转化生长因子β在胰腺癌发生发展中的作用
DOI: 10.3969/j.issn.1001-5256.2022.12.041
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:张浩负责课题设计,资料分析,撰写论文;侯晓凡、霍峥负责查阅文献;刘林勋、赵占学、潘洪帅负责校正,修改论文,最后定稿。
Role of transforming growth factor-β in the development and progression of pancreatic cancer
-
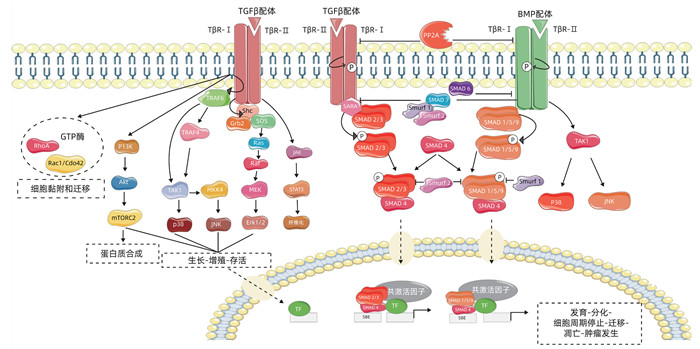
摘要: 胰腺导管腺癌恶性程度高,起病隐匿,病情进展快,在早期无明显的腹部表现及体征。多数患者就诊时已发展为中晚期,并常伴远处脏器转移,因此手术治疗、放化疗及药物靶向治疗效果通常不佳。近年来研究表明,转化生长因子β(TGFβ)与肿瘤的发生、发展密不可分,在肿瘤细胞的增殖、侵袭、迁移和血管的生成等进程中发挥关键性的调节作用。尽管TGFβ信号能够利用SMAD介导的细胞周期阻滞起到强大的肿瘤抑制作用,但是TGFβ信号也可以通过增强上皮-间质转化、纤维化和免疫逃逸来加速胰腺癌的发生。本文所述胰腺癌均特指为胰腺导管腺癌,旨在对TGFβ在其信号传导中的作用及其相关因子和免疫反应等方面关系的研究予以综述,为今后胰腺癌的靶向治疗提供理论依据。Abstract: Pancreatic ductal adenocarcinoma has a high degree of malignancy, an insidious onset, and rapid progression, with no obvious abdominal manifestations and signs in the early stage. Most patients are already in the advanced stage and have distant organ metastasis at the time of diagnosis, and thus surgical treatment, chemoradiotherapy, and targeted drug therapy often have an unsatisfactory clinical effect. Recent studies have shown that transforming growth factor-β (TGF-β) is closely associated with the development and of tumors and plays a key role in the processes of tumor cell proliferation, invasion, migration, and angiogenesis. Although TGF-β signal can exert a powerful inhibitory effect on tumors through SMAD-mediated cell cycle arrest, TGF-β signal can also accelerate the development of pancreatic cancer by enhancing epithelial-mesenchymal transition, fibrosis, and immune escape. In this article, pancreatic cancer specifically refers to pancreatic ductal adenocarcinoma, and this article reviews the role of TGF-β in its signal transduction and the association of TGF-β with related factors and immune response, so as to provide a theoretical basis for targeted therapy for pancreatic cancer in the future.
-
[1] General Office of National Health Commission. Standard for diagnosis and treatment of pancreatic cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(5): 1006-1030. DOI: 10.3969/j.issn.1001-5256.2022.05.007.国家卫生健康委办公厅. 胰腺癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38(5): 1006-1030. DOI: 10.3969/j.issn.1001-5256.2022.05.007. [2] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [3] SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70(1): 7-30. DOI: 10.3322/caac.21590. [4] TANG D, WU Q, ZHANG J, et al. Galectin-1 expression in activated pancreatic satellite cells promotes fibrosis in chronic pancreatitis/pancreatic cancer via the TGF-β1/Smad pathway[J]. Oncol Rep, 2018, 39(3): 1347-1355. DOI: 10.3892/or.2018.6202. [5] KAJDANIUK D, MAREK B, BORGIEL-MAREK H, et al. Transforming growth factor β1 (TGFβ1) in physiology and pathology[J]. Endokrynol Pol, 2013, 64(5): 384-396. DOI: 10.5603/EP.2013.0022. [6] KUMARI A, SHONIBARE Z, MONAVARIAN M, et al. TGFβ signaling networks in ovarian cancer progression and plasticity[J]. Clin Exp Metastasis, 2021, 38(2): 139-161. DOI: 10.1007/s10585-021-10077-z. [7] GALLO-OLLER G, DI SCALA M, ARANDA F, et al. Transforming growth factor beta (TGF-β) activity in immuno-oncology studies[J]. Methods Enzymol, 2020, 636: 129-172. DOI: 10.1016/bs.mie.2019.06.008. [8] ZHAO Y, QIAO W, WANG X, et al. 14-3-3ζ/TGFβR1 promotes tumor metastasis in lung squamous cell carcinoma[J]. Oncotarget, 2016, 7(50): 82972-82984. DOI: 10.18632/oncotarget.12690. [9] FAN JY, WANG J. Expression of Mir-196b and TGFβR1 in pancreatic ductal adenocarcinoma and its significance[J]. Shandong Med J, 2021, 61(10): 53-56. DOI: 10.3969/j.issn.1002-266X.范婧怡, 王健. 胰腺导管腺癌组织miR-196b、TGFβR1表达变化及其意义[J]. 山东医药, 2021, 61(10): 53-56. DOI: 10.3969/j.issn.1002-266X. [10] PRINCIPE DR, DOLL JA, BAUER J, et al. TGF-β: duality of function between tumor prevention and carcinogenesis[J]. J Natl Cancer Inst, 2014, 106(2): djt369. DOI: 10.1093/jnci/djt369. [11] WEISS A, ATTISANO L. The TGFbeta superfamily signaling pathway[J]. Wiley Interdiscip Rev Dev Biol, 2013, 2(1): 47-63. DOI: 10.1002/wdev.86. [12] JIA EC, ZHU WL. Research progress of transforming growth factor β Ⅱ receptor in digestive system tumors. [J]. Chongqing Med J, 2016, 45(23): 3293-3295, 3299. DOI: 10.3969/j.issn.1671-8348.2016.23.046.贾恩朝, 朱武凌. 转化生长因子β Ⅱ型受体在消化系统肿瘤中的研究进展[J]. 重庆医学, 2016, 45(23): 3293-3295, 3299. DOI: 10.3969/j.issn.1671-8348.2016.23.046. [13] GUO W, DONG Z, GUO Y, et al. Association of polymorphisms in transforming growth factor-β receptors with susceptibility to gastric cardia adenocarcinoma[J]. Mol Biol Rep, 2012, 39(4): 4301-4309. DOI: 10.1007/s11033-011-1217-0. [14] BIANKIN AV, WADDELL N, KASSAHN KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes[J]. Nature, 2012, 491(7424): 399-405. DOI: 10.1038/nature11547. [15] CHEN YN, ZHANG XY. Research progress on the role of transforming growth factor β Ⅲ receptor in the development of human tumors[J]. J Modern Oncol, 2022, 30(10): 1907-1910. DOI: 10.3969/j.issn.1672-4992.陈亚楠, 张学彦. 转化生长因子βⅢ型受体在人类肿瘤发生发展中作用的研究进展[J]. 现代肿瘤医学, 2022, 30(10): 1907-1910. DOI: 10.3969/j.issn.1672-4992. [16] TZAVLAKI K, MOUSTAKAS A. TGF-β Signaling[J]. Biomolecules, 2020, 10(3). DOI: 10.3390/biom10030487. [17] YIN Z, MA T, HUANG B, et al. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway[J]. J Exp Clin Cancer Res, 2019, 38(1): 310. DOI: 10.1186/s13046-019-1313-x. [18] ZOU ML, CHEN ZH, TENG YY, et al. The Smad dependent TGF-β and BMP signaling pathway in bone remodeling and therapies[J]. Front Mol Biosci, 2021, 8: 593310. DOI: 10.3389/fmolb.2021.593310. [19] MARTIN-MALPARTIDA P, BATET M, KACZMARSKA Z, et al. Structural basis for genome wide recognition of 5-bp GC motifs by SMAD transcription factors[J]. Nat Commun, 2017, 8(1): 2070. DOI: 10.1038/s41467-017-02054-6. [20] PRINCIPE DR, DECANT B, MASCARIÑAS E, et al. TGFβ signaling in the pancreatic tumor microenvironment promotes fibrosis and immune evasion to facilitate tumorigenesis[J]. Cancer Res, 2016, 76(9): 2525-2539. DOI: 10.1158/0008-5472.CAN-15-1293. [21] WANG F, XIA X, YANG C, et al. SMAD4 gene mutation renders pancreatic cancer resistance to radiotherapy through promotion of autophagy[J]. Clin Cancer Res, 2018, 24(13): 3176-3185. DOI: 10.1158/1078-0432.CCR-17-3435. [22] SHUGANG X, HONGFA Y, JIANPENG L, et al. Prognostic value of SMAD4 in pancreatic cancer: A meta-analysis[J]. Transl Oncol, 2016, 9(1): 1-7. DOI: 10.1016/j.tranon.2015.11.007. [23] DU Y, ZHOU X, HUANG Z, et al. Meta-analysis of the prognostic value of smad4 immunohistochemistry in various cancers[J]. PLoS One, 2014, 9(10): e110182. DOI: 10.1371/journal.pone.0110182. [24] WANG JD, JIN K, CHEN XY, et al. Clinicopathological significance of SMAD4 loss in pancreatic ductal adenocarcinomas: a systematic review and meta-analysis[J]. Oncotarget, 2017, 8(10): 16704-16711. DOI: 10.18632/oncotarget.14335. [25] KANG Y, LING J, SUZUKI R, et al. SMAD4 regulates cell motility through transcription of N-cadherin in human pancreatic ductal epithelium[J]. PLoS One, 2014, 9(9): e107948. DOI: 10.1371/journal.pone.0107948. [26] LEUNG L, RADULOVICH N, ZHU CQ, et al. Loss of canonical Smad4 signaling promotes KRAS driven malignant transformation of human pancreatic duct epithelial cells and metastasis[J]. PLoS One, 2013, 8(12): e84366. DOI: 10.1371/journal.pone.0084366. [27] LI Y, BASTI A, YALÇIN M, et al. Circadian dysregulation of the TGFβ/SMAD4 pathway modulates metastatic properties and cell fate decisions in pancreatic cancer cells[J]. iScience, 2020, 23(10): 101551. DOI: 10.1016/j.isci.2020.101551. [28] DARDARE J, WITZ A, MERLIN JL, et al. SMAD4 and the TGFβ pathway in patients with pancreatic ductal adenocarcinoma[J]. Int J Mol Sci, 2020, 21(10). DOI: 10.3390/ijms21103534. [29] NEUZILLET C, HAMMEL P, TIJERAS-RABALLAND A, et al. Targeting the Ras-ERK pathway in pancreatic adenocarcinoma[J]. Cancer Metastasis Rev, 2013, 32(1-2): 147-162. DOI: 10.1007/s10555-012-9396-2. [30] BUONATO JM, LAZZARA MJ. ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition[J]. Cancer Res, 2014, 74(1): 309-319. DOI: 10.1158/0008-5472.CAN-12-4721. [31] PRINCIPE DR, DIAZ AM, TORRES C, et al. TGFβ engages MEK/ERK to differentially regulate benign and malignant pancreas cell function[J]. Oncogene, 2017, 36(30): 4336-4348. DOI: 10.1038/onc.2016.500. [32] GUO Q. Changes in mitochondrial function during EMT induced by TGFβ-1 in pancreatic cancer[J]. Oncol Lett, 2017, 13(3): 1575-1580. DOI: 10.3892/ol.2017.5613. [33] CHANG CH, PAUKLIN S. ROS and TGFβ: from pancreatic tumour growth to metastasis[J]. J Exp Clin Cancer Res, 2021, 40(1): 152. DOI: 10.1186/s13046-021-01960-4. [34] SANJABI S, OH SA, LI MO. Regulation of the immune response by TGF-β: From conception to autoimmunity and infection[J]. Cold Spring Harb Perspect Biol, 2017, 9(6): a022236. DOI: 10.1101/cshperspect.a022236. [35] MA B, LI JG, WANG J, et al. Promotion effect of miR-106b on invasion and migration of colon cancer cells through targeting TGF-β/Smad pathway[J]. J Jilin Univ(Med Edit), 2021, 47(3): 636-636. DOI: 10.13481/j.1671-587Ⅹ.20210312.马博, 李建刚, 王俊, 等. miR-106b靶向调控TGF-β/Smad通路对结肠癌细胞侵袭和迁移的促进作用[J]. 吉林大学学报(医学版), 2021, 47(3): 636-636. DOI: 10.13481/j.1671-587Ⅹ.20210312. [36] DAVID CJ, MASSAGUÉ J. Contextual determinants of TGFβ action in development, immunity and cancer[J]. Nat Rev Mol Cell Biol, 2018, 19(7): 419-435. DOI: 10.1038/s41580-018-0007-0. [37] ANDERTON MJ, MELLOR HR, BELL A, et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors[J]. Toxicol Pathol, 2011, 39(6): 916-924. DOI: 10.1177/0192623311416259. [38] MELISI D, GARCIA-CARBONERO R, MACARULLA T, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer[J]. Br J Cancer, 2018, 119(10): 1208-1214. DOI: 10.1038/s41416-018-0246-z. [39] FAIVRE S, SANTORO A, KELLEY RK, et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma[J]. Liver Int, 2019, 39(8): 1468-1477. DOI: 10.1111/liv.14113. [40] KOVACS RJ, MALDONADO G, AZARO A, et al. Cardiac safety of TGF-β receptor i kinase inhibitor LY2157299 monohydrate in cancer patients in a First-in-Human dose study[J]. Cardiovasc Toxicol, 2015, 15(4): 309-323. DOI: 10.1007/s12012-014-9297-4. -



 PDF下载 ( 2378 KB)
PDF下载 ( 2378 KB)

 下载:
下载:


