慢性乙型肝炎患者停用核苷(酸)类似物后循环血清中HBV pgRNA、HBcrAg表达水平与复发的相关性分析
DOI: 10.3969/j.issn.1001-5256.2023.01.009
Expression levels of HBV pregenomic RNA and hepatitis B core-related antigen in circulating serum and their association with recurrence in chronic hepatitis B patients after withdrawal from nucleos(t)ide analogues
-
摘要:
目的 检测慢性乙型肝炎(CHB)患者停用核苷(酸)类似物(NUC)后循环血清中HBV前基因组RNA(pgRNA)以及乙型肝炎核心相关抗原(HBcrAg)的表达水平,分析停药后CHB患者循环血中不同时段HBV pgRNA、HBcrAg水平与复发之间的相关性。 方法 选取2019年12月—2022年7月川北医学院附属医院门诊就诊者中抗HBV治疗至少5年到达完全应答并满足2017年版欧洲肝病学会指南停药标准的CHB患者108例,根据停药时间分为停药后4、12、24周组;根据复发情况分为复发组与未复发组。运用实时荧光定量PCR法检测CHB患者循环血清中HBV pgRNA水平,运用ELISA检测研究对象静脉血中HBcrAg的表达水平,采用实时荧光定量PCR法高精度检测HBV DNA载量。计量资料两组间比较采用t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。采用Pearson相关检验分析循环血中各指标间的相关性。 结果 CHB患者停药后在随访4~12周时复发率17.1%,24周后累积复发率达到29.3%,其中单独HBV DNA复阳者各占64.3%、60.0%,HBeAg单独复阳者各占28.6%、20.0%,HBV DNA、HBeAg同时复阳者各占7.1%、20.0%。停药24周时,CHB患者循环血清中HBV pgRNA、HBcrAg、HBV DNA表达水平较停药时及停药后4周时明显高表达,不同时段比较差异具有统计学意义(P值均<0.05)。复发组循环血清中HBV pgRNA、HBcrAg、HBV DNA水平较未复发组显著高表达(t值分别2.549、8.654、27.429,P值均<0.05);对复发组进一步分析发现,在12~24周时复发组患者循环血清中HBV pgRNA、HBcrAg与HBV DNA水平较4~12周时高表达(P值均<0.05)。复发组停药时循环血清中HBV pgRNA、HBcrAg的表达水平较未复发组停药时显著高表达(t值分别为18.561、6.152,P值均<0.001)。相关性分析显示,CHB患者停药后复发组循环血清中HBV pgRNA、HBcrAg与HBV DNA呈正相关(r值分别为0.82、0.66,P值均<0.001);未复发组循环血清中HBV pgRNA、HBcrAg与HBV DNA均无相关性(r值分别为0.14、0.04,P值均>0.05)。 结论 停药时复发组HBV pgRNA、HBcrAg水平较未复发组高表达,提示未复发组在停药时的HBV pgRNA、HBcrAg水平可能作为CHB患者可安全停药的参考临界值指标,HBV pgRNA、HBcrAg水平检测可能是未来抗HBV治疗终点选择的潜在参考指标之一。 -
关键词:
- 慢性乙型肝炎 /
- HBV前基因组RNA /
- 乙型肝炎核心相关抗原 /
- 复发
Abstract:Objective To investigate the expression levels of HBV pregenomic RNA (pgRNA) and hepatitis B core-related antigen (HBcrAg) in circulating serum of chronic hepatitis B (CHB) patients after withdrawal from nucleos(t)ide analogues (NUC), as well as the correlation of HBV pgRNA and HBcrAg levels in circulating blood in different periods of time with recurrence in CHB patients after drug withdrawal. Methods Among the patients who attended the outpatient service of Affiliated Hospital of North Sichuan Medical College from December 2019 to July 2022, a total of 108 CHB patients who received anti-HBV therapy for at least 5 years and met the criteria for drug withdrawal in 2017 EASL Guidelines were enrolled. According to the time of drug withdrawal, the patients were divided into 4-, 12-, and 24-week groups after drug withdrawal, and according to the presence or absence of recurrence, they were divided into recurrence group and non-recurrence group. Quantitative real-time PCR was used to measure the level of HBV pgRNA in circulating serum of CHB patients; ELISA was used to measure the expression level of HBcrAg in peripheral venous blood; quantitative real-time PCR was used to measure HBV DNA load with high accuracy. The t-test was used for comparison of continuous data between two groups. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. The Pearson correlation test was used to investigate the correlation between the indices in circulating blood. Results For the CHB patients after drug withdrawal, the recurrence rate was 17.1% at 4-12 weeks, cumulative recurrence rate reached 29.3% after 24 weeks of follow-up, the patients with positive HBV DNA alone accounted for 64.3% and 60.0%, respectively, those with positive HBeAg alone accounted for 28.5% and 20.0%, respectively, and those with positive HBV DNA and HBeAg accounted for 7.1% and 20.0%, respectively. The expression levels of HBV pgRNA, HBcrAg, and HBV DNA in circulating serum of CHB patients at 24 weeks after drug withdrawal were significantly higher than those at the time of drug withdrawal and at 4 weeks after drug withdrawal, and there was a significant difference between groups at different time points (all P < 0.05). Compared with the non-recurrence group, the recurrence group had significantly higher expression levels of HBV pgRNA, HBcrAg, and HBV DNA in circulating serum (t=2.549, 8.654, and 27.429, all P < 0.05), and further analysis of the recurrence group showed that the levels of HBV pgRNA, HBcrAg, and HBV DNA in circulating serum at 12-24 weeks were significantly higher than those at 4-12 weeks (all P < 0.05). At the time of drug withdrawal, the recurrence group had significantly higher expression levels of HBV pgRNA and HBcrAg in circulating serum than the non-recurrence group (t=18.561 and 6.152, both P < 0.001). The Pearson correlation analysis showed that in the recurrence group after drug withdrawal, HBV pgRNA and HBcrAg were positively correlated with HBV DNA in circulating serum (r=0.82 and 0.66, both P < 0.001), while no such correlation was observed in the non-recurrence group (r=0.14 and 0.04, both P > 0.05). Conclusion The recurrence group had significantly higher expression levels of HBV pgRNA and HBcrAg than the non-recurrence group at the time of drug withdrawal, suggesting that the levels of HBV pgRNA and HBcrAg in the CHB patients of the non-recurrence group at the time of drug withdrawal may be used as the reference thresholds for safe drug withdrawal in CHB patients, and measurement of HBV pgRNA and HBcrAg may be one of the potential reference indicators for the selection of anti-HBV treatment endpoints in the future. -
Key words:
- Hepatitis B, Chronic /
- HBV Pregenomic RNA /
- Hepatitis B Core Related Antigen /
- Recurrence
-
目前我国慢性乙型肝炎(CHB)患者主要使用核苷(酸)类似物(NUC)抗病毒治疗[1],作为目前最重要的抗HBV治疗手段,NUC主要以抑制HBV的逆转录来降低循环血中的HBV DNA水平。目前的NUC药物并不能完全清除肝细胞内的HBV共价闭合环状DNA(cccDNA)[2-3],cccDNA的存在是CHB患者HBV持续感染的关键因素,因此肝内HBV cccDNA的消失是目前国内公认的CHB患者最可能达到病毒学治愈的状态。HBV cccDNA存在于肝细胞内,其检测具有创伤性,在临床难以广泛开展[4]。目前HBV相关的血清学标志物包括HBV DNA、HBeAg、HBsAg,经NUC治疗后HBV DNA阴转并不代表HBV cccDNA的清除,即使HBV cccDNA转录沉默,整合HBV S基因区也可转录翻译成HBsAg[5],在前C区以及基本cp区变异后可导致HBeAg载量下调甚至消失,但检测HBV cccDNA仍存在一定的转录活性。因此,目前常用的血清学标志物反映肝内HBV cccDNA的转录水平存在一定的局限性。
新型生物学标志物HBV前基因组RNA(pgRNA)、乙型肝炎核心相关抗原(HBcrAg)已引起广泛关注,HBV pgRNA作为cccDNA的直接转录产物,HBcAg作为HBV衣壳的主要结构蛋白,表达于完整的HBV Dane颗粒中[6],其水平与cccDNA的转录活性和水平具有良好的相关性[7-8]。本团队在研究了经NUC治疗CHB患者各时间段的HBV pgRNA、HBcrAg水平之后,收集到治疗至少5年到达完全应答并满足2017年版欧洲肝病学会(EASL)指南[9]停药标准的CHB患者,研究其停药后各时间段HBV pgRNA、HBcrAg水平,分析pgRNA、HBcrAg水平变化与复发的相关性,探索其在临床的应用价值。
1. 资料与方法
1.1 研究对象
研究对象来自2019年12月—2022年7月川北医学院附属医院门诊中抗HBV治疗至少5年到达完全应答并满足2017年版EASL指南停药标准的随访队列。所有研究对象年龄均满18周岁且具有民事行为能力。排除标准:合并其他嗜肝/非嗜肝病毒感染及其他原因所致肝组织炎症;合并肝硬化、肝癌等其他疾病。完全应答标准:满足生化学、血清学以及病毒学联合应答,生化指标ALT、AST正常,e抗原阴转,HBV DNA检测不到。复发标准:病毒学复发指的是获得病毒学应答的患者停药后,间隔1个月2次检测HBV DNA均>2×103 IU/mL;临床复发指的是病毒学复发且ALT>2倍正常值上限,但排除其他因素引起的ALT增高。
1.2 标本的采集与处理
收集CHB患者停药时、停药4周、12周以及24周时的空腹静脉血样本3 mL于肝素抗凝管内,在6 h内以1000 r/min离心15 min并获取上清液,置于-80 ℃冰箱保存。
1.3 检测方法
1.3.1 HBV pgRNA检测
采用湖南圣湘公司HBV pgRNA定量测定试剂盒检测血清中HBV RNA含量。
1.3.2 HBcrAg检测
采用ELISA酶联免疫分析试剂盒,运用CObase601全自动免疫分析系统检测。
1.3.3 HBV DNA水平检测
运用实时荧光定量法高精度检测样品中HBV DNA载量,标准值>20 IU/mL,如低于检测值下限按20 IU/mL进行统计学计算,严格按照说明书专人操作。
1.4 统计学方法
数据运用SPSS 21.0统计软件进行分析。符合正态分布的计量资料以x±s表示,两组间比较采用t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。采用Pearson相关检验分析循环血中各指标间的相关性。P<0.05表示差异有统计学意义。
2. 结果
2.1 CHB患者停药后随访24周内病毒学复发人数及累积复发率
本研究收集CHB患者108例,其中失访者26例,82例随访者在4周、12周、24周的复发情况见表 1,累积复发率达到29.3%(24/82)。复发者均为病毒学复发,无临床复发者,病毒学复发的患者从复发时结束随访观察。
表 1 CHB患者停药后随访24周内病毒学复发人数及累积复发率Table 1. The number of viral recurrences and cumulative recurrence rate of CHB patients within 24 weeks of follow-up after drug withdrawal组别 复发率(%) 停药4周 0 停药12周 17.1(14/82) HBV DNA复阳 64.3(9/14) HBeAg复阳 28.6(4/14) HBV DNA、HBeAg同时复阳 7.1(1/14) 停药24周 14.7(10/68) HBV DNA复阳 60.0(6/10) HBeAg复阳 20.0(2/10) HBV DNA、HBeAg同时复阳 20.0(2/10) 2.2 CHB患者停药后各时段循环血清中HBV pgRNA、HBcrAg、HBV DNA的表达水平
停药24周时,CHB患者循环血清中HBV pgRNA、HBcrAg、HBV DNA表达水平较停药时及停药4周表达明显升高(P值均<0.05);停药后各时段组间CHB患者循环血清中HBV pgRNA、HBcrAg、HBV DNA表达水平差异均有统计学意义(P值均<0.05)(表 2)。
表 2 CHB患者停药后各时段循环血清中HBV pgRNA、HBcrAg、HBV DNA表达水平比较Table 2. Comparison of HBV pgRNA, HBcrAg and HBV DNA expression levels in circulating serum of CHB patients at different periods after drug withdrawal组别 例数 HBV pgRNA (log10拷贝/mL) HBcrAg (log10 U/L) HBV DNA (log10 IU/mL) 停药时 82 1.36±0.12 1.31±0.16 1.30±0.18 停药4周 82 1.37±0.10 1.31±0.17 1.61±0.20 停药12周 82 1.39±0.12 1.40±0.13 2.38±0.26 停药24周 68 1.42±0.141)2) 1.51±0.161)2) 2.50±0.351)2) F值 10.276 14.359 21.473 P值 0.022 0.013 < 0.001 注:与停药时相比,1)P<0.05;与停药4周相比,2)P<0.05。 2.3 CHB患者停药后复发组与未复发组循环血清中HBV pgRNA、HBcrAg、HBV DNA水平比较
对CHB患者停药后复发组选择复发当时循环血清中各指标水平状态,未复发组选择随访24周后循环血清中各指标的水平状态,复发组循环血清中HBV pgRNA、HBcrAg、HBV DNA水平较未复发组显著高表达(P值均<0.05)(表 3)。对复发组进一步分析发现,在12~24周时复发患者循环血清中HBV pgRNA[(1.59±0.16)log10拷贝/mL vs (1.42±0.14)log10拷贝/mL,t=2.903,P<0.05]、HBcrAg[(2.43±0.12)log10 U/L vs (1.73±0.06)log10 U/L,t=18.163,P<0.05]以及HBV DNA[(3.97±0.44)log10 IU/mL vs (3.57±0.31)log10 IU/mL,t=2.640,P<0.05]水平较4~12周时高表达。
表 3 CHB患者停药后复发组与未复发组循环血清中HBV pgRNA、HBcrAg、HBV DNA、HBsAg水平比较Table 3. Comparison of HBV pgRNA, HBcrAg, HBV DNA and HBsAg levels in circulating serum between relapse group and non relapse group of CHB patients after drug withdrawal组别 例数 HBV pgRNA (log10拷贝/mL) HBcrAg (log10 U/L) HBV DNA (log10 IU/mL) 复发组 24 1.49±0.15 2.02±0.11 3.74±0.39 未复发组 58 1.39±0.13 1.35±0.17 2.24±0.15 t值 2.549 8.654 25.680 P值 0.015 < 0.001 < 0.001 2.4 CHB患者停药后复发组与未复发组在停药时循环血清中HBV pgRNA、HBcrAg表达水平比较
复发组时循环血清中HBV pgRNA、HBcrAg的表达水平较未复发组显著升高(P值均<0.05)(表 4)。
表 4 停药时复发组与未复发组CHB患者循环血清中HBV pgRNA、HBcrAg表达水平比较Table 4. Comparison of HBV pgRNA, HBcrAg expression levels in circulating serum of CHB patients in relapse and non relapse groups at the time of drug withdrawal组别 例数 HBV pgRNA (log10拷贝/mL) HBcrAg (log10 U/L) 复发组 24 1.42±0.11 1.55±0.20 未复发组 58 1.34±0.07 1.21±0.11 t值 18.561 6.152 P值 < 0.001 < 0.001 2.5 CHB患者复发组和未复发组循环血清中HBV pgRNA、HBcrAg与HBV DNA的相关性分析
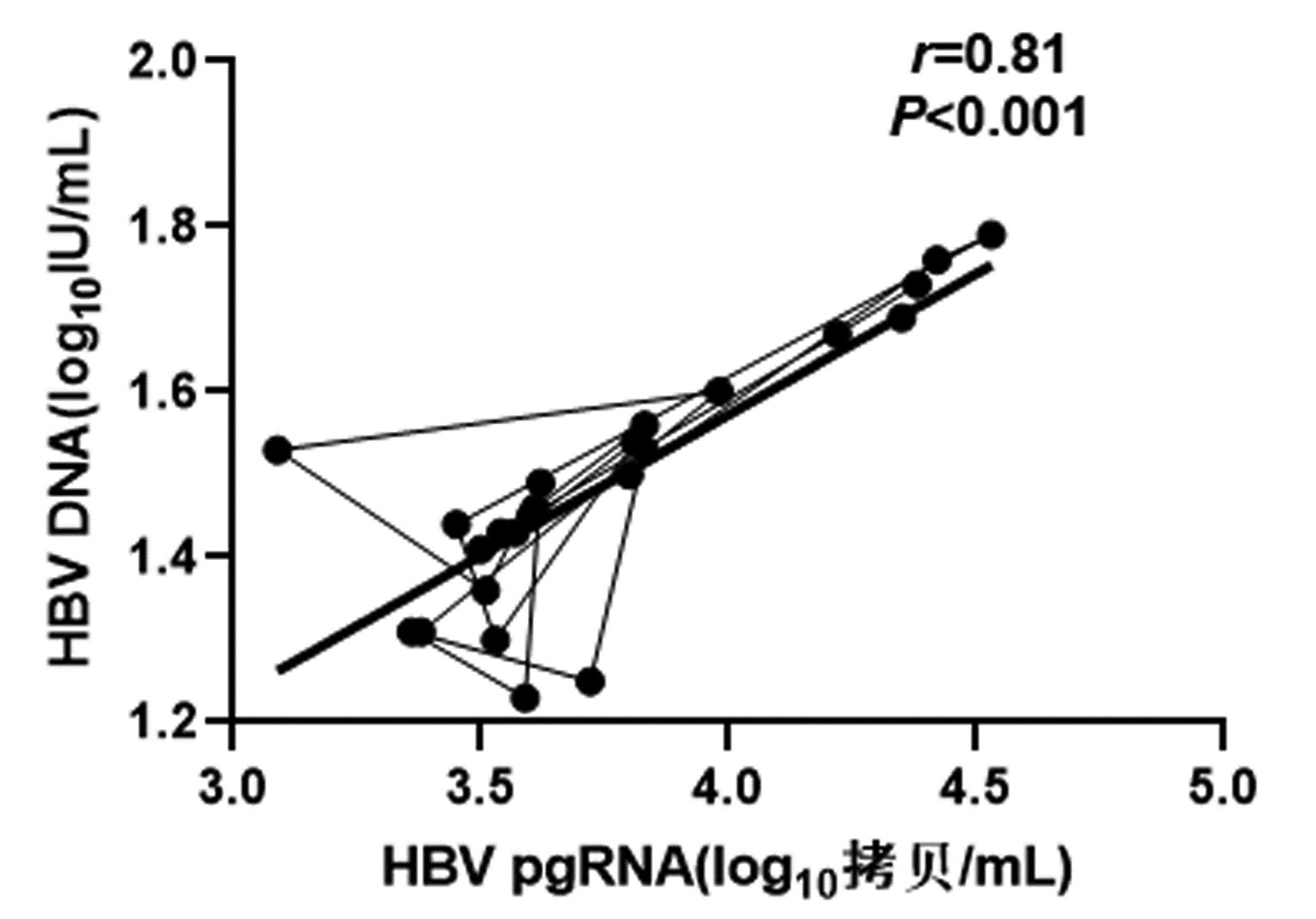
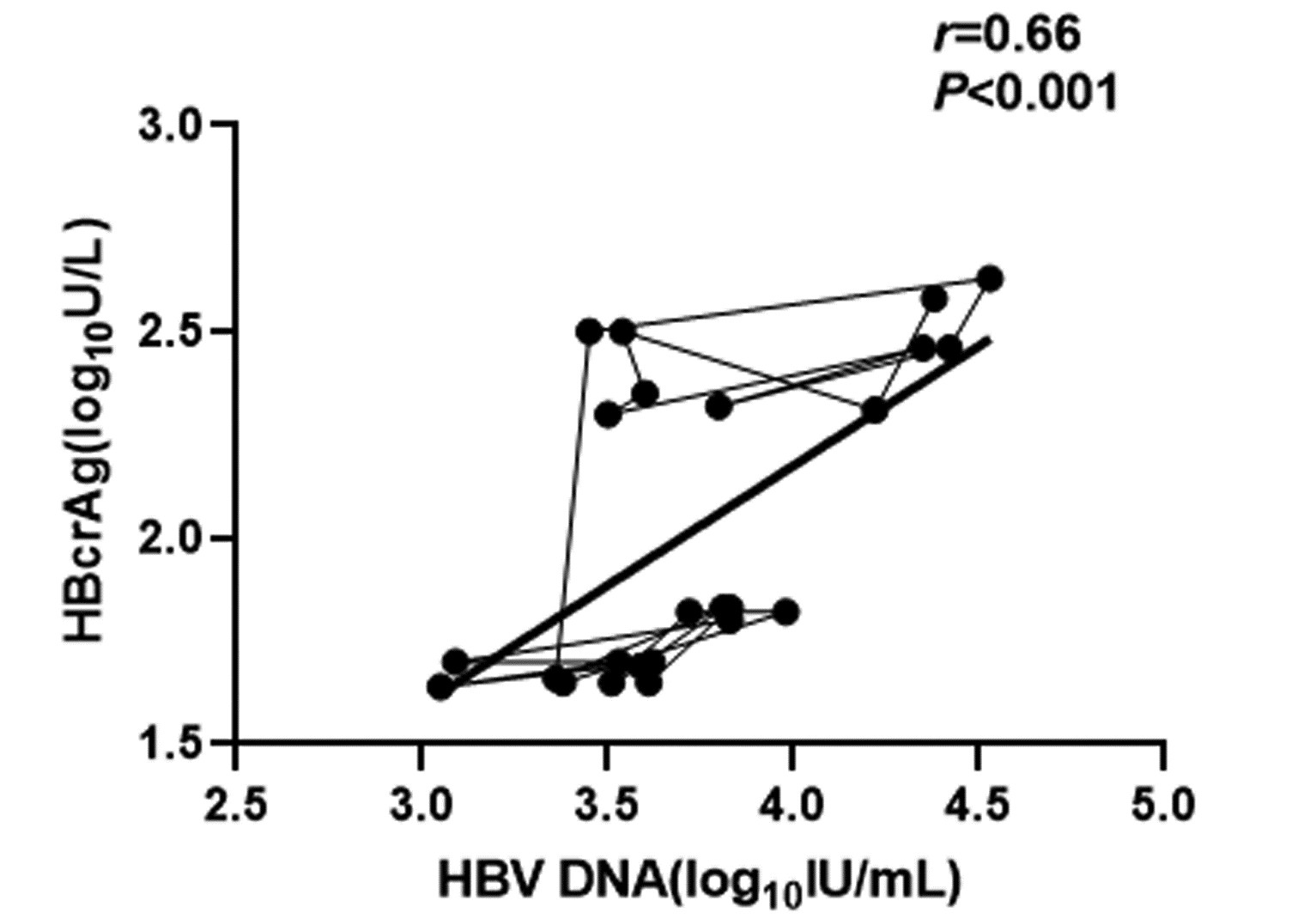
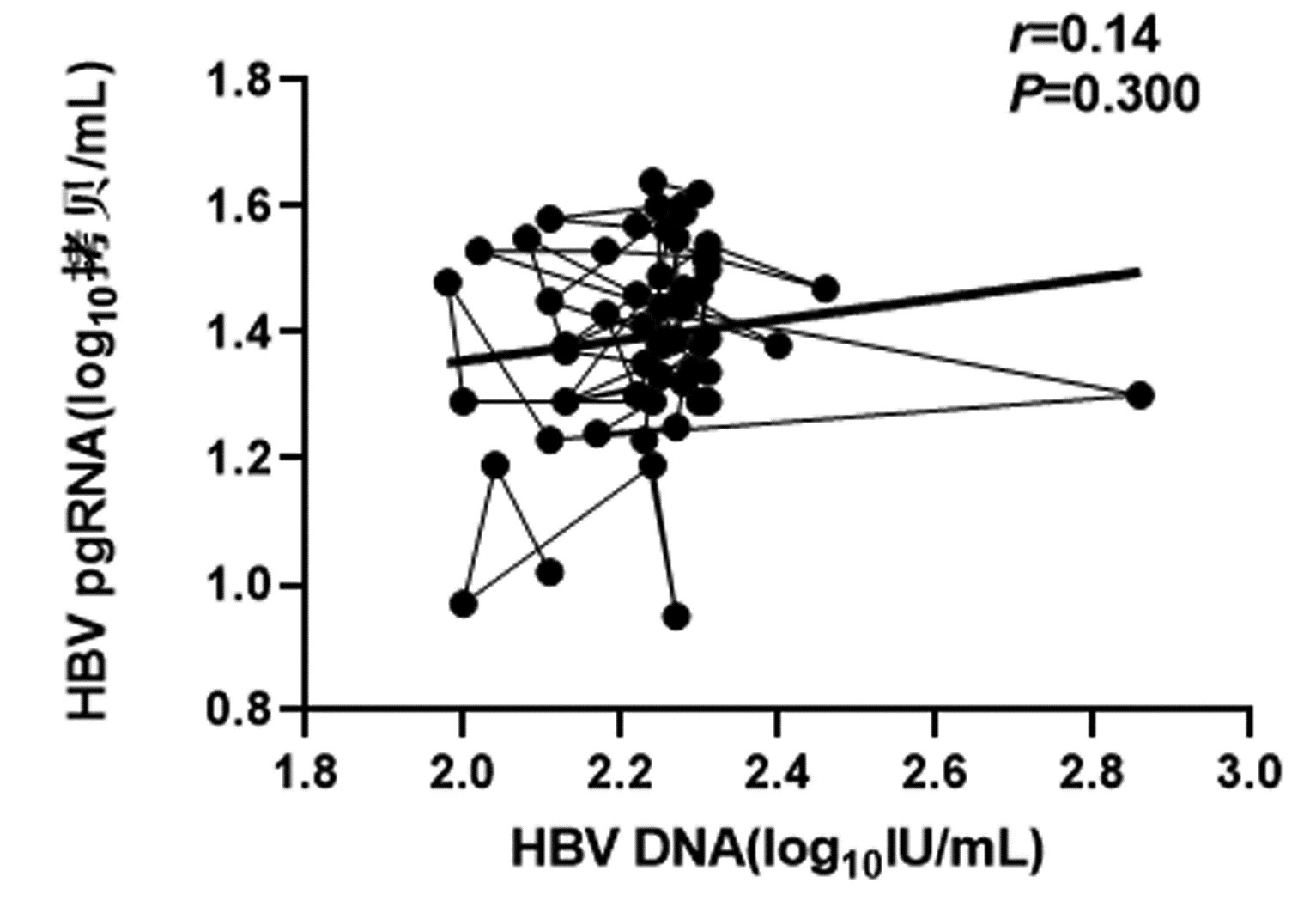
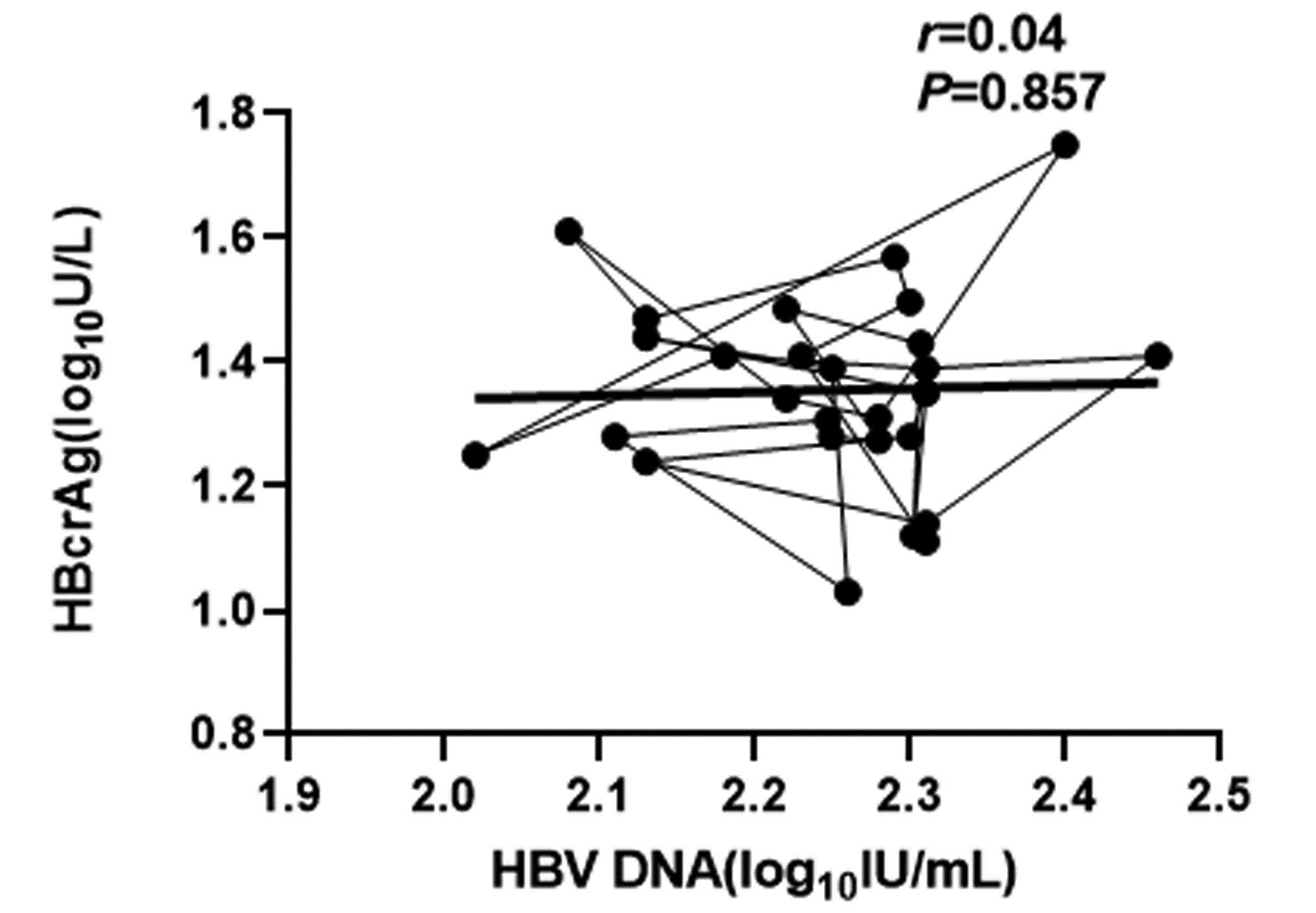
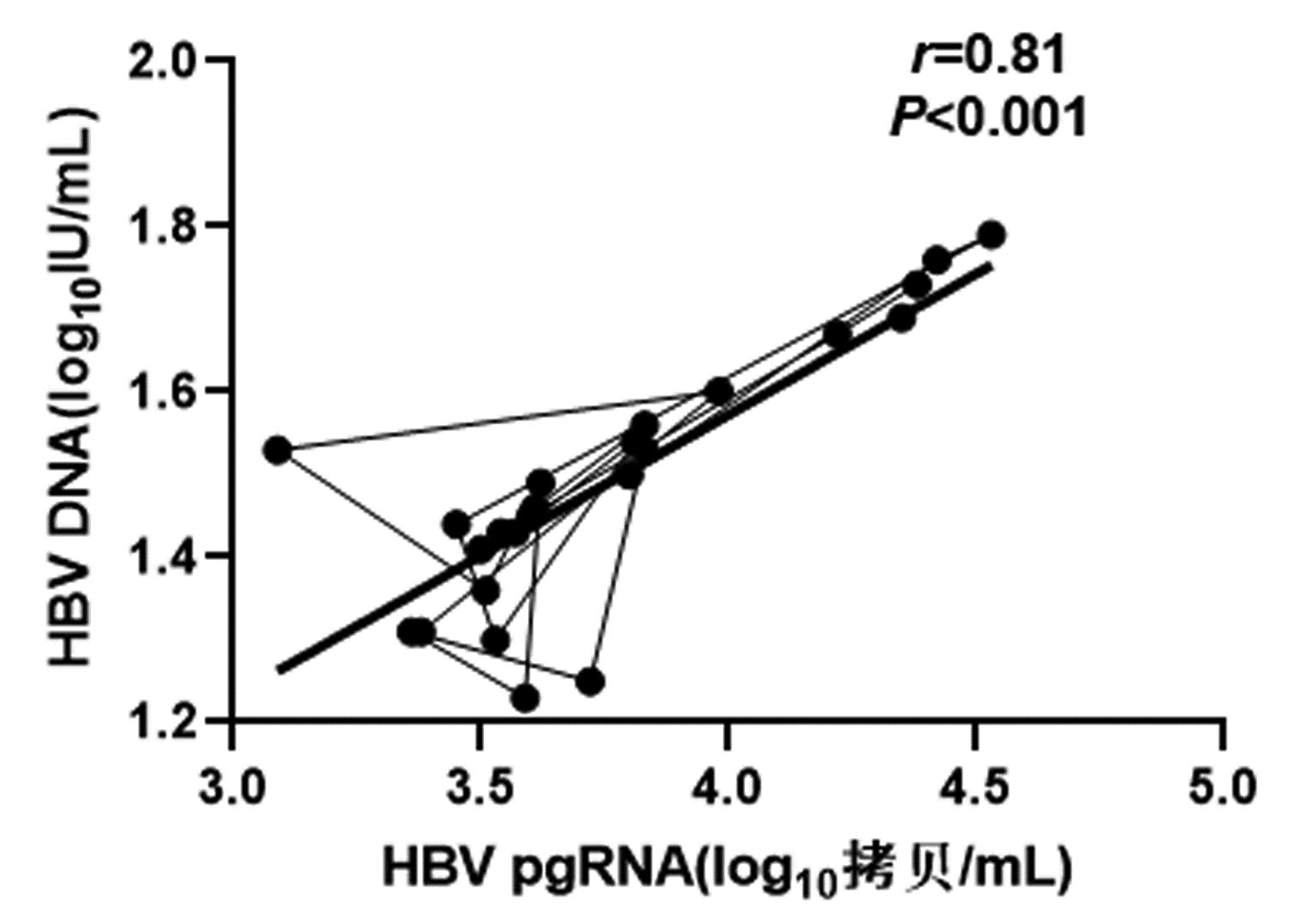
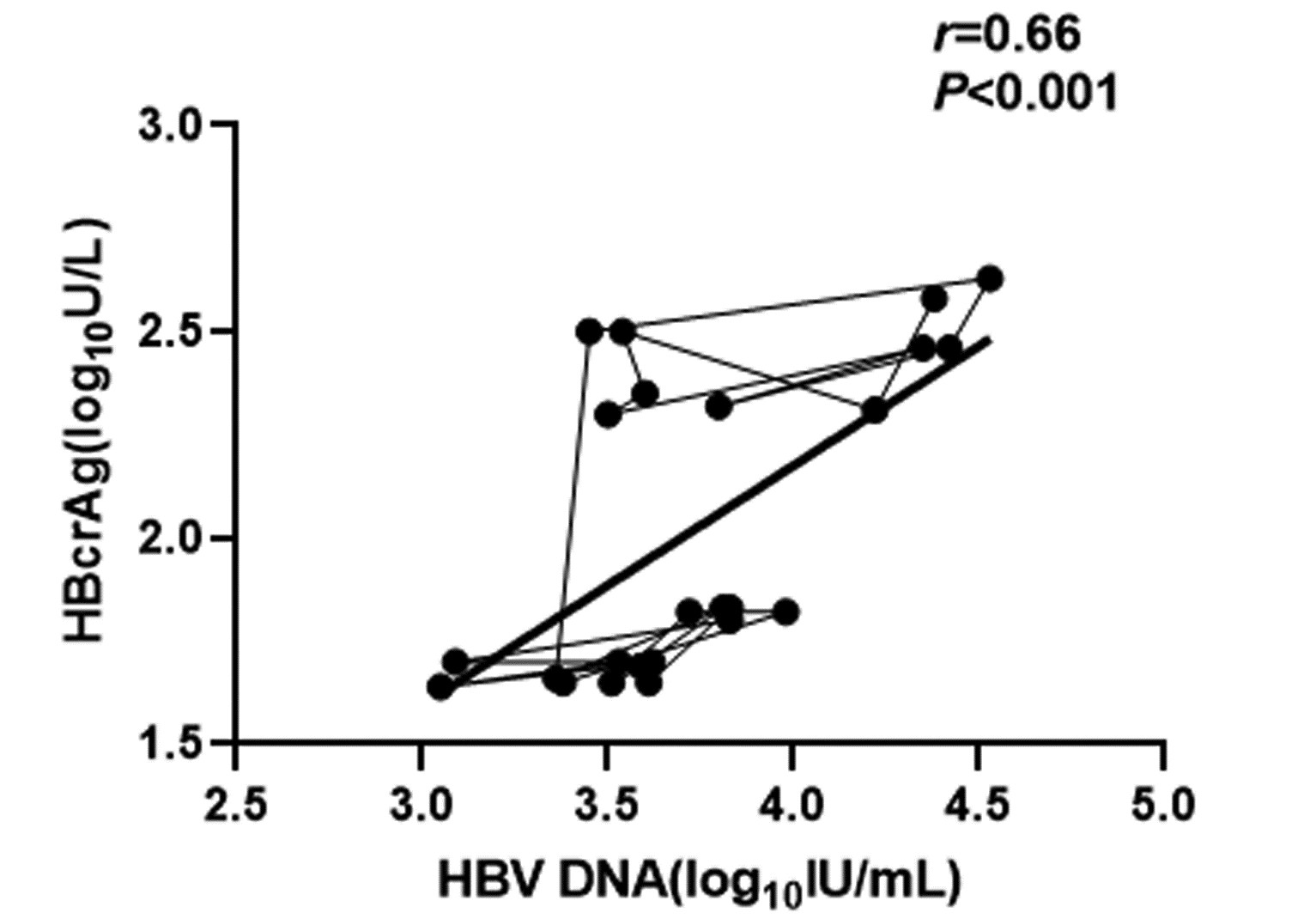
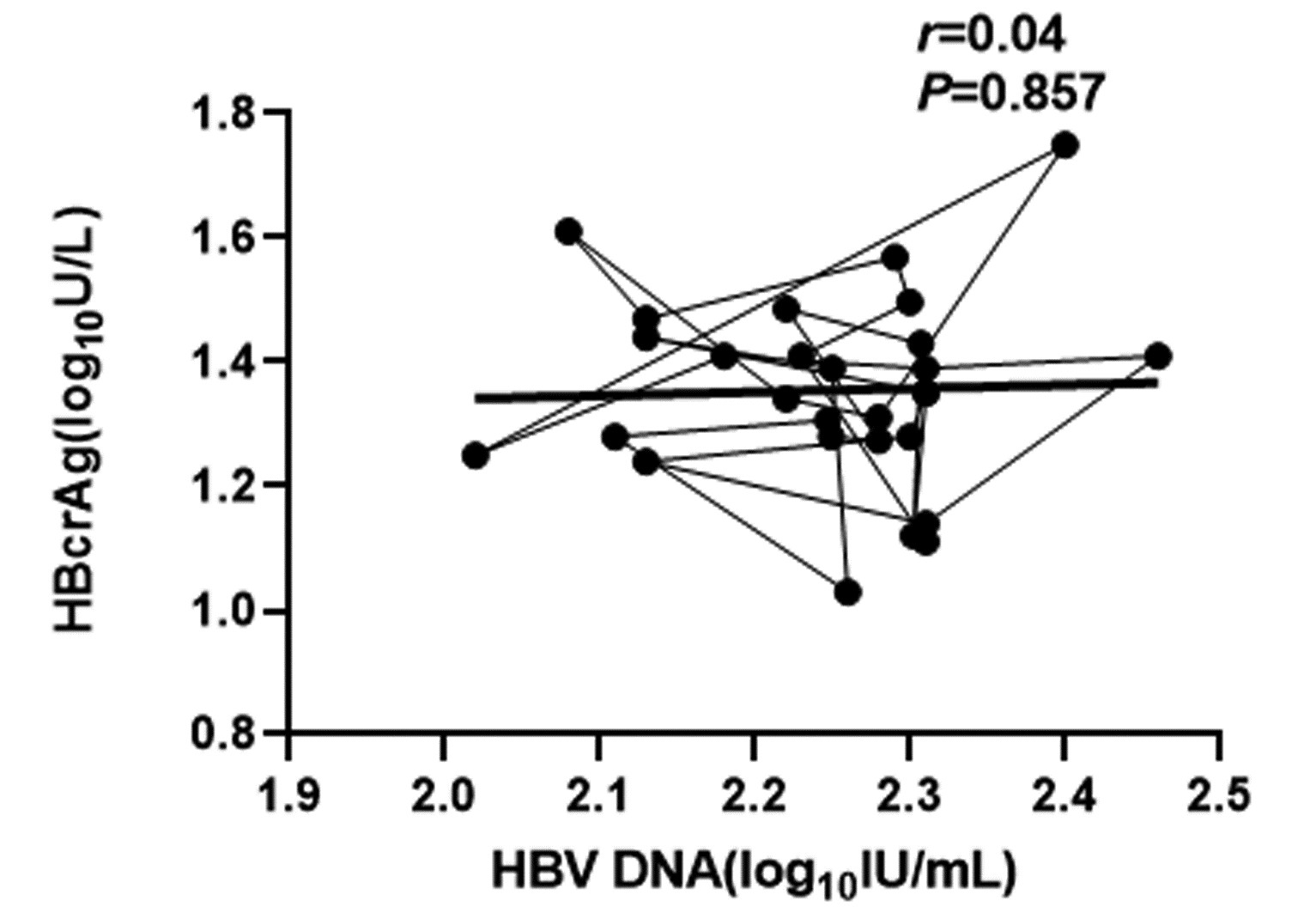
CHB患者停药后复发组循环血清中HBV pgRNA、HBcrAg与HBV DNA均呈正相关(r值分别为0.81、0.66,P值均<0.001);未复发组循环血清中HBV pgRNA、HBcrAg与HBV DNA均无相关性(r值分别为0.14、0.04,P值均>0.05)(图 1~4)。
3. 讨论
CHB患者经NUC药物治疗后可以达到HBV DNA阴转,但CHB患者体内仍存在一定量的HBV cccDNA,HBV cccDNA的持续低表达是HBV不断复制的根源,目前只有HBV cccDNA彻底清除才是CHB等肝病患者根治的核心指标[5]。已有研究[10]表明,CHB患者循环血清中HBV pgRNA水平检测可以反映肝细胞内cccDNA水平;也有研究[11-12]发现,HBcrAg表达水平与HBV cccDNA水平呈正相关。因此,本研究通过检测CHB患者停药后各时段循环血清中HBV pgRNA、HBcrAg的表达水平以及复发组与未复发组循环血清中HBV pgRNA、HBcrAg的表达差异,分析HBV pgRNA、HBcrAg停药时水平与复发的相关性以及其水平能否作为停药的参考临界值指标,HBV pgRNA、HBcrAg的检测可能为后续选择停药时机、评估药物疗效等提供临床价值基础。
本研究显示,CHB患者停药后随访4~12周时复发率17.1%,随访24周时累积复发率达到29.3%。其中单独HBV DNA复阳者各占64.3%、60.0%,所有复发者均为病毒学复发,未发现临床复发患者。目前CHB患者抗病毒治疗主要依赖于NUC药物。NUC药物主要是抑制HBV逆转录酶来抑制HBV DNA的转录,降低CHB患者循环血清中HBV DNA的表达水平,对肝细胞内的HBV cccDNA清除能力较低,HBV pgRNA作为HBV cccDNA的直接转录产物即可长期以低水平存在于CHB患者体内。本研究的CHB患者经过至少5年的NUC抗病毒治疗,满足2017年版EASL指南停药标准后停药,由结果可见,尽管CHB患者经治疗后HBV DNA阴转,e抗原发生转换,在停药后随访中发现,仍有一部分患者发生HBV DNA和/或HBeAg复阳,其中HBV DNA单独复阳者占多数,这可能是由于CHB患者循环血清中的HBV DNA来源于HBV pgRNA,pgRNA再经过逆转录形成HBV rcDNA,被病毒蛋白包裹后释放到肝细胞外成为新的HBV DNA,HBV DNA形成与HBV pgRNA关系密切,在后续研究结果中发现低水平的HBV pgRNA是HBV DNA复阳的关键,既往也有研究[13]证实,停止NUC治疗后,血清pgRNA水平与病毒复发风险有密切关系。
本研究在CHB患者停药24周内的随访中发现,随着停药时间的延长,CHB患者循环血清中HBV pgRNA、HBcrAg、HBV DNA表达水平成上升趋势,差异具有统计学意义;进一步对复发组进行分析,复发组CHB患者循环血清中HBV pgRNA、HBcrAg、HBV DNA表达水平较未复发组高表达,且12~14周复发组患者循环血清中HBV pgRNA、HBcrAg、HBV DNA表达水平较4~12周复发组高表达;停药24周后未复发组患者循环血中HBV pgRNA、HBcrAg、HBV DNA的表达水平相比较于最初停药时有所上升,但未达到复发组患者水平。由于本研究观察时间较短,在后续的停药过程中,未复发组患者循环血中各指标水平是否会进一步上升甚至达到复发组患者水平还需要进一步观察;根据未复发组患者24周时循环血中各指标的表达水平能否预测其未来复发的可能性还需要进一步研究证明。本研究的CHB患者停药时HBV DNA阴转,但检测结果提示其循环血清中HBV pgRNA、HBcrAg仍微量存在,在停药后CHB患者循环血清中各指标水平逐渐升高,以HBV DNA水平上升为著,这可能是由于CHB患者体内HBV pgRNA、HBcrAg的持续存在,在失去NUC药物的抑制后,HBV pgRNA经过逆转录酶重新生成HBV DNA,导致循环血清中HBV DNA表达水平上升明显。也有研究[14]显示,在CHB患者达到2017年版EASL指南停药标准后,仍有约半数的患者循环血清中pgRNA未阴转,这表明肝细胞内HBV cccDNA仍有一定的转录活性,在停药后其复制活跃程度增高,导致患者发生病毒学复发。
进一步分析复发组和未复发组在停药时其循环血清中HBV pgRNA、HBcrAg的水平差异,研究结果提示复发组在停药时其循环血清中HBV pgRNA、HBcrAg表达水平较未复发组高表达。这表明,相比较于未复发患者,复发患者在停药时,其循环血清中存在较高水平的HBV pgRNA、HBcrAg,这可能是导致复发组患者体内HBV再活跃的关键指标,因本研究停药后观察时间较短,未复发组循环血清中HBV pgRNA、HBcrAg停药时水平是否能作为停药的临界指标尚需进一步观察研究。免疫系统的激活与应答对乙型肝炎的进展和临床结局起着关键作用,清除CHB患者体内HBV需要固有免疫以及获得性免疫的协同作用。在大多数CHB患者体内免疫细胞处于“无应答”状态,经NUC治疗后,部分免疫细胞激活,免疫系统重新构建。近期研究[15-16]表明,经长期NUC治疗后停药复发与患者体内各种细胞因子激活等也有一定关系。
复发组以及未复发组循环血清中HBV pgRNA、HBcrAg与HBV DNA之间的相关性分析发现,复发组循环血清中HBV pgRNA、HBcrAg与HBV DNA呈正相关,未复发组循环血清中HBV pgRNA、HBcrAg与HBV DNA无相关性,这与部分学者[17-18]研究结果一致。作者认为在CHB患者经NUC药物治疗至HBV DNA阴转后,其HBV pgRNA、HBcrAg水平下降滞后于HBV DNA,此时CHB患者体内HBV pgRNA、HBcrAg水平不能反映HBV DNA活性,而更能准确反映HBV cccDNA的转录活性与药物治疗疗效;在复发组患者循环血清中存在较高水平的HBV pgRNA、HBcrAg,HBV cccDNA的转录活性更强,经逆转录酶生成HBV DNA能力提高,HBV DNA载量越高,HBV cccDNA活性越强,因此HBV DNA与HBV pgRNA、HBcrAg呈正相关。
综上所述,目前CHB患者治疗主要依赖NUC药物,根据2017年版EASL指南停药标准,即使HBV DNA阴转,循环血清中仍存在一定量的HBV pgRNA、HBcrAg,本研究发现复发组与未复发组停药时循环血清中HBV pgRNA、HBcrAg水平存在差异,但由于随访时间较短,未复发组患者在停药时HBV pgRNA、HBcrAg水平是否能代表安全停药的临界值指标尚需进一步观察研究。本研究仅纳入了HBV DNA阴转检测HBV pgRNA仍呈阳性的患者,未检测出HBV pgRNA阴性的患者,两者未予以比较,对于停药时HBV pgRNA阴性的患者停药后其水平是否会进一步升高、是否发生病毒学复发、其水平是否更能代表安全停药的临界值指标等诸多问题需要进一步扩大样本,延长随访时间进行研究观察。
-
表 1 CHB患者停药后随访24周内病毒学复发人数及累积复发率
Table 1. The number of viral recurrences and cumulative recurrence rate of CHB patients within 24 weeks of follow-up after drug withdrawal
组别 复发率(%) 停药4周 0 停药12周 17.1(14/82) HBV DNA复阳 64.3(9/14) HBeAg复阳 28.6(4/14) HBV DNA、HBeAg同时复阳 7.1(1/14) 停药24周 14.7(10/68) HBV DNA复阳 60.0(6/10) HBeAg复阳 20.0(2/10) HBV DNA、HBeAg同时复阳 20.0(2/10) 表 2 CHB患者停药后各时段循环血清中HBV pgRNA、HBcrAg、HBV DNA表达水平比较
Table 2. Comparison of HBV pgRNA, HBcrAg and HBV DNA expression levels in circulating serum of CHB patients at different periods after drug withdrawal
组别 例数 HBV pgRNA (log10拷贝/mL) HBcrAg (log10 U/L) HBV DNA (log10 IU/mL) 停药时 82 1.36±0.12 1.31±0.16 1.30±0.18 停药4周 82 1.37±0.10 1.31±0.17 1.61±0.20 停药12周 82 1.39±0.12 1.40±0.13 2.38±0.26 停药24周 68 1.42±0.141)2) 1.51±0.161)2) 2.50±0.351)2) F值 10.276 14.359 21.473 P值 0.022 0.013 < 0.001 注:与停药时相比,1)P<0.05;与停药4周相比,2)P<0.05。 表 3 CHB患者停药后复发组与未复发组循环血清中HBV pgRNA、HBcrAg、HBV DNA、HBsAg水平比较
Table 3. Comparison of HBV pgRNA, HBcrAg, HBV DNA and HBsAg levels in circulating serum between relapse group and non relapse group of CHB patients after drug withdrawal
组别 例数 HBV pgRNA (log10拷贝/mL) HBcrAg (log10 U/L) HBV DNA (log10 IU/mL) 复发组 24 1.49±0.15 2.02±0.11 3.74±0.39 未复发组 58 1.39±0.13 1.35±0.17 2.24±0.15 t值 2.549 8.654 25.680 P值 0.015 < 0.001 < 0.001 表 4 停药时复发组与未复发组CHB患者循环血清中HBV pgRNA、HBcrAg表达水平比较
Table 4. Comparison of HBV pgRNA, HBcrAg expression levels in circulating serum of CHB patients in relapse and non relapse groups at the time of drug withdrawal
组别 例数 HBV pgRNA (log10拷贝/mL) HBcrAg (log10 U/L) 复发组 24 1.42±0.11 1.55±0.20 未复发组 58 1.34±0.07 1.21±0.11 t值 18.561 6.152 P值 < 0.001 < 0.001 -
[1] TANG L, COVERT E, WILSON E, et al. Chronic hepatitis B infection: a review[J]. JAMA, 2018, 319(17): 1802-1813. DOI: 10.1001/jama.2018.3795. [2] BAI L, ZHANG X, KOZLOWSKI M, et al. Extracellular hepatitis B virus RNAs are heterogeneous in length and circulate as capsid-antibody complexes in addition to virions in chronic hepatitis B patients[J]. J Virol, 2018, 92(24): e00798-18. DOI: 10.1128/JVI.00798-18. [3] NING X, LUCKENBAUGH L, LIU K, et al. Common and distinct capsid and surface protein requirements for secretion of complete and genome-free hepatitis B virions[J]. J Virol, 2018, 92(14): e00272-18. DOI: 10.1128/JVI.00272-18. [4] LU L, ZHANG HY, YUENG YH, et al. Intracellular levels of hepatitis B virus DNA and pregenomic RNA in peripheral blood mononuclear cells of chronically infected patients[J]. J Viral Hepat, 2009, 16(2): 104-112. DOI: 10.1111/j.1365-2893.2008.01054.x. [5] LARSSON SB, MALMSTRÖM S, HANNOUN C, et al. Mechanisms downstream of reverse transcription reduce serum levels of HBV DNA but not of HBsAg in chronic hepatitis B virus infection[J]. Virol J, 2015, 12: 213. DOI: 10.1186/s12985-015-0447-5. [6] TANG H. The development history of new biomarkers for hepatitis B virus infection[J]. J Clin Hepatol, 2019, 35(10): 2137-2139. DOI: 10.3969/j.issn.1001-5256.2019.10.001.唐红. HBV感染"新型"生物标志物的"前世今生"[J]. 临床肝胆病杂志, 2019, 35(10): 2137-2139. DOI: 10.3969/j.issn.1001-5256.2019.10.001. [7] WANG XR, HU XJ, WANG XR, et al. Correlation between the expressions of HBV pgRNA and HBV-LP in serum and the degree of liver fibrosis in patients with chronic HBV infection[J]. Int J Virol, 2021, 28(2): 149-153. DOI: 10.3760/cma.j.issn.1673-4092.2021.02.014.王鲜茹, 胡新俊, 王雪茹, 等. 慢性HBV感染者血清HBV pgRNA、HBV-LP表达与肝脏纤维化程度的相关性研究[J]. 国际病毒学杂志, 2021, 28(2): 149-153. DOI: 10.3760/cma.j.issn.1673-4092.2021.02.014. [8] 陈来印, 齐聪幸, 安伟娜, 等. HBeAg阳性CHB患者恩替卡韦治疗中HBcrAg水平变化及其对疗效预测价值[J/CD]. 中国肝脏病杂志(电子版), 2022, 14(2): 50-56. DOI: 10.3969/j.issn.1674-7380.2022.02.008.CHEN LY, QI CX, AN WN, et al. Levels and predictive value of HBcrAg in CHB patients with HBeAg positive in treatment with entecavir[J/CD]. Chin J Liver Dis (Electronic Version), 2022, 14(2): 50-56. DOI: 10.3969/j.issn.1674-7380.2022.02.008. [9] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. [10] LIU RX, PAN XC, GENG J. Clinical value of serum HBV RNA[J]. J Clin Hepatol, 2017, 33(11): 2196-2199. DOI: 10.3969/j.issn.1001-5256.2017.11.032.刘瑞霞, 潘修成, 耿建. 血清HBV RNA检测的临床价值[J]. 临床肝胆病杂志, 2017, 33(11): 2196-2199. DOI: 10.3969/j.issn.1001-5256.2017.11.032. [11] NASSAL M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B[J]. Gut, 2015, 64(12): 1972-1984. DOI: 10.1136/gutjnl-2015-309809. [12] CHEN EQ, FENG S, WANG ML, et al. Serum hepatitis B core-related antigen is a satisfactory surrogate marker of intrahepatic covalently closed circular DNA in chronic hepatitis B[J]. Sci Rep, 2017, 7(1): 173. DOI: 10.1038/s41598-017-00111-0. [13] WANG J, SHEN T, HUANG X, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound[J]. J Hepatol, 2016, 65(4): 700-710. DOI: 10.1016/j.jhep.2016.05.029. [14] LING XZ, WANG RM, SU MH, et al. Clinical significance of dynamic monitoring of serum HBV pgRNA in CHB patients treated with Nas[J]. Chin J Pract Intern Med, 2022, 42(5): 404-408. DOI: 10.19538/j.nk2022050112.零小樟, 王荣明, 苏明华, 等. 慢性乙型肝炎患者核苷酸类似物治疗中动态监测血清乙型肝炎病毒前基因组RNA的临床意义[J]. 中国实用内科杂志, 2022, 42(5): 404-408. DOI: 10.19538/j.nk2022050112. [15] HÖNER ZU SIEDERDISSEN C, RINKER F, MAASOUMY B, et al. Viral and host responses after stopping long-term nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B[J]. J Infect Dis, 2016, 214(10): 1492-1497. DOI: 10.1093/infdis/jiw412. [16] ZIMMER CL, RINKER F, HÖNER ZU SIEDERDISSEN C, et al. Increased NK cell function after cessation of long-term nucleos(t)ide analogue treatment in chronic hepatitis B is associated with liver damage and HBsAg loss[J]. J Infect Dis, 2018, 217(10): 1656-1666. DOI: 10.1093/infdis/jiy097. [17] LIAO H, LIU Y, LI X, et al. Monitoring of serum HBV RNA, HBcrAg, HBsAg and anti-HBc levels in patients during long-term nucleoside/nucleotide analogue therapy[J]. Antivir Ther, 2019, 24(2): 105-115. DOI: 10.3851/IMP3280. [18] BUTLER EK, GERSCH J, MCNAMARA A, et al. Hepatitis B virus serum DNA and RNA levels in nucleos(t)ide analog-treated or untreated patients during chronic and acute infection[J]. Hepatology, 2018, 68(6): 2106-2117. DOI: 10.1002/hep.30082. 期刊类型引用(14)
1. 刘波,陈昌兰. 研究ELISA联合荧光定量PCR检测丙型肝炎病毒的效果. 系统医学. 2024(02): 79-82 .  百度学术
百度学术2. 王学英. HBV pgRNA联合cccDNA对慢性乙型肝炎患者抗病毒疗效的预测价值. 罕少疾病杂志. 2024(04): 54-56 .  百度学术
百度学术3. 陈春燕,樊子勉. 慢性乙型肝炎者外周血SAA/CRP、NLR水平与HBV-DNA载量、病情程度的相关性分析. 昆明医科大学学报. 2024(05): 144-150 .  百度学术
百度学术4. 隋娟,钟芳芳,郑秀霞. 乙肝患者HBV-DNA载量与血清标志物水平的相关性及其对抗病毒疗效的预测价值. 中国民康医学. 2024(10): 148-150+154 .  百度学术
百度学术5. 周芳,王永平,欧阳宇. HBV pgRNA联合HBcrAg对慢性乙型肝炎患者停药后复发的预测价值. 中国肝脏病杂志(电子版). 2024(02): 42-47 .  百度学术
百度学术6. 朱启泰,黄帆,宋华峰,陈慧,周芬,胥萍. 苏州地区HBV感染患者血清中乙型肝炎核心相关抗原表达特点分析. 标记免疫分析与临床. 2024(09): 1648-1652+1764 .  百度学术
百度学术7. 蒋贤静,潘小平,周颖,潘强,池林峰,饶芳. 慢性乙型肝炎中西医双重诊疗体系的实践与应用进展. 中西医结合肝病杂志. 2024(11): 1041-1044 .  百度学术
百度学术8. 王丽莉. 不同抗病毒方案对慢性乙型肝炎患者的影响. 实用中西医结合临床. 2024(22): 42-44 .  百度学术
百度学术9. 巫翟,祝达. TDF与TAF交替使用治疗慢性乙型肝炎的临床效果观察. 现代养生. 2024(24): 1849-1853 .  百度学术
百度学术10. 田晓晓,张娟,李琳彬,赵小芬,刘力瑜,余焰. Th1/Th2细胞因子表达水平对慢性乙型肝炎患者停用核苷(酸)类似物后复发的预测价值. 中国药物经济学. 2024(12): 59-63+67 .  百度学术
百度学术11. 陈柏伶. 动态护理模式对门诊慢性乙型肝炎患者疾病认知及遵医行为的影响. 现代养生. 2023(18): 1410-1413 .  百度学术
百度学术12. 刘虹,王鹏雁,刘友德. 监测血清HBV RNA水平变化对于NAs治疗CHB停药复发的预测价值. 国际医药卫生导报. 2023(19): 2762-2766 .  百度学术
百度学术13. 陈长红. 肝爽颗粒联合恩替卡韦对乙型肝炎肝硬化患者HBV-DNA水平及肝功能的影响. 黑龙江医药. 2023(06): 1349-1351 .  百度学术
百度学术14. 戎云清,徐敬轩. 加味补肾生髓成肝方联合聚乙二醇干扰素α对慢性乙型肝炎患者肝纤维化的影响. 世界复合医学. 2023(12): 98-102 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2010 KB)
PDF下载 ( 2010 KB)

 下载:
下载:




 下载:
下载:

 百度学术
百度学术