慢性乙型肝炎相关慢加急性肝衰竭前期免疫细胞的表达及意义
DOI: 10.3969/j.issn.1001-5256.2023.01.012
Expression and significance of immune cells in patients with hepatitis B virus-related acute-on-chronic pre-liver failure
-
摘要:
目的 探讨髓系抑制性细胞(MDSC)、调节性T淋巴细胞(Treg)、分泌IL-17的CD4(Th17)和CD8 T淋巴细胞(Tc17)在HBV相关慢加急性肝衰竭前期(pre-ACHBLF)的表达, 为ACHBLF的早期治疗提供思路。 方法 纳入2018年8月—2019年5月石家庄市第五医院收治的pre-ACHBLF和ACHBLF患者各15例,同时选择慢性乙型肝炎(CHB)患者15例和健康体检者(HC)15例为对照,流式细胞术检测外周血中MDSC、Th17、Treg和Tc17细胞水平,血液分析仪检测血常规并计算中性粒细胞/淋巴细胞比值(NLR)、单核细胞/淋巴细胞比值(MLR)、血小板/淋巴细胞比值(PLR)与全身免疫炎症指数(SIRS)评估患者炎症程度,分析免疫细胞表达与炎症程度的关系。符合正态分布的计量资料多组间比较采用独立样本方差分析,组间进一步两两比较采用LSD-t检验;不服从正态分布的计量资料多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Nemenyi检验。变量间的相关性采用Pearson线性相关或Spearman秩相关分析。 结果 与CHB组比较,ACHBLF和pre-ACHBLF组的Th17、Treg和Tc17细胞水平明显升高,同时pre-ACHBLF患者的MDSC细胞水平也明显升高(P值均<0.05)。相关性分析结果显示,在pre-ACHBLF患者中,MDSC与白细胞数、中性粒细胞数及NLR、MLR、SIRS呈正相关(r值分别为0.775、0.727、0.571、0.786、0.846,P值均<0.05),Treg细胞仅与白细胞数呈正相关(r=0.618,P=0.043),而Th17/Treg值和Tc17细胞水平与淋巴细胞数呈负相关(r值分别为-0.790、-0.795,P值均<0.05)。 结论 pre-ACHBLF患者已存在细胞免疫功能紊乱,MDSC表达与炎症程度密切相关,应早期关注。 Abstract:Objective To investigate the expression of myeloid-derived suppressor cells (MDSC), regulatory T cells (Treg), IL-17-producing CD4+ T cells (Th17), and CD8+ T cells (Tc17) in hepatitis B virus-related acute-on-chronic pre-liver failure (pre-ACHBLF), and to provide ideas for the early treatment of acute-on-chronic hepatitis B liver failure (ACHBLF). Methods A total of patients with pre-ACHBLF and 15 patients with ACHBLF who were hospitalized in Shijiazhuang Fifth Hospital, from August 2018 to May 2019 were enrolled as subjects, and 15 patients with chronic hepatitis B (CHB) and 15 healthy controls (HC) who underwent physical examination were enrolled as controls. Flow cytometry was used to measure the expression levels of MDSC and Th17, Treg, and Tc17 cells in peripheral blood; a blood analyzer was used to measure routine blood parameters and calculate neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index(SIRS) to evaluate the degree of inflammation, and the correlation between the expression of immune cells and the degree of inflammation was analyzed. An analysis of variance for independent samples was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups; the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups, and the Nemenyi test was used for further comparison between two groups. A Pearson linear correlation analysis or Spearman's rank correlation analysis was used to investigate the correlation between variables. Results Compared with the CHB group, the ACHBLF and pre-ACHBLF groups had significant increases in the expression levels of Th17, Treg, and Tc17 cells, and the pre-ACHBLF group also had a significant increase in the expression level of MDSC (all P < 0.05). The correlation analysis showed that in pre-ACHBLF patients, MDSC were positively correlated with leukocyte count, neutrophil count, NLR, MLR, and SII (r=0.775, 0.727, 0.571, 0.786, and 0.846, all P < 0.05), and Treg cells were only positively correlated with leukocyte count (r=0.618, P=0.043); Th17/Treg ratio and Tc17 cells were negatively correlated with the number of lymphocytes (r=-0.790 and -0.795, both P < 0.05). Conclusion Cellular immune dysfunction is observed in patients with pre-ACHBLF, and the expression of MDSC is closely associated with the degree of inflammation and should be taken seriously in the early stage. -
Key words:
- Acute-on-Chronic Liver Failure /
- Hepatitis B, Chronic /
- T-Lymphocytes
-
慢性乙型肝炎(CHB)相关的慢加急性肝衰竭(acute-on-chronic hepatitis B liver failure,ACHBLF)是在慢性HBV感染引起的CHB基础上出现的急性严重肝功能障碍临床综合征,病死率极高。因我国慢性HBV的高感染率,ACHBLF已成为影响患者生存质量的重要因素[1]。在CHB向ACHBLF进展过程中,存在着患者肝功能急剧恶化,但尚未达到肝衰竭的“肝衰竭前期(pre-ACHBLF)”阶段[2],如能在此阶段进行预警及干预,则有可能预防进一步发展为肝衰竭。
目前普遍认为细胞免疫功能紊乱是ACHBLF发生的病理机制之一,许多免疫细胞如髓系抑制性细胞(myeloid-derived suppressor cells, MDSC)、调节性T淋巴细胞(Treg)、分泌IL-17的CD4 T淋巴细胞(IL-17-producing CD4 T cells,Th17)和细胞毒性T淋巴细胞等在肝衰竭的发病中发挥重要作用[3-5]。尽管既往许多研究已证实肝衰竭发病与免疫密切相关,但pre-ACHBLF阶段的免疫状态及其与疾病进展的关系尚不清楚,因此本研究探讨了MDSC、Th17、Treg和分泌IL-17的CD8 T淋巴细胞(IL-17-producing CD8 T cells, Tc17)在pre-ACHBLF和ACHBLF患者中的表达,以期为ACHBLF的早期治疗提供思路。
1. 资料与方法
1.1 研究对象
选取2018年8月—2019年5月于石家庄市第五医院住院患者45例,其中ACHBLF患者、pre-ACHBLF患者和CHB患者各15例,同时选取于本院健康体检者15例作为健康对照组(HC组)。ACHBLF和pre-ACHBLF的临床诊断均符合《肝衰竭诊治指南(2018年版)》[6];CHB的临床诊断符合《慢性乙型肝炎防治指南(2015年版)》[7]。排除标准为合并其他病毒性肝炎或由酒精、药物等原因导致的肝脏疾病;肝癌或其他恶性肿瘤伴有肝脏转移;患有自身免疫性疾病、HIV感染、妊娠或有严重的心、肺、肾脏、神经系统等疾病。
1.2 标本采集与储存
采用EDTA-K2抗凝的真空采血管于清晨空腹采集外周静脉血约3 mL,取100 μL用于流式细胞仪检测外周血MDSC频数,剩余全血分离外周血单个核细胞(peripheral blood mononuclear cells, PBMC)。所有标本均为新鲜抗凝血,且在采集后4 h内处理。
1.3 流式细胞仪检测外周血免疫细胞的水平
MDSC细胞检测:取100 μL全血加至流式管中,然后分别加入CD11b-APC、CD33-PE和HLA-DR-PE/Cy7流式抗体各2 μL,避光孵育15 min后,每管加入溶血素500 μL,室温避光静置15 min,离心洗涤,加入流式鞘液300 μL重悬细胞,流式细胞仪检测MDSC细胞比例。
Treg细胞检测:收集PBMC,加入CD4-FITC和CD25-APC抗体各2 μL,4 ℃避光孵育30 min。经固定破膜处理后,离心洗涤2次,重悬细胞,然后加入10 μL Foxp3-PE抗体,4 ℃避光孵育45 min,离心洗涤,加入流式鞘液200 μL重悬细胞,流式细胞仪检测。
Th17、Tc17细胞检测:收集PBMC,用佛波醇酯和离子霉素刺激,同时加入GolgiStop,4 h后收集细胞至流式管,加入CD4-FITC和CD8a-PerCP/Cy5.5抗体各2 μL,4 ℃避光孵育30 min。经固定破膜处理后,离心洗涤2次,然后加入10 μL IL-17-APC抗体,避光孵育30 min,离心洗涤,加入流式鞘液200 μL重悬细胞,流式细胞仪检测。
1.4 临床检验指标的测定
肝功能检测采用美国雅培公司C8000型生化分析仪,HBeAg检测采用瑞士罗氏公司全自动Cobase601型电化学发光免疫分析仪,血常规检测采用日本希森美康公司XN1000血液分析仪,并计算炎症指标,包括中性粒细胞/淋巴细胞比值(neutrophil-to-lymphocyte ratio, NLR)、单核细胞/淋巴细胞比值(monocyte-to-lymphocyte ratio, MLR)、血小板/淋巴细胞比值(platelet-to-lymphocyte ratio, PLR)与全身免疫炎症指数(systemic immune inflammation index,SIRS),SIRS=中性粒细胞×血小板/淋巴细胞。
1.5 统计学方法
采用SPSS 21.0统计软件进行数据分析。符合正态分布的计量资料以x±s表示,多组间比较采用独立样本方差分析,组间进一步两两比较采用LSD-t检验;不服从正态分布的计量资料以M(P25~P75)表示,多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Nemenyi检验。变量间的相关性采用Pearson线性相关或Spearman秩相关分析,P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
四组间年龄与性别构成具有可比性,差异均无统计学意义(P值均>0.05)。ACHBLF组、pre-ACHBLF组和CHB组患者ALT与AST水平、HBeAg阳性比均明显高于HC组(P值均<0.05)。ACHBLF组和pre-ACHBLF组患者TBil水平均明显高于HC组(P值均<0.05)。ACHBLF组和pre-ACHBLF组患者PTA水平均明显低于CHB组(P值均<0.05)(表 1)。
表 1 四组研究对象一般特征比较Table 1. Comparison of general characteristics of four groups组别 例数 年龄(岁) 性别(男/女,例) ALT(U/L) AST(U/L) HBeAg (+/-) TBil(μmol/L) PTA(%) ACHBLF组 15 45.00±10.86 11/4 67.00(33.00~132.00)1) 88.00(57.70~117.00)1) 10/51) 279.00(241.00~337.00)1) 38.00(25.00~65.50)2) pre-ACHBLF组 15 48.50±7.47 11/4 57.40(31.20~114.00)1) 54.90(34.00~112.00)1) 10/51) 76.40(54.70~129.00)1) 43.90(37.50~51.00)2) CHB组 15 43.10±11.72 12/3 57.00(44.00~261.00)1) 46.00(29.00~135.00)1) 12/31) 20.00(12.00~33.00)1) 84.00(70.50~91.20) HC组 15 42.10±6.37 12/3 21.10(11.00~32.30) 21.70(17.20~27.90) 0/15 11.60(9.90~14.40) - 统计值 F=1.326 χ2=0.373 F=18.594 F=27.525 χ2=23.571 F=48.781 F=26.217 P值 0.275 0.946 <0.001 <0.001 <0.001 <0.001 <0.001 注:与HC组比较,1)P<0.05;与CHB组比较,2)P<0.05。 2.2 MDSC、Th17、Treg和Tc17细胞的表达
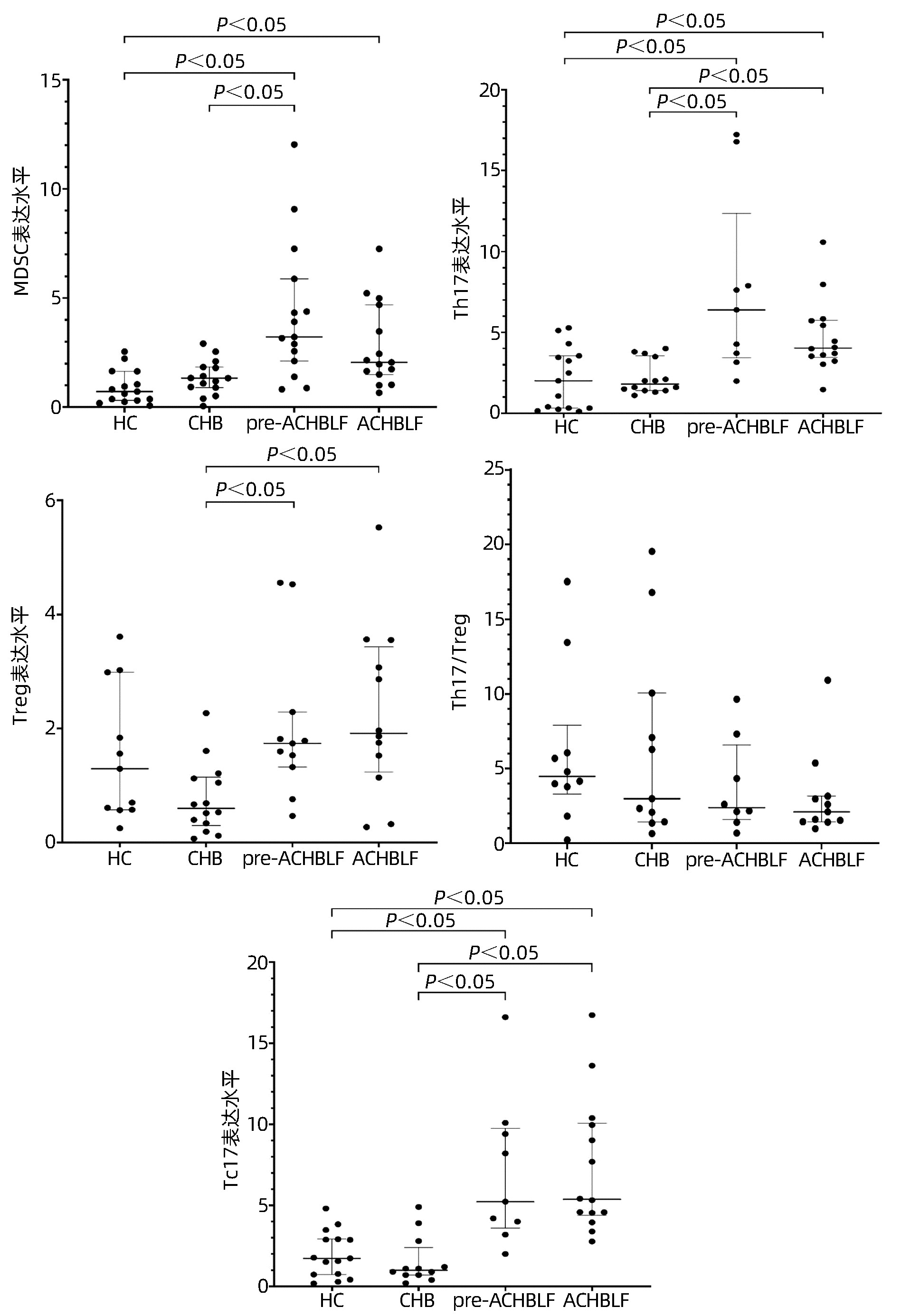
MDSC、Th17、Treg和Tc17细胞水平在四组间差异均有统计学意义(P值均<0.01)。与CHB组比较,ACHBLF和pre- ACHBLF组的Th17、Treg和Tc17细胞水平均明显升高(P值均<0.05),同时pre-ACHBLF患者的MDSC细胞水平明显升高,差异有统计学意义(P<0.05)(图 1)。
2.3 pre-ACHBLF患者中免疫细胞与炎症指标的关系
相关性分析显示,在pre-ACHBLF患者中,MDSC与白细胞数、中性粒细胞数及NLR、MLR、SIRS呈正相关(P值均<0.05),而Treg细胞仅与白细胞数呈正相关(P=0.043)。相反,Th17/Treg值和Tc17细胞水平与淋巴细胞数呈负相关(P值均<0.05)(表 2)。
表 2 pre-ACHBLF患者免疫细胞水平与炎症指标的关系Table 2. The relationship between the expression of immune cells and the degree of inflammation in patients with pre-ACHBLF炎症指标 MDSC Th17 Treg Th17/Treg Tc17 r值 P值 r值 P值 r值 P值 r值 P值 r值 P值 白细胞 0.775 0.001 0.167 0.668 0.618 0.043 -0.286 0.493 -0.533 0.139 中性粒细胞 0.727 0.002 0.250 0.516 0.491 0.125 -0.238 0.570 -0.417 0.265 淋巴细胞 0.079 0.781 -0.611 0.081 0.333 0.318 -0.790 0.020 -0.795 0.010 NLR 0.571 0.026 0.483 0.187 0.255 0.450 0.048 0.911 -0.150 0.700 MLR 0.786 0.001 0.233 0.546 0.236 0.484 0.024 0.955 -0.433 0.244 PLR 0.161 0.567 0.600 0.088 0.018 0.958 0.429 0.289 0.106 0.787 SIRS 0.846 <0.001 0.233 0.546 0.336 0.312 0.024 0.955 -0.433 0.244 3. 讨论
ACHBLF的发生发展涉及固有免疫和获得性免疫的多个环节,已有许多研究[8]证实免疫细胞如树突状细胞、巨噬细胞、MDSC、T淋巴细胞等与ACHBLF的进展及预后密切相关,但尚未有研究评价这些细胞在pre-ACHBLF中的变化。本研究发现pre-ACHBLF患者中MDSC、Th17、Treg和Tc17均比CHB患者明显升高,提示ACHBLF前期已存在严重的细胞免疫功能紊乱。因此,关注患者的病期变化,对pre-ACHBLF患者及早进行干预,维持细胞免疫平衡,可能对防止疾病进展起到重要作用。
MDSC是机体重要的免疫抑制细胞,具有抑制固有免疫和适应性免疫应答的作用,参与多种炎症和免疫性疾病的发生发展。近年来,一些研究[9]相继分析了MDSC在ACHBLF中的作用,发现ACHBLF患者MDSC频数明显升高,且随患者病期程度加重,本研究也证实了以上结果。为了探讨MDSC在ACHBLF疾病进程中的变化,本研究还观察了pre-ACHBLF患者中MDSC的频数,结果发现pre-ACHBLF患者中MDSC频数明显高于CHB患者,且高于ACHBLF患者。推测随着各种诱因导致CHB患者病情加重,肝细胞坏死及炎症反应产生的多种因子诱导机体MDSC快速升高,以抑制过强的免疫反应;而在ACHBLF发生时,机体以免疫炎症反应占主导,使MDSC频数相应降低。目前国内外关于MDSC在CHB-pre-ACHBLF-ACHBLF进程中如何发挥作用的研究还很少,尚需要进一步深入探讨。
值得注意的是,笔者团队观察到MDSC和Treg细胞的变化过程存在差异,MDSC水平在pre-ACHBLF患者中最高,而Treg细胞在ACHBLF患者中更高,此结果提示MDSC的增殖具有相对前置性,这两类重要的抑制性细胞在ACHBLF进展的不同时期分别发挥作用。此外还发现pre-ACHBLF患者中MDSC水平与炎症指标之间存在正相关性,而Treg水平仅与白细胞数呈正相关。目前炎症和免疫反应是ACHBLF发生发展过程中的重要病理生理机制,随着系统性炎症反应综合征的发生,炎症介质的持续释放导致ACHBLF患者出现血管内皮障碍,毛细血管渗漏和组织灌注不足而引起微循环紊乱, 继而引发器官衰竭。既往研究表明循环炎症介质及肝细胞坏死释放的损伤相关模式分子等可促进MDSC的增殖和分化[10],而MDSC具有诱导Treg细胞扩增和分化的作用[11-12]。因此,这些研究提示在肝衰竭前期,过强的炎症反应激活MDSC使其表达升高,形成以MDSC为主的免疫抑制状态;随着疾病进展,MDSC诱导Treg细胞表达,转变为以Treg细胞为主的状态。
Th17和Tc17是以分泌IL-17为主要特征的T淋巴细胞亚群,他们有着共同的分化刺激因子及分化调节通路,共同在炎症性疾病、病毒感染、自身免疫性疾病、肿瘤等疾病中发挥作用[13]。但是,这两类细胞在许多方面如分子和代谢调节网络、可塑性、表达变化也存在差异[14]。一项肝癌相关的研究[15]显示,肝癌组织中分泌IL-17的细胞主要是Tc17细胞,而外周血中则主要是Th17细胞。在ACHBLF病理损伤过程中,已有大量研究证实ACHBLF患者的Th17细胞明显升高,且与预后密切相关[16],但仅有极少数研究报道了Tc17细胞在ACHBLF发生发展中的作用[17-18], 特别是罕有研究观察Tc17细胞在pre-ACHBLF患者的表达。本研究发现pre-ACHBLF和ACHBLF患者外周血Th17与Tc17细胞频数显著高于CHB患者,且Tc17的变化更明显,推测Th17与Tc17细胞可能协同参与ACHBLF的疾病进程。
综上所述,本研究发现pre-ACHBLF患者外周血MDSC、Treg、Th17细胞和Tc17细胞水平均明显升高,特别是MDSC表达与炎症指标呈密切相关性。尽管本研究病例数偏少,但研究结果对理解ACHBLF的疾病进展具有重要意义。将来进一步扩大样本量进行验证,并深入探讨这些免疫细胞的作用机制,将为ACHBLF早期抗免疫治疗提供更多的理论依据。
-
表 1 四组研究对象一般特征比较
Table 1. Comparison of general characteristics of four groups
组别 例数 年龄(岁) 性别(男/女,例) ALT(U/L) AST(U/L) HBeAg (+/-) TBil(μmol/L) PTA(%) ACHBLF组 15 45.00±10.86 11/4 67.00(33.00~132.00)1) 88.00(57.70~117.00)1) 10/51) 279.00(241.00~337.00)1) 38.00(25.00~65.50)2) pre-ACHBLF组 15 48.50±7.47 11/4 57.40(31.20~114.00)1) 54.90(34.00~112.00)1) 10/51) 76.40(54.70~129.00)1) 43.90(37.50~51.00)2) CHB组 15 43.10±11.72 12/3 57.00(44.00~261.00)1) 46.00(29.00~135.00)1) 12/31) 20.00(12.00~33.00)1) 84.00(70.50~91.20) HC组 15 42.10±6.37 12/3 21.10(11.00~32.30) 21.70(17.20~27.90) 0/15 11.60(9.90~14.40) - 统计值 F=1.326 χ2=0.373 F=18.594 F=27.525 χ2=23.571 F=48.781 F=26.217 P值 0.275 0.946 <0.001 <0.001 <0.001 <0.001 <0.001 注:与HC组比较,1)P<0.05;与CHB组比较,2)P<0.05。 表 2 pre-ACHBLF患者免疫细胞水平与炎症指标的关系
Table 2. The relationship between the expression of immune cells and the degree of inflammation in patients with pre-ACHBLF
炎症指标 MDSC Th17 Treg Th17/Treg Tc17 r值 P值 r值 P值 r值 P值 r值 P值 r值 P值 白细胞 0.775 0.001 0.167 0.668 0.618 0.043 -0.286 0.493 -0.533 0.139 中性粒细胞 0.727 0.002 0.250 0.516 0.491 0.125 -0.238 0.570 -0.417 0.265 淋巴细胞 0.079 0.781 -0.611 0.081 0.333 0.318 -0.790 0.020 -0.795 0.010 NLR 0.571 0.026 0.483 0.187 0.255 0.450 0.048 0.911 -0.150 0.700 MLR 0.786 0.001 0.233 0.546 0.236 0.484 0.024 0.955 -0.433 0.244 PLR 0.161 0.567 0.600 0.088 0.018 0.958 0.429 0.289 0.106 0.787 SIRS 0.846 <0.001 0.233 0.546 0.336 0.312 0.024 0.955 -0.433 0.244 -
[1] XIAO LL, WU XX, CHEN JJ, et al. Progress in hepatitis B virus-related acute-on-chronic liver failure treatment in China: A large, multicenter, retrospective cohort study using a propensity score matching analysis[J]. Hepatobiliary Pancreat Dis Int, 2021, 20(6): 535-541. DOI: 10.1016/j.hbpd.2021.05.010. [2] LIU M, ZHANG XQ, MAO Q. Features of acute-on-chronic pre-liver failure and establishment of a predictive model for risk of acute-on-chronic liver failure[J]. J Clin Hepatol, 2012, 28(10): 732-734. https://www.cnki.com.cn/Article/CJFDTOTAL-LCGD201210005.htm刘明, 张绪清, 毛青. 慢加急性肝衰竭前期的概念及预警模型[J]. 临床肝胆病杂志, 2012, 28(10): 732-734. https://www.cnki.com.cn/Article/CJFDTOTAL-LCGD201210005.htm [3] TAN NH, CHEN B, PENG J, et al. Treg/Th17 cell balance in patients with hepatitis B virus-related acute-on-chronic liver failure at different disease stages[J]. Biomed Res Int, 2021, 2021: 9140602. DOI: 10.1155/2021/9140602. [4] ZENG Y, LI Y, XU Z, et al. Myeloid-derived suppressor cells expansion is closely associated with disease severity and progression in HBV-related acute-on-chronic liver failure[J]. J Med Virol, 2019, 91(8): 1510-1518. DOI: 10.1002/jmv.25466. [5] WEISS E, de la GRANGE P, DEFAYE M, et al. Characterization of blood immune cells in patients with decompensated cirrhosis including ACLF[J]. Front Immunol, 2020, 11: 619039. DOI: 10.3389/fimmu.2020.619039. [6] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [7] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. [8] TRIANTAFYLLOU E, WOOLLARD KJ, MCPHAIL M, et al. The role of monocytes and macrophages in acute and acute-on-chronic liver failure[J]. Front Immunol, 2018, 9: 2948. DOI: 10.3389/fimmu.2018.02948. [9] ZENG YF, XU Z, LU LR, et al. The possible role and clinical implication of myeloid-derived suppressor cells in the peripheral blood of patients with hepatitis B virus-related acute-on- chronic liver failure[J]. Chin J Infect Dis, 2016, 34(4): 215-222. DOI: 10.3760/cma.j.issn.1000-6680.2016.04.003.曾映夫, 许镇, 陆丽蓉, 等. 髓源性抑制细胞在乙型肝炎病毒相关慢加急性(亚急性)肝衰竭患者外周血中的作用及临床意义[J]. 中华传染病杂志, 2016, 34(4): 215-222. DOI: 10.3760/cma.j.issn.1000-6680.2016.04.003. [10] CHIU DK, XU IM, LAI RK, et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26[J]. Hepatology, 2016, 64(3): 797-813. DOI: 10.1002/hep.28655. [11] REN JP, WANG L, ZHAO J, et al. Decline of miR-124 in myeloid cells promotes regulatory T-cell development in hepatitis C virus infection[J]. Immunology, 2017, 150(2): 213-220. DOI: 10.1111/imm.12680. [12] HAIST M, STEGE H, GRABBE S, et al. The functional crosstalk between myeloid-derived suppressor cells and regulatory T cells within the immunosuppressive tumor microenvironment[J]. Cancers (Basel), 2021, 13(2): 210. DOI: 10.3390/cancers13020210. [13] BAO J, CUI D, WANG X, et al. Decreased frequencies of Th17 and Tc17 cells in patients infected with avian influenza A (H7N9) virus[J]. J Immunol Res, 2019, 2019: 1418251. DOI: 10.1155/2019/1418251. [14] LVCKEL C, PICARD F, HUBER M. Tc17 biology and function: Novel concepts[J]. Eur J Immunol, 2020, 50(9): 1257-1267. DOI: 10.1002/eji.202048627. [15] ZHANG JP, YAN J, XU J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients[J]. J Hepatol, 2009, 50(5): 980-989. DOI: 10.1016/j.jhep.2008.12.033. [16] LIANG XS, LI CZ, ZHOU Y, et al. Changes in circulating Foxp3(+) regulatory T cells and interleukin-17-producing T helper cells during HBV-related acute-on-chronic liver failure[J]. World J Gastroenterol, 2014, 20(26): 8558-8571. DOI: 10.3748/wjg.v20.i26.8558. [17] ZHANG GL, XU SC, ZHANG T, et al. Effects of Tc17 cells on disease severity and HBV-DNA elimination in patients with HBV related acute-on-chronic liver failure[J]. J Sun Yat-sen Univ(Med Sci), 2019, 40(5): 739-746. DOI: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2019.0103.张耿林, 徐士丞, 张婷, 等. Tc17细胞对乙肝相关慢加急性肝衰竭病情进展及乙肝病毒清除的影响[J]. 中山大学学报(医学科学版), 2019, 40(5): 739-746. DOI: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2019.0103. [18] ZHANG GL, ZHANG T, ZHAO QY, et al. Increased IL-17-producing CD8+ T cell frequency predicts short-term mortality in patients with hepatitis B virus-related acute-on-chronic liver failure[J]. Ther Clin Risk Manag, 2018, 14: 2127-2136. DOI: 10.2147/TCRM.S184809. 期刊类型引用(2)
1. 张雄乐,陈芬兰,林文. 甲泼尼龙联合抗病毒药物治疗对乙型肝炎早期肝衰竭患者肝功能及炎性因子的影响. 中国医药指南. 2023(34): 98-101 .  百度学术
百度学术2. 吴刚,叶晓玲,张宇,石磬. 单免疫球蛋白-白介素1受体蛋白在慢性乙肝患者中的表达水平变化及临床意义研究. 罕少疾病杂志. 2023(12): 72-74 .  百度学术
百度学术其他类型引用(3)
-




 PDF下载 ( 1963 KB)
PDF下载 ( 1963 KB)

 下载:
下载:

 下载:
下载:
 百度学术
百度学术


