.9) χ2=7.507 0.024 合并呼吸衰竭[例(%)] 0(0.0) 1(2.1) 4(7.5) χ2=11.241 0.002 合并高血压病[例(%)] 19(9.5) 5(10.4) 6(11.3) χ2=0.300 0.880 合并代谢性酸中毒[例(%)] 0(0.0) 1(2.1) 4(7.5) χ2=11.241 0.002 合并SIRS[例(%)] 0(0.0) 0(0.0) 4(7.5) χ2=11.139 0.002 合并脓毒症[例(%)] 0(0.0) 0(0.0) 4(7.5) χ2=11.139 0.002 合并MODS[例(%)] 0(0.0) 0(0.0) 4(7.5) χ2=11.139 0.002 合并胸腔积液[例(%)] 1(0.5) 3(6.3) 8(15.1) χ2=19.961 <0.001 合并腹腔积液[例(%)] 2(1.0) 0(0.0) 2(3.8) χ2=2.331 0.265 代谢综合征[例(%)] 5(2.5) 2(4.2) 1(1.9) χ2=0.958 0.749 注:SIRS,全身炎症反应综合征;MODS,多器官功能障碍综合征。 2.2 临床资料比较
本研究回顾性分析患者入院24 h的实验室检查资料,根据统计分析结果提示三组间白细胞计数(WBC)、中性粒细胞绝对值(NEUT#)、D-二聚体(D-dimer)、国际标准化比值(INR)、凝血酶原时间(PT)、空腹血糖(GLU)、血钙(Ca)、血尿素(BUN)、白蛋白(Alb)、乳酸脱氢酶(LDH)、血淀粉酶(AMY)、血脂肪酶(LIP)、C反应蛋白(CRP)、淋巴细胞绝对值(LYM) 差异均有统计学意义(P值均<0.05),提示上述指标为重症HTGAP发生的危险因素(表 2)。
表 2 三组患者实验室检验结果比较Table 2. Comparison of laboratory results among the three groups指标 MAP(n=199) MSAP(n=48) SAP(n=53) 统计值 P值 WBC(×109/L) 110.41±18.41 20.78±1.09 22.05±3.69 F=9.424 <0.001 NEUT#(%) 100.44±74.12 20.90±2.36 20.61±1.97 F=10.758 <0.001 LYM(×109/L) 1.520(1.180~2.210) 1.410(1.115~1.920) 1.250(0.800~1.620) χ2=11.328 0.003 MXD(×109/L) 0.705(0.520~0.900) 0.700(0.570~1.000) 0.690(0.553~0.983) χ2=1.486 0.476 PTL(×109/L) 7.35±0.09 105.09±15.74 110.41±18.42 F=1.544 0.215 HCT(%) 45.70(42.80~48.40) 46.70(43.30~49.20) 46.95(41.55~50.60) χ2=1.959 0.376 D-dimer(μg/mL) 0.30(0.20~0.64) 0.32(0.20~0.76) 0.82(0.34~1.96) χ2=25.235 <0.001 PT(s) 11.00(10.00~11.00) 11.20(10.15~11.75) 11.80(10.80~13.00) χ2=19.045 <0.001 INR 0.95(0.89~1.00) 0.960(0.865~1.025) 1.02(0.94~1.12) χ2=25.617 <0.001 GLU(mmol/L) 8.235(6.190~12.163) 9.440(6.895~14.325) 12.770(9.325~17.588) χ2=27.509 <0.001 Ca(mmol/L) 2.310(2.210~2.390) 2.250(2.090~2.360) 2.060(1.785~2.218) χ2=45.766 <0.001 K(mmol/L) 4.165(3.948~4.445) 4.210(3.910~4.350) 4.150(3.905~4.810) χ2=0.613 0.736 BUN(mmol/L) 4.275(3.450~5.393) 4.320(3.645~5.285) 5.390(3.915~6.635) χ2=11.603 0.003 CREA(μmol/L) 61.900(54.100~71.300) 58.600(51.350~68.950) 62.000(50.025~83.750) χ2=2.224 0.329 UA(μmol/L) 405.50(346.75~484.25) 375.00(329.50~480.50) 414.00(307.00~493.00) χ2=0.516 0.773 Alb(g/L) 43.850(40.780~46.930) 42.800(39.500~46.900) 38.700(34.350~43.025) χ2=24.657 <0.001 LDH(U/L) 531.00(432.25~633.25) 659.00(513.50~819.00) 780.00(480.75~1 268.75) χ2=39.743 <0.001 TC(mmol/L) 6.880(5.770~8.520) 7.460(6.200~10.425) 7.000(6.268~9.540) χ2=2.885 0.236 TG(mmol/L) 12.635(10.770~21.683) 11.860(11.850~24.975) 13.910(8.503~27.740) χ2=0.669 0.716 AMY(U/L) 196.00(90.75~358.00) 292.00(140.50~440.00) 411.00(190.00~607.50) χ2=17.024 <0.001 LIP(U/L) 1 161.5(434.0~3 641.0) 922.0(554.0~3 109.5) 2 283.0(1 016.0~5 589.0) χ2=9.880 0.007 CRP(mg/L) 89.00(45.00~136.00) 136.00(79.75~273.75) 154.50(90.00~255.50) χ2=28.317 <0.001 2.3 五种评分系统及临床结局比较
本研究结果显示,三组间PASS评分、Ranson评分、APACHE-Ⅱ评分和BISAP评分系统差异均有统计学意义(P值均<0.01);对三组患者的疾病转归进行比较,根据统计分析结果提示三组间住院天数、住院费用、入住ICU率和复发率差异均有统计学意义(P值均<0.05),但三组间死亡率差异均无统计学意义(P值均>0.05)(表 3)。
表 3 三组患者评分系统和疾病转归及预后比较Table 3. Comparison of scoring systems, disease outcome, and prognosis among the three groups of patients指标 MAP(n=199) MSAP(n=48) SAP(n=53) χ2值 P值 PASS评分 90.0(90.0~90.0) 140.0(115.0~140.0) 200.0(152.5~220.0) 219.351 <0.001 Ranson评分 1(1~2) 2(2~2) 2(2~2) 83.084 <0.001 HAPS评分 1(1~1) 1(1~1) 1(0~1) 0.480 0.799 APACHE-Ⅱ评分 1(1~1) 1(1~2) 2(1~2) 43.388 <0.001 BISAP评分 0(0~0) 0(0~0) 0(0~1) 50.785 <0.001 住院天数(d) 7.00(4.00~9.00) 11.00(8.00~14.50) 10.00(6.25~15.75) 48.281 <0.001 住院费用(元) 10 713(7 200~17 224) 17 027(11 800~24 020) 31 189(21 395~47 700) 93.894 <0.001 ICU[例(%)] 0(0.0) 0(0.0) 6(11.3) 17.317 <0.001 死亡[例(%)] 0(0.0) 0(0.0) 1(1.9) 3.662 0.338 复发[例(%)] 91(45.7) 14(29.2) 36(67.9) 11.357 0.003 2.4 五种评分对HTGAP病情严重程度、并发症和死亡的评估
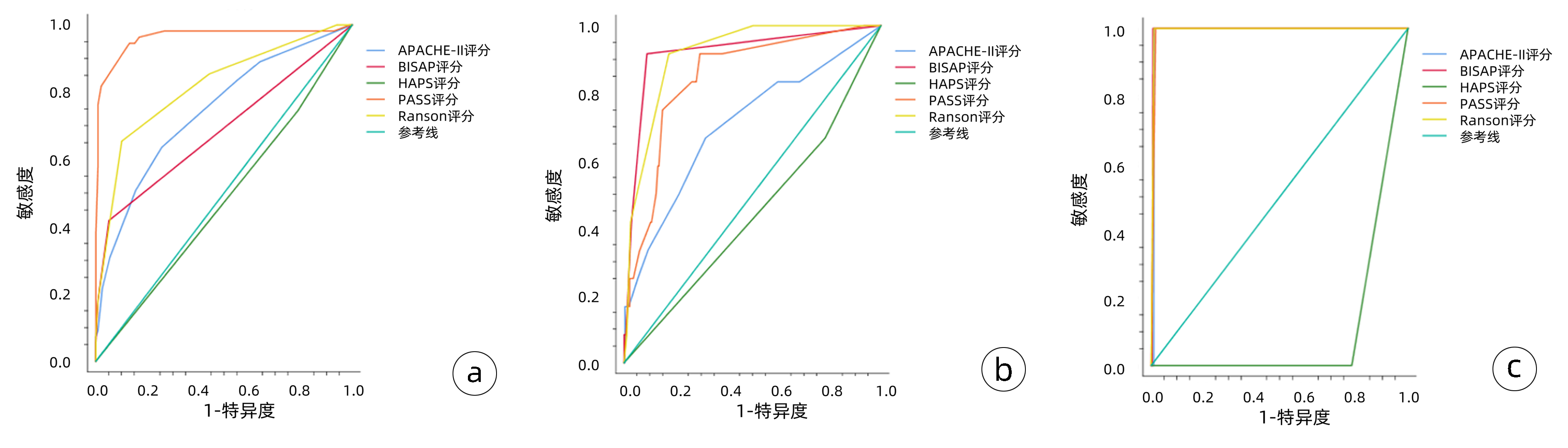
(1) 对病情严重程度(MSAP、SAP) 的预测: PASS评分的敏感度(0.945)和AUC(0.963)高于其他四种评分系统,Ranson评分的敏感度(0.655)和AUC(0.819)次之,HAPS评分AUC仅为0.478;(2)对并发症的预测:对于胸腔积液评估的AUC比较,BISAP评分>Ranson评分>PASS评分>APACHE-Ⅱ评分;(3)对于死亡的预测:BISAP评分和Ranson评分的AUC最高,均为0.995(表 4)。
表 4 五种评分系统对HTGAP患者病情严重程度、并发症和死亡的评估准确性比较Table 4. Comparison of the accuracy of the five scoring systems in evaluating patients' severity of disease, complications, and mortality评分系统及预后指标 AUC P值 敏感度 特异度 阳性预测值 阳性似然比 约登指数 APACHE-Ⅱ MSAP、SAP 0.744 <0.001 0.636 0.743 0.712 2.472 0.379 胸腔积液 0.704 0.016 0.683 0.350 0.678 2.105 0.350 死亡 0.990 <0.001 1.000 0.990 0.990 97.333 0.990 BISAP MSAP、SAP 0.687 <0.001 0.418 0.949 0.892 8.259 0.368 胸腔积液 0.919 <0.001 0.917 0.911 0.912 10.303 0.828 死亡 0.995 <0.001 1.000 0.993 0.993 146.000 0.993 PASS MSAP、SAP 0.963 <0.001 0.945 0.869 0.878 7.288 0.815 胸腔积液 0.846 <0.001 0.917 0.705 0.756 3.103 0.621 死亡 0.991 <0.001 1.000 0.983 0.983 58.400 0.983 Ranson MSAP、SAP 0.819 <0.001 0.655 0.899 0.866 6.464 0.553 胸腔积液 0.916 <0.001 0.917 0.826 0.840 5.257 0.742 死亡 0.995 <0.001 1.000 0.990 0.990 97.333 0.990 HAPS MSAP、SAP 0.418 0.619 0.745 0.211 0.486 0.945 -0.044 胸腔积液 0.442 0.512 0.512 0.217 0.460 0.852 -0.016 死亡 0.110 <0.001 0.000 0.219 0.000 0.000 -0.781 五种评分系统预测AP患者病情严重程度、并发症和死亡的ROC曲线见图 1,图 1a中ROC曲线对五种评分系统预测病情严重程度的准确度进行了比较,其中PASS评分预测病情严重程度的敏感度最高,其次是Ranson评分,HAPS评分对预测病情严重程度无统计学意义。图 1b中ROC曲线对五种评分系统预测并发症的准确度进行了比较,BISAP评分预测并发症的敏感度和特异度最高,其次是Ranson评分,HAPS评分对预测并发症无统计学意义。图 1c中ROC曲线对五种评分系统预测死亡的准确度进行了比较,除外HAPS评分,另外四种评分系统对预测死亡的敏感度、特异度均较高(均>0.90)。
3. 讨论
多个研究[6-7]表明,相较于其他病因所致的AP,HTGAP患者多为年轻人,病情进展更快更重,预后差且复发率高,正因为HTGAP特殊的临床表现,要求临床医师准确并高效的确定疾病的严重程度,对于潜在的重症患者尽早给予特殊的监护措施及治疗方案。目前虽无完美的预测HTGAP病情的工具,但存在一些多因素评分系统(基于临床、实验室、影像学证据)以及单因素评价指标(多为生物标志物),其侧重点和临床价值各不相同[8]。
HTGAP病程具有高度可变的特点, 早期患者可能表现为轻症, 但会迅速发展为危急重症状态, 为了更好地研究、评估和预测疾病发展,2017年国际专家提出了新的胰腺炎活动评分系统(PASS)[9-10],本研究结果显示对于HTGAP病情严重程度的预测: PASS评分的敏感度和AUC均高于其他四种评分系统。既往多数评分系统,大部分聚焦于早期评估HTGAP患者的病情严重程度,PASS评分的提出旨在对患者病程中以12 h为增量进行一次评分,不仅客观评估患者实验室指标,还加入了患者主观感受(如疼痛耐受程度、经口进食耐受程度),从理论上来说更能综合全面评估患者病情[11-12],动态预测不同时间点患者的病情严重程度等能力更优,综上,PASS可能对HTGAP患者的病情预测和干预具有更重要的临床意义,是一项很有前景的定量测量HTGAP患者疾病活动的评分系统。
Ranson评分纳入了11项临床和实验室参数,最初主要用于评估酒精性AP患者的病情。有些研究认为,Ranson评分耗时较长,内容相对繁琐,对临床应用价值不大,但随着对HTGAP发病机制的进一步了解,以及对于HTGAP的规范化治疗,近年来多项临床研究[13-14]证实了Ranson评分系统对于AP的病情严重程度评估的准确性和有效性。本研究发现SAP组的Ranson评分值显著高于其余两组, 其次,Ranson评分对于胸腔积液和死亡的预测能力也较高,这说明采用Ranson评分系统水平能够对HTGAP严重程度及预后进行一定的评价,但不足之处在于第2次评分需在入院48 h后进行,故难以早期预测HTGAP病情的走势。
同多数研究结果一致,BISAP在预测HTGAP严重程度、局部并发症等方面具有较高的准确性,且本研究提示BISAP评分对HTGAP的病情严重程度的预测特异度达94.5%。同时,BISAP评分系统临床数据易获取,操作相对简便,有利于早期指导临床治疗决策[15-17]。因此BISAP评分系统目前仍广泛在临床应用,但该评分系统在是否需要介入或手术干预等方面预测价值有限[18]。
HAPS评分是由Lankisch等经过一项前瞻性研究于2009年提出,该评分将无反跳痛和肌紧张、正常红细胞压积水平和正常血清肌酐水平这3项对非严重病程有最强预测的参数结合起来[8],多数研究提示入院时HAPS评分预测MAP的效能较高[19],但国内外鲜有关于HAPS评分对于HTGAP的大样本评价,本研究结果显示HAPS评分对于HTGAP严重程度的预测AUC仅为0.478,提示HAPS评分对于识别非重症急性胰腺炎有较高的临床价值,但其预测SAP的可靠性较低,对于重症HTGAP的预测价值有待于进一步研究。
本研究存在一定的局限性,单中心研究且样本量有限,回顾性的研究分析数据可能存在偏差,所以目前还需要大样本的、前瞻性、多中心的研究来验证以上评分系统对HTGPA患者病情严重程度及预后的预测作用。
综上所述,对于HTGAP患者,相较于其他四种评分系统,PASS评分可以更准确地评估HTGAP患者的病情严重程度,有望在临床中更好的推广应用。临床医师需要多结合各评分系统的优点,灵活使用每种评分系统的优势,以便及时、有效地评估病情,指导临床医师采取积极的治疗措施,从而降低HTGAP的病死率、改善预后。
-
表 1 426例患者基线资料
Table 1. Baseline data of 426 patients
特征 数值 年龄(岁) 64.59±10.79 性别[例(%)] 男 291(68.3) 女 135(31.7) 吸烟史[例(%)] 有 149(35.0) 无 277(65.0) 饮酒史[例(%)] 有 92(21.6) 无 334(78.4) 高血压史[例(%)] 有 137(32.2) 无 289(67.8) 血脂异常[例(%)] 有 290(68.1) 无 70(16.4) 不详 66(15.5) 体质量下降[例(%)] 有 240(56.3) 无 185(43.4) 不详 1(0.3) ΔWt(kg) 2.50(0~5.00) BMI(kg/m2) 21.69±3.25 首发症状[例(%)] 腹痛 190(44.6) 腹胀及不适 132(31.0) 黄疸 73(17.1) 体检 22(5.2) 肿瘤部位[例(%)] 胰头 257(60.3) 胰体尾 157(36.9) 其他 12(2.8) 胰管扩张[例(%)] 有 254(59.6) 无 150(35.2) 不详 22(5.2) 手术切除[例(%)] 是 90(21.1) 否 336(78.9) CA19-9(U/mL) 203.50(57.05~750.5) CA125(U/mL) 39.50(15.66~203.93) 表 2 新发DM组和长期DM组基本特征比较
Table 2. Comparison of basic characteristics between the new-onset DM group and the long-term DM group
特征 新发DM组(n=74) 长期DM组(n=50) 统计值 P值 年龄(岁) 62.04±11.77 66.22±10.26 t=-2.041 0.043 性别[例(%)] χ2=0.170 0.681 男 50(67.6) 32(64.0) 女 24(32.4) 18(36.0) 吸烟史[例(%)] χ2=7.032 0.008 有 30(40.5) 9(18.0) 无 44(59.5) 41(82.0) 饮酒史[例(%)] χ2=1.976 0.160 有 18(24.3) 7(14.0) 无 56(75.7) 43(86.0) BMI(kg/m2) 22.40±3.28 21.82±2.98 0.319 ΔWt(kg) 5.00(0~7.63) 3.00(0~5.00) Z=-2.161 0.031 高血压史[例(%)] χ2=3.950 0.047 有 37(50.0) 34(68.0) 无 37(50.0) 16(32.0) 血脂异常[例(%)] χ2=1.089 0.297 有 57(77.0) 32(64.0) 无 12(16.2) 11(22.0) 不详 5(6.8) 7(14.0) 胆石症史[例(%)] χ2=0.402 0.526 有 17(23.0) 14(28.0) 无 57(77.0) 36(72.0) 胆囊切除术[例(%)] χ2=0.168 0.682 有 6(8.1) 6(12.0) 无 68(91.9) 44(88.0) PC家族史[例(%)] χ2=1.330 0.249 有 4(5.4) 0 无 70(94.6) 50(100.0) DM家族史[例(%)] χ2=3.893 0.048 有 3(4.1) 8(16.0) 无 71(95.9) 42(84.0) 表 3 新发DM组和长期DM组临床特征比较
Table 3. Comparison of clinical characteristics between the new-onset DM group and the long-term DM group
特征 新发DM组(n=74) 长期DM组(n=50) 统计值 P值 首发症状[例(%)] 腹痛 27(36.5) 22(44.0) χ2=0.705 0.401 腹胀及不适 19(25.7) 13(26.0) χ2=0.002 0.968 黄疸 11(14.9) 6(12.0) χ2=0.207 0.649 体检 7(9.5) 4(8.0) χ2=0.000 1.0 肿瘤直径(cm) 4.00(2.92~4.92) 3.5(2.50~4.30) Z=-2.269 0.023 肿瘤部位[例(%)] χ2=2.869 0.090 头部 52(70.3) 27(54.0) 体尾部 21(28.4) 21(42.0) 不详 1(1.3) 2(4.0) 胰管扩张[例(%)] χ2=4.870 0.027 有 54(73.0) 25(50.0) 无 19(25.7) 21(42.0) 不详 1(1.3) 4(8.0) 手术切除[例(%)] χ2=0.045 0.833 是 19(25.7) 12(24.0) 否 55(74.3) 38(76.0) 用药情况[例(%)] χ2=1.976 <0.001 口服药 20(27.0) 21(42.0) 胰岛素 21(28.4) 16(32.0) 口服药+胰岛素 7(9.5) 11(22.0) 未治疗 26(35.1) 2(4.0) FPG(mmol/L) 9.00(7.00~10.16) 11.07(7.57~13.65) Z=-2.740 0.005 CA19-9(U/mL) 290.88(58.68~1238.94) 336.50(64.48~1038.40) Z=-0.020 0.984 CA125(U/mL) 38.50(15.30~192.03) 48.90(12.90~292.65) Z=-0.190 0.850 表 4 新发DM与长期DM行手术患者血糖随访情况
Table 4. Follow-up of blood glucose in patients with new onset DM and long-term DM undergoing surgery
分组 例数 血糖改善[例(%)] 新发DM手术组 19 7(36.8) 长期DM手术组 12 0 表 5 新发DM组与单纯PC组基本特征比较
Table 5. Comparison of basic characteristics between the new-onset DM group and the PC-only group
特征 新发DM组(n=74) 单纯PC组(n=302) 统计值 P值 年龄(岁) 62.04±11.77 64.95±10.56 t=-0.273 0.039 性别[例(%)] χ2=0.074 0.785 男 50(67.6) 209(69.2) 女 24(32.4) 93(30.8) 吸烟史[例(%)] χ2=0.431 0.511 有 30(40.5) 110(36.4) 无 44(59.5) 192(63.6) 饮酒史[例(%)] χ2=0.155 0.693 有 18(24.3) 67(22.2) 无 56(75.7) 235(77.8) ΔWt(kg) 5.00(0~7.63) 2.50(0~5.00) Z=-2.262 0.024 BMI(kg/m2) 22.40±3.28 21.49±3.27 t=-2.139 0.033 高血压史[例(%)] χ2=17.438 <0.001 有 37(50.0) 76(25.2) 无 37(50.0) 226(74.8) 血脂异常[例(%)] χ2=0.087 0.768 有 57(77.0) 201(66.6) 无 12(16.2) 47(15.6) 不详 5(6.8) 54(17.8) 胆石症史[例(%)] χ2=0.317 0.573 有 17(23.0) 79(26.2) 无 57(77.0) 223(73.8) 胆囊切除术[例(%)] χ2=0.852 0.098 有 6(8.1) 16(5.3) 无 68(91.9) 286(94.7) 表 6 新发DM组与单纯PC组临床特征比较
Table 6. Comparison of clinical characteristics between the new-onset DM group and the PC-only group
特征 新发DM组(n=74) 单纯PC组(n=302) 统计值 P值 首发症状[例(%)] 腹痛 27(36.5) 141(46.7) χ2=2.503 0.114 腹胀及不适 19(25.7) 100(33.1) χ2=1.520 0.218 黄疸 11(14.9) 56(18.5) χ2=0.549 0.459 体检 7(9.5) 11(3.6) χ2=4.412 0.036 肿瘤直径(cm) 4.00(2.92~4.92) 3.90(3.00~4.80) Z=-1.012 0.311 肿瘤部位[例(%)] χ2=2.750 0.097 头部 52(70.3) 178(59.0) 体尾部 21(28.4) 115(38.1) 不详 1(1.3) 9(2.9) 胰管扩张[例(%)] χ2=3.983 0.046 有 54(73.0) 175(58.0) 无 19(25.7) 110(36.4) 不详 1(1.3) 17(5.6) 手术切除[例(%)] χ2=1.363 0.243 是 19(25.7) 59(19.5) 否 55(74.3) 243(80.5) FPG(mmol/L) 9.00(7.00~10.16) 5.70(5.00~6.82) Z=-8.322 <0.001 CA19-9(U/mL) 290.88(58.68~1238.94) 166.00(56.44~628.37) Z=-1.070 0.285 CA125(U/mL) 38.50(15.30~192.03) 38.00(15.80~158.61) Z=-0.081 0.936 -
[1] SIEGEL RL, MILLER KD, FUCHS HE, et al. Cancer Statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. DOI: 10.3322/caac.21654. [2] CAO W, CHEN HD, YU YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020[J]. Chin Med J (Engl), 2021, 134(7): 783-791. DOI: 10.1097/CM9.0000000000001474. [3] SHI CG, LIU XH, XIE YX, et al. Research advances in pancreatic exocrine insufficiency secondary to pancreatic cancer[J]. J Clin Hepatol, 2021, 37(4): 982-984. DOI: 10.3969/j.issn.1001-5256.2021.04.057.史晨光, 刘晓欢, 谢亚兴, 等. 继发于胰腺癌的胰腺外分泌功能不全的研究进展[J]. 临床肝胆病杂志, 2021, 37(4): 982-984. DOI: 10.3969/j.issn.1001-5256.2021.04.057. [4] ZHANG LX, ZHAO HR, ZHANG ZQ. On the effect of Roy adaptation mode nursing on negative emotion and postoperative pain in patients with pancreatic cancer[J]. J Changchun Univ Chin Med, 2021, 37(1): 171-173. DOI: 10.13463/j.cnki.cczyy.2021.01.046.张连香, 赵海蓉, 张志琴. Roy适应模式护理对胰腺癌患者负性情绪及术后疼痛程度的影响分析[J]. 长春中医药大学学报, 2021, 37(1): 171-173. DOI: 10.13463/j.cnki.cczyy.2021.01.046. [5] ZHANG JJ, JIA JP, SHAO Q, et al. Diabetes mellitus and risk of pancreatic cancer in China: A meta-analysis based on 26 case-control studies[J]. Prim Care Diabetes, 2019, 13(3): 276-282. DOI: 10.1016/j.pcd.2018.11.015. [6] ANDERSEN DK, KORC M, PETERSEN GM, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer[J]. Diabetes, 2017, 66(5): 1103-1110. DOI: 10.2337/db16-1477. [7] HUANG BZ, PANDOL SJ, JEON CY, et al. New-onset diabetes, longitudinal trends in metabolic markers, and risk of pancreatic cancer in a heterogeneous population[J]. Clin Gastroenterol Hepatol, 2020, 18(8): 1812-1821.e7. DOI: 10.1016/j.cgh.2019.11.043. [8] LI Y, TENG D, SHI X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study[J]. BMJ, 2020, 369: m997. DOI: 10.1136/bmj.m997. [9] BOND-SMITH G, BANGA N, HAMMOND TM, et al. Pancreatic adenocarcinoma[J]. BMJ, 2012, 344: e2476. DOI: 10.1136/bmj.e2476. [10] BOURSI B, FINKELMAN B, GIANTONIO BJ, et al. A clinical prediction model to assess risk for pancreatic cancer among patients with prediabetes[J]. Eur J Gastroenterol Hepatol, 2022, 34(1): 33-38. DOI: 10.1097/MEG.0000000000002052. [11] HUANG J, LOK V, NGAI CH, et al. Worldwide burden of, risk factors for, and trends in pancreatic cancer[J]. Gastroenterology, 2021, 160(3): 744-754. DOI: 10.1053/j.gastro.2020.10.007. [12] MIDHA S, CHAWLA S, GARG PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review[J]. Cancer Lett, 2016, 381(1): 269-277. DOI: 10.1016/j.canlet.2016.07.022. [13] MAISONNEUVE P. Epidemiology and burden of pancreatic cancer[J]. Presse Med, 2019, 48(3 Pt 2): e113, e123. DOI: 10.1016/j.lpm.2019.02.030. [14] DONG X, LOU YB, MU YC, et al. Predictive factors for differentiating pancreatic cancer-associated diabetes mellitus from common type 2 diabetes mellitus for the early detection of pancreatic cancer[J]. Digestion, 2018, 98(4): 209-216. DOI: 10.1159/000489169. [15] CAI J, CHEN H, LU M, et al. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis[J]. Cancer Lett, 2021, 520: 1-11. DOI: 10.1016/j.canlet.2021.06.027. [16] MATSUBAYASHI H, KIYOZUMI Y, ISHIWATARI H, et al. Surveillance of individuals with a family history of pancreatic cancer and inherited cancer syndromes: A strategy for detecting early pancreatic cancers[J]. Diagnostics(Basel), 2019, 9(4). DOI: 10.3390/diagnostics9040169. [17] LANG J, KUNOVSKÝ L, KALA Z, et al. Risk factors of pancreatic cancer and their possible uses in diagnostics[J]. Neoplasma, 2021, 68(2): 227-239. DOI: 10.4149/neo_2020_200706N699. [18] ZHANG FY, ADILA YKP, ZHAO JM, et al. New advances in the treatment of pancreatic cancer by targeting tumor microenvironment[J]. J Clin Hepatol, 2021, 37(9): 2246-2248. DOI: 10.3969/j.issn.1001-5256.2021.09.049.张飞宇, 阿迪拉·亚克普, 赵金明, 等. 靶向肿瘤微环境治疗胰腺癌的新进展[J]. 临床肝胆病杂志, 2021, 37(9): 2246-2248. DOI: 10.3969/j.issn.1001-5256.2021.09.049. [19] MA ML, ZHOU MC, YANG J, et al. Clinical characteristics of pancreatic cancer with different duration of diabetes and impact of related risk factors on onset age of pancreatic cancer[J]. Med J Peking Union Med Coll Hosp, 2015, 6(6): 419-426. DOI: 10.3969/j.issn.1674-9081.2015.06.005.马明磊, 周美岑, 杨婧, 等. 合并不同糖尿病病程的胰腺癌患者临床特征及相关危险因素对胰腺癌发病年龄的影响[J]. 协和医学杂志, 2015, 6(6): 419-426. DOI: 10.3969/j.issn.1674-9081.2015.06.005. [20] Collaborative Group of Pancreatic Diseases, Gastrointestinal Endoscopy Branch. Chinese consensus on the early screening and surveillance for pancreatic cancer in the high-risk individuals(2021, Nanjing)[J]. Chin J Pancretol, 2022, 22(1): 1-13. DOI: 10.3760/cma.j.cn115667-20211224-00224.中华医学会消化内镜学分会胰腺疾病协作组. 中国胰腺癌高危人群早期筛查和监测共识意见(2021, 南京)[J]. 中华胰腺病杂志, 2022, 22(1): 1-13. DOI: 10.3760/cma.j.cn115667-20211224-00224. [21] PANNALA R, LEIRNESS JB, BAMLET WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus[J]. Gastroenterology, 2008, 134(4): 981-987. DOI: 10.1053/j.gastro.2008.01.039. [22] SAH RP, NAGPAL SJ, MUKHOPADHYAY D, et al. New insights into pancreatic cancer-induced paraneoplastic diabetes[J]. Nat Rev Gastroenterol Hepatol, 2013, 10(7): 423-433. DOI: 10.1038/nrgastro.2013.49. [23] SHAO CW. Focus on the diagnosis of pancreatic diseases from the perspective of pancreatic duct changes[J]. Chin J Pancretol, 2021, 21(6): 401-405. DOI: 10.3760/cma.j.cn115667-20211016-00184.邵成伟. 从胰管改变谈胰腺疾病的诊断[J]. 中华胰腺病杂志, 2021, 21(6): 401-405. DOI: 10.3760/cma.j.cn115667-20211016-00184. -

 本文二维码
本文二维码
计量
- 文章访问数: 1551
- HTML全文浏览量: 1103
- PDF下载量: 64
- 被引次数: 0



 PDF下载 ( 1916 KB)
PDF下载 ( 1916 KB)

 下载:
下载:

 下载:
下载: