-
摘要:
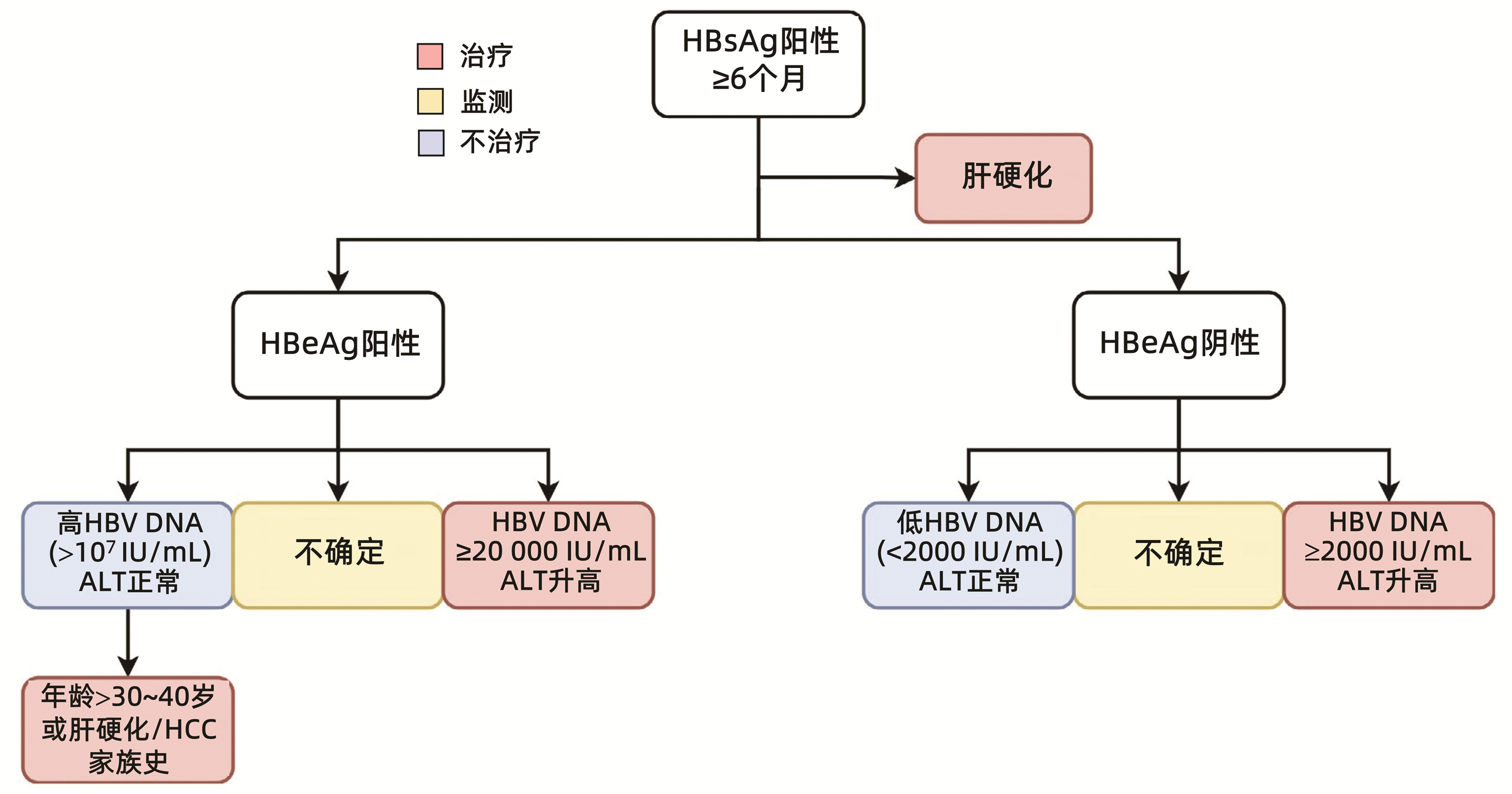
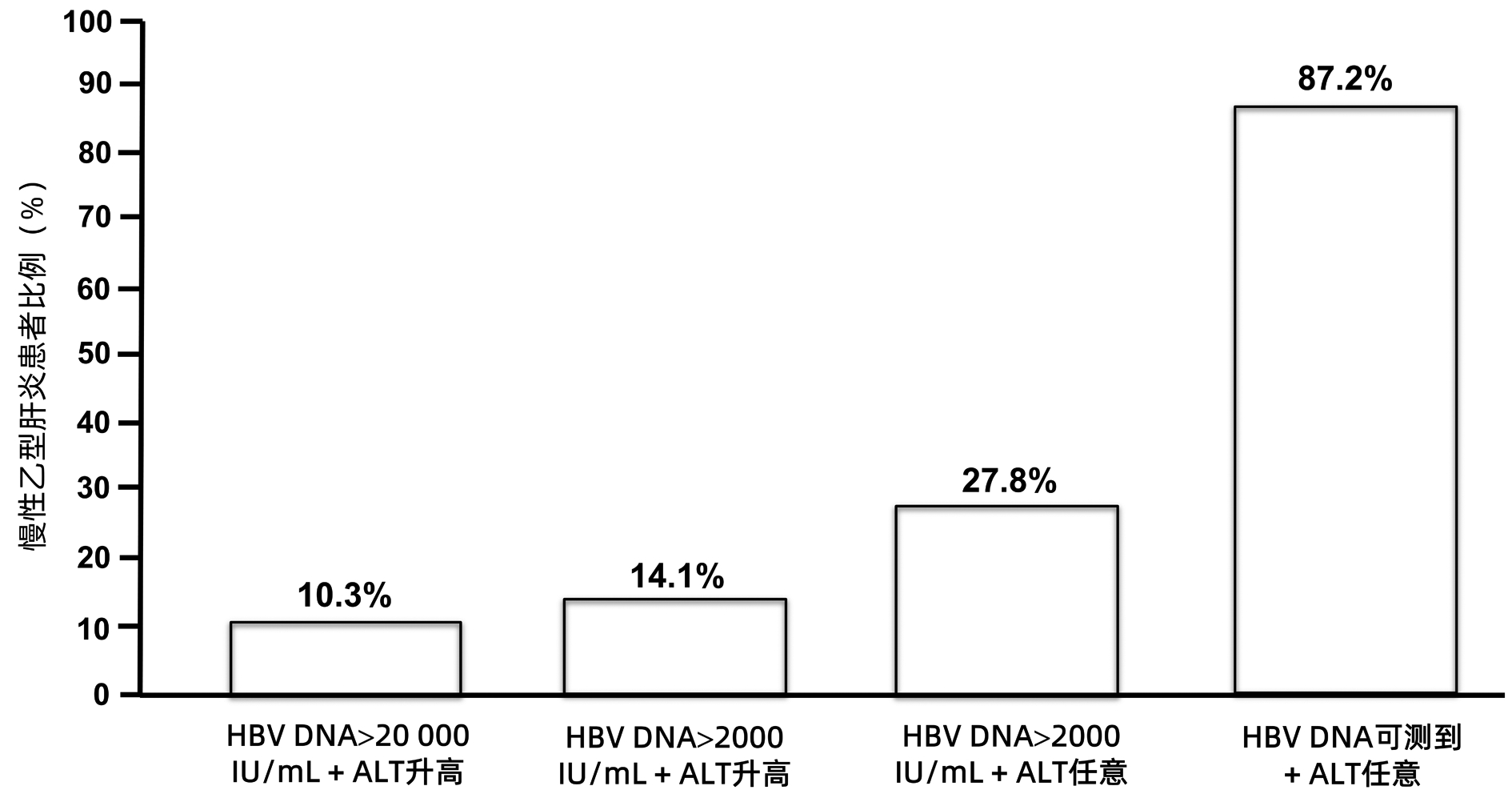
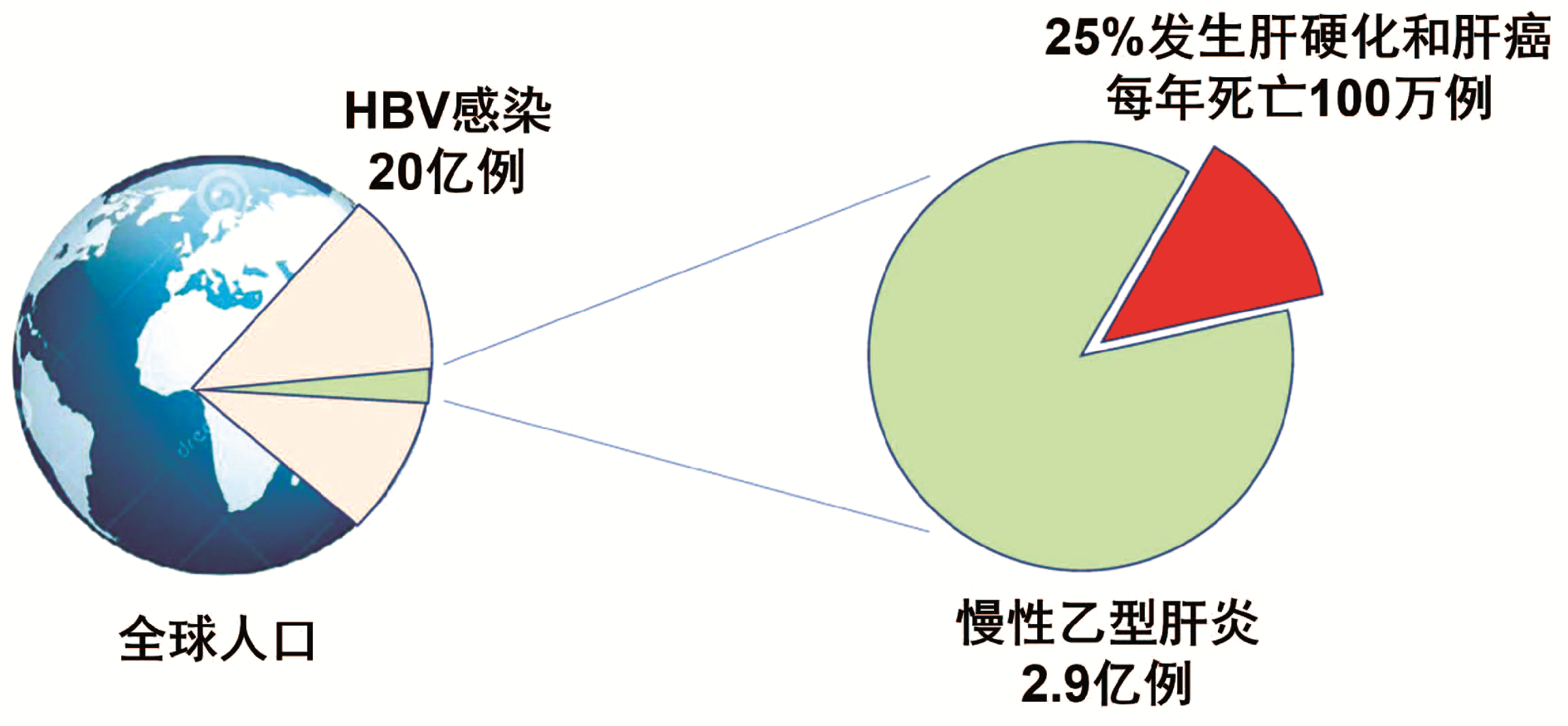
全球符合治疗标准的慢性乙型肝炎患者中,仅5%接受抗病毒治疗。现行指南治疗标准过严是慢性乙型肝炎患者治疗率低的原因之一。扩大现行指南确定的慢性乙型肝炎治疗标准将减轻疾病负担。普遍乙型肝炎疫苗免疫、普遍筛查及对所有HBV DNA阳性者治疗,将有助于达到世界卫生组织提出的2030年消除乙型肝炎目标。
Abstract:Among chronic hepatitis B patients eligible for treatment, only 5% have received antiviral therapy worldwide. The strictness of treatment criteria in current guidelines is one of the contributing factors for the low treatment rate of patients with chronic hepatitis B, and expanding the treatment criteria for chronic hepatitis B established in current guidelines may reduce disease burden. Universal hepatitis B vaccination, universal screening, and treatment of all HBV DNA-positive patients will help to achieve the goal of HBV elimination by 2030 proposed by the World Health Organization.
-
Key words:
- Hepatitis B, Chronic /
- Hepatitis B Vaccine /
- Therapeutics
-
-
[1] SORIANO V, ALVAREZ C, EDAGWA B, et al. Ultra-long-acting (XLA) antivirals for chronic viral hepatitis[J]. Int J Infect Dis, 2022, 114: 45-50. DOI: 10.1016/j.ijid.2021.10.052. [2] World Health Organization. Global hepatitis report, 2017[R]. Geneva: World Health Organization, 2017. [3] World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030[R]. Geneva: World Health Organization, 2022. [4] Polaris Observatory HBV Collaborators[EB/OL]. [2022-11-10]. https://cdafound.org/dashboard/polaris/dashboard.html. [5] SINN DH, KIM SE, KIM BK, et al. The risk of hepatocellular carcinoma among chronic hepatitis B virus-infected patients outside current treatment criteria[J]. J Viral Hepat, 2019, 26(12): 1465-1472. DOI: 10.1111/jvh.13185. [6] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. [7] TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. [8] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. [9] PAPATHEODORIDIS GV, LAMPERTICO P, MANOLAKOPOULOS S, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review[J]. J Hepatol, 2010, 53(2): 348-356. DOI: 10.1016/j.jhep.2010.02.035. [10] PAPATHEODORIDIS GV, IDILMAN R, DALEKOS GN, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B[J]. Hepatology, 2017, 66(5): 1444-1453. DOI: 10.1002/hep.29320. [11] CHEN YC, CHU CM, LIAW YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B[J]. Hepatology, 2010, 51(2): 435-444. DOI: 10.1002/hep.23348. [12] YUEN MF, WONG DK, FUNG J, et al. HBsAg seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma[J]. Gastroenterology, 2008, 135(4): 1192-1199. DOI: 10.1053/j.gastro.2008.07.008. [13] CHOI H, TONTHAT A, JANSSEN H, et al. Aiming for functional cure with established and novel therapies for chronic hepatitis B[J]. Hepatol Commun, 2022, 6(5): 935-949. DOI: 10.1002/hep4.1875. [14] WONG RJ, KAUFMAN HW, NILES JK, et al. Simplifying treatment criteria in chronic hepatitis B: Reducing barriers to elimination[J]. Clin Infect Dis, 2022. DOI: 10.1093/cid/ciac385.[Onlineaheadofprint] [15] TONG MJ, PAN CQ, HAN SB, et al. An expert consensus for the management of chronic hepatitis B in Asian Americans[J]. Aliment Pharmacol Ther, 2018, 47(8): 1181-1200. DOI: 10.1111/apt.14577. [16] RAZAVI-SHEARER D, ESTES C, GAMKRELIDZE I, et al. Cost-effectiveness analysis of treating all HBsAg+ individuals in the United States[J]. Hepatology, 2021, 74(Suppl 1): 22A. [17] LIM YS, AHN SH, SHIM JJ, et al. Impact of expanding hepatitis B treatment guidelines: A modelling and economic impact analysis[J]. Aliment Pharmacol Ther, 2022, 56(3): 519-528. DOI: 10.1111/apt.17052. [18] Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403. DOI: 10.1016/S2468-1253(18)30056-6. [19] TOY M, HUTTON D, HARRIS AM, et al. Cost-effectiveness of 1-time universal screening for chronic hepatitis B infection in adults in the United States[J]. Clin Infect Dis, 2022, 74(2): 210-217. DOI: 10.1093/cid/ciab405. [20] RAMRAKHIANI NS, CHEN VL, LE M, et al. Optimizing hepatitis B virus screening in the United States using a simple demographics-based model[J]. Hepatology, 2022, 75(2): 430-437. DOI: 10.1002/hep.32142. [21] SU S, WONG WC, ZOU Z, et al. Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation[J]. Lancet Glob Health, 2022, 10(2): e278-e287. DOI: 10.1016/S2214-109X(21)00517-9. [22] WENG MK, DOSHANI M, KHAN MA, et al. Universal hepatitis B vaccination in adults aged 19-59 years: Updated recommendations of the advisory committee on immunization practices-United States, 2022[J]. MMWR Morb Mortal Wkly Rep, 2022, 71(13): 477-483. DOI: 10.15585/mmwr.mm7113a1. -



 PDF下载 ( 3154 KB)
PDF下载 ( 3154 KB)

 下载:
下载: