基因表达谱分析非酒精性脂肪性肝病中谷胱甘肽转移酶的作用
DOI: 10.3969/j.issn.1001-5256.2023.01.014
Role of glutathione transferase in nonalcoholic fatty liver disease: An analysis based on gene expression profile
-
摘要:
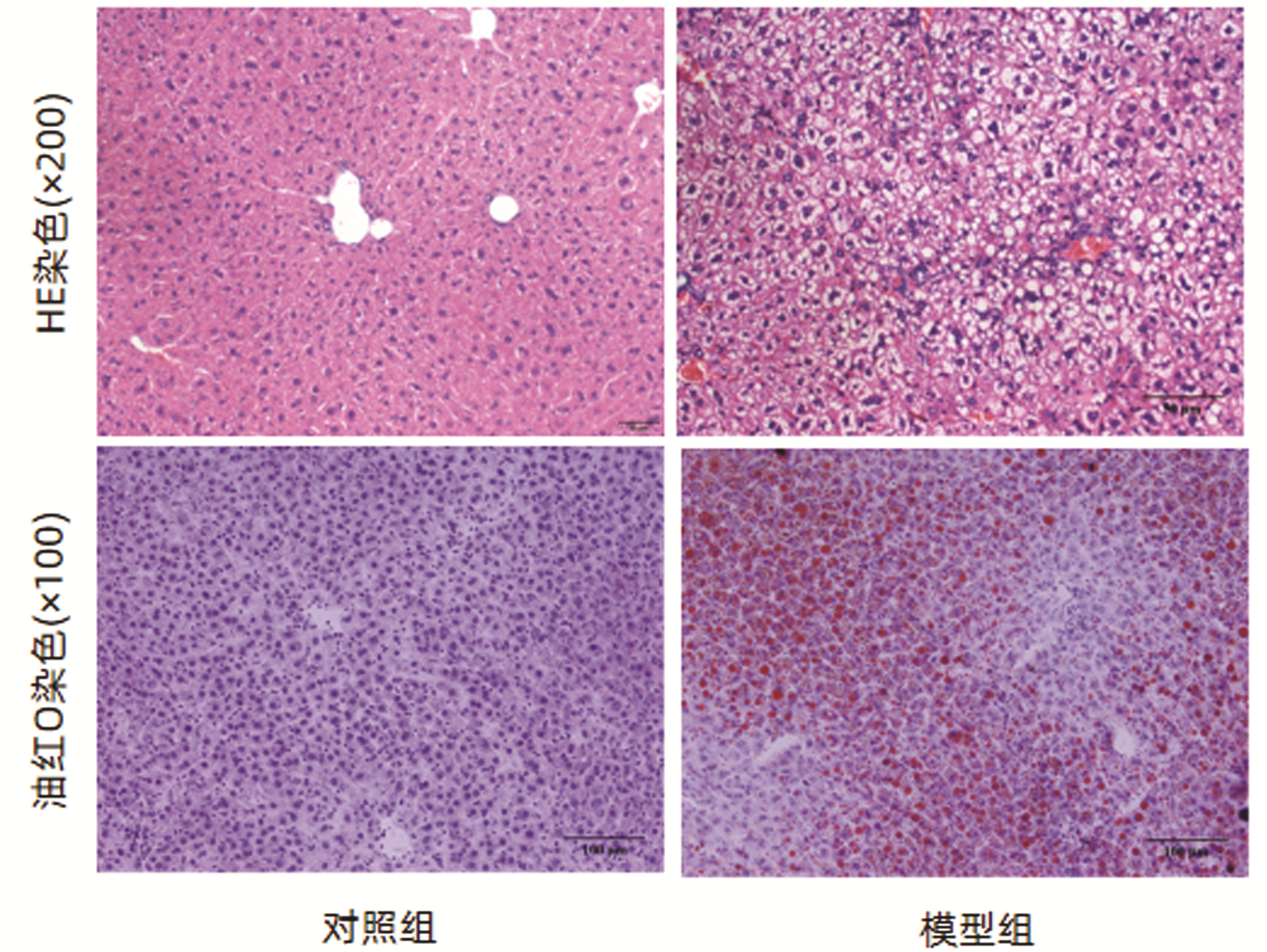
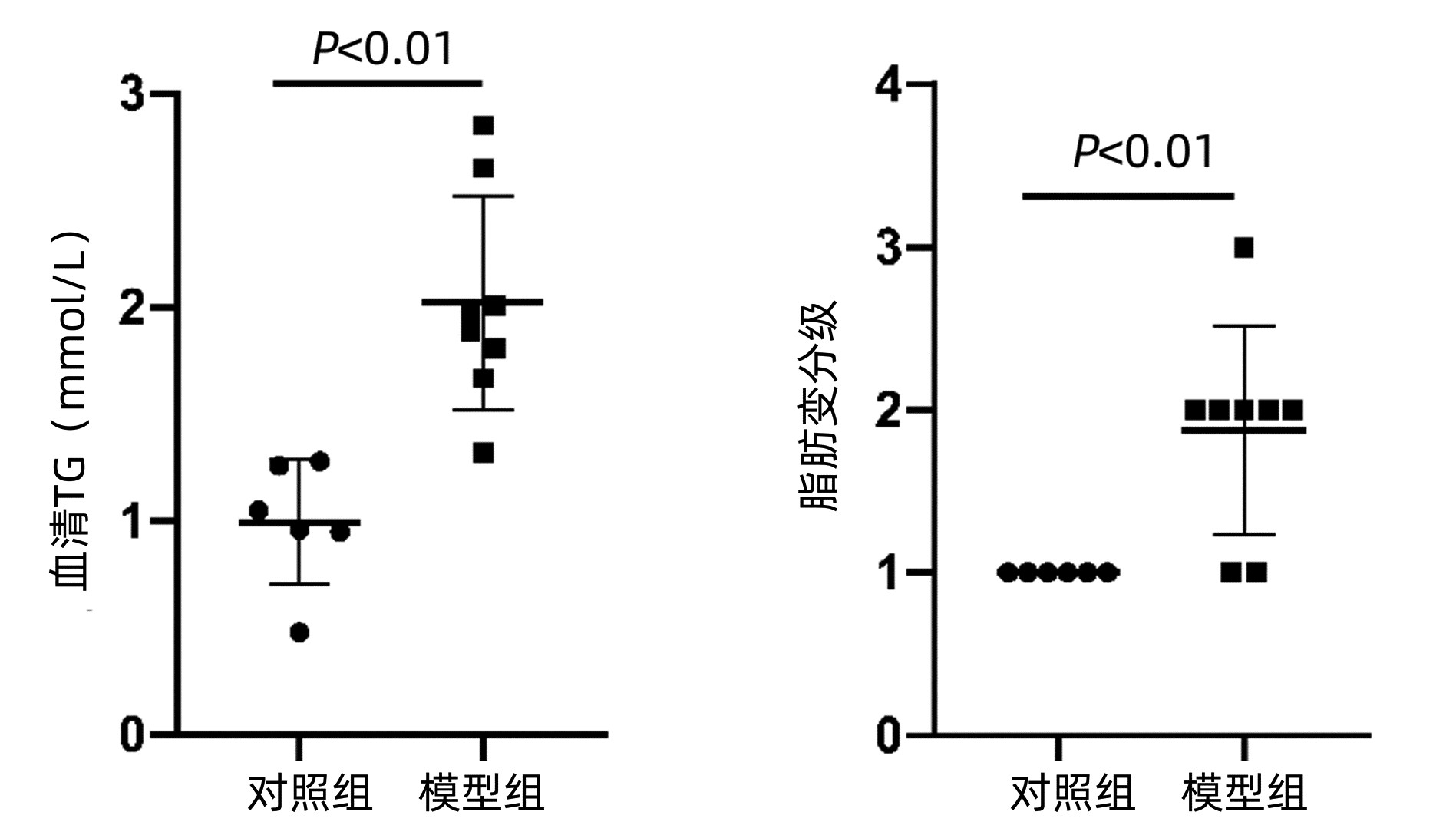
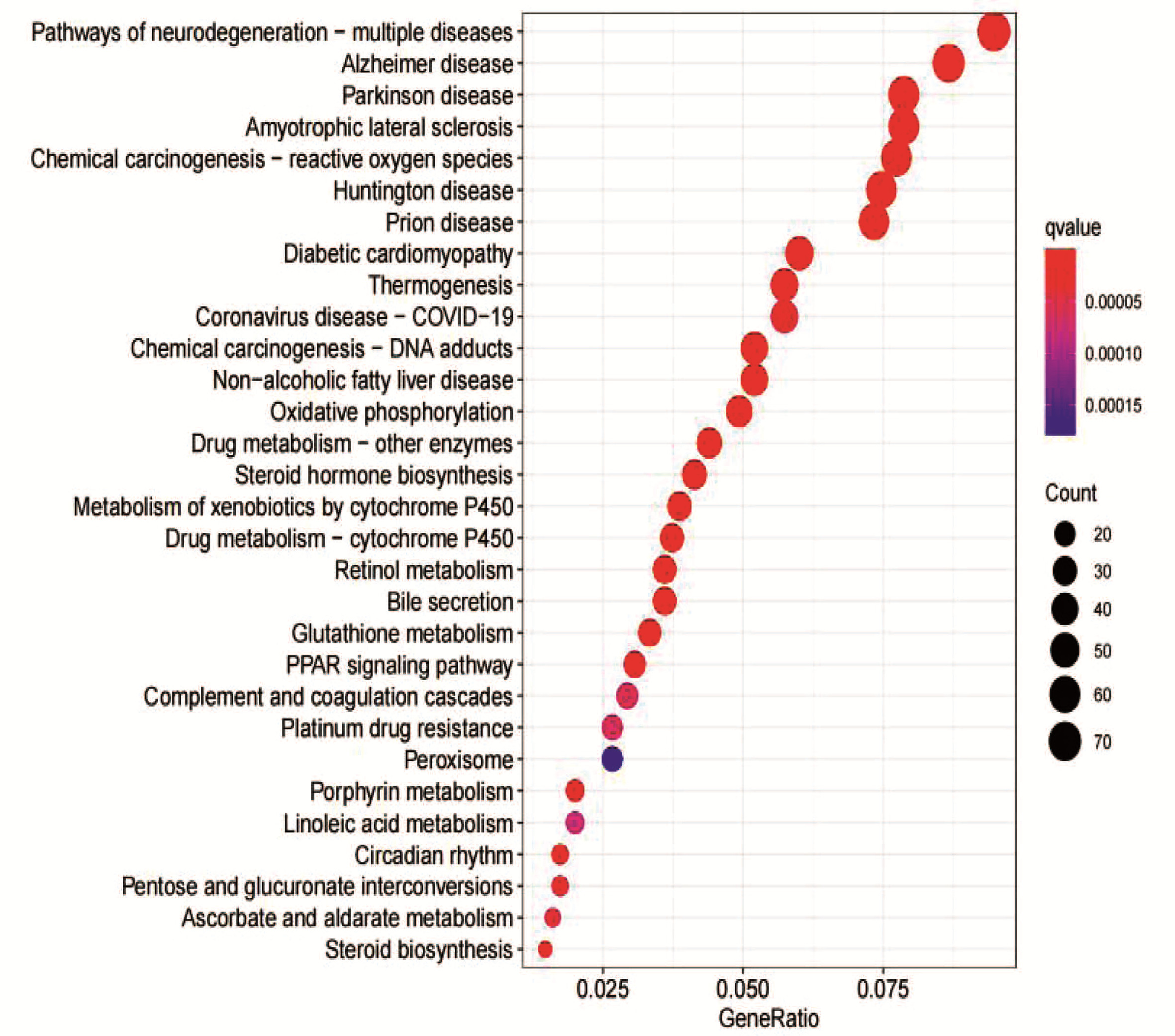
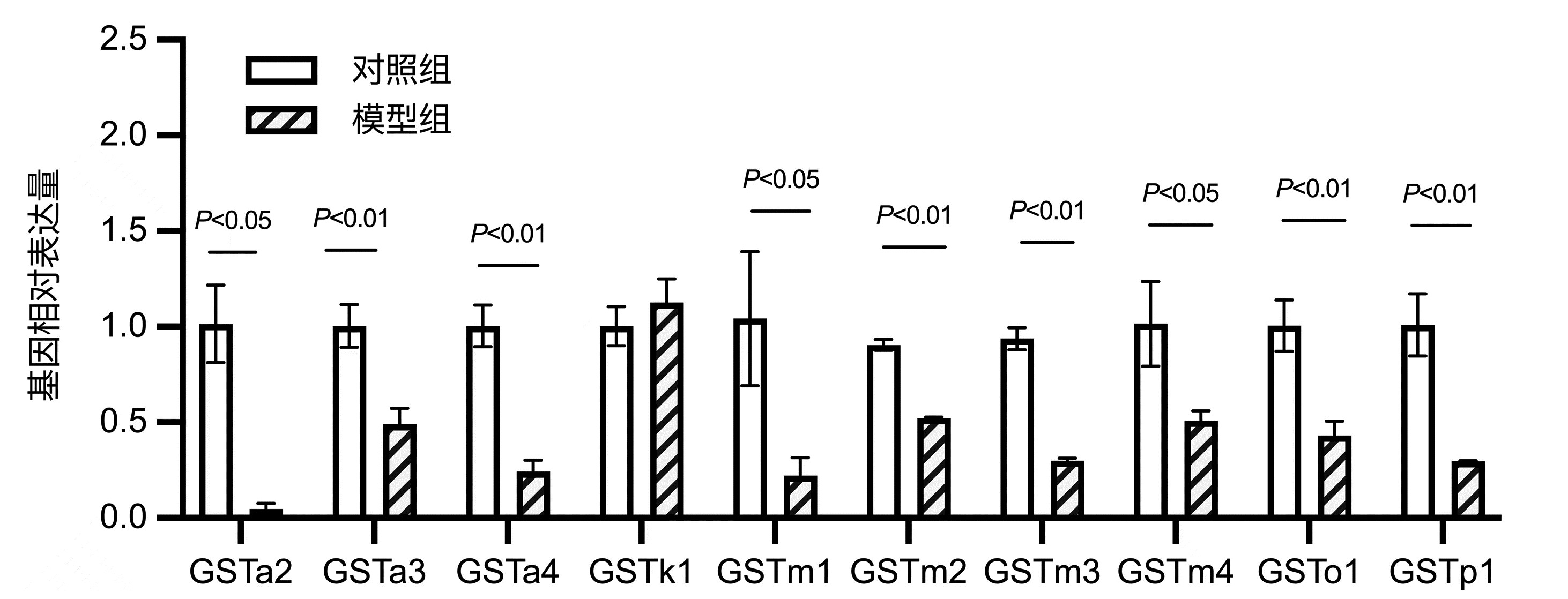
目的 用二代转录组测序(RNA-seq)技术,结合差异基因GO分析、KEGG富集分析,探讨谷胱甘肽转移酶(GST)在高脂饮食诱导的小鼠非酒精性脂肪性肝病(NAFLD)中的作用。 方法 14只雄性C57BL/6J小鼠随机抽样分为对照组(n=6)和模型组(n=8)。对照组小鼠喂养普通饲料;模型组喂养高脂饲料,连续7周,构建NAFLD模型。试剂盒检测血清ALT、AST活性及TG水平、苏木精-伊红(HE)和油红染色观察肝组织病理和脂滴沉积情况;提取肝组织RNA进行高通量转录组测序,将基因表达量差异倍数≥2.0且P<0.05定义为差异基因,筛选对照组与模型组肝组织差异基因,应用GO、KEGG数据库进行功能分析,并采用qRT-PCR验证差异基因表达。符合正态分布的计量资料两组比较采用独立样本t检验。 结果 对照组与模型组小鼠体质量、血清ALT、AST差异无统计学意义(P值均>0.05)。与对照组相比,模型组血清TG水平明显高于对照组[(2.02±0.50)mmol/L vs (1.00±0.29)mmol/L,t=-4.45,P=0.001]。HE染色提示:模型组可见弥漫性脂肪变性和气球样变。油红染色显示:模型组肝细胞胞浆内橘红色脂滴明显增多,且肝细胞脂肪变分级明显高于对照组(1.88±0.64 vs 1.00±0.00,t=-3.86,P=0.006)。转录组测序提示两组差异基因1367个,其中上调基因数608个、下调基因数759个;且两组中GST基因差异表达17个。选择差异倍数最明显的前10个GST基因进行验证。与对照组相比,GSTa2、GSTa3、GSTa4、GSTm1、GSTm2、GSTm3、GSTm4、GSTp1、GSTo1表达下调,GSTk1表达上调。实验结果与测序结果一致。 结论 GST通过参与类固醇代谢过程、脂肪酸代谢过程、胆固醇代谢过程等多个生物学过程影响脂质代谢,与NAFLD发病密切相关。 Abstract:Objective To investigate the role of glutathione transferase in nonalcoholic fatty liver disease (NAFLD) induced by high-fat diet using the RNA-Seq technique in combination with gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of differentially expressed genes. Methods A total of 14 male C57BL/6J mice were divided into control group with 6 mice and model group with 8 mice by random sampling. The mice in the control group were fed with normal diet, and those in the model group were fed with high-fat diet for 7 consecutive weeks to establish a model of NAFLD. Kits were used to measure the activities of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and the level of triglyceride (TG), and HE staining and oil red staining were used to observe liver pathology and deposition of lipid droplets. Liver tissue RNA was extracted for RNA-Seq, and genes with a fold change of ≥2.0 and a P value of < 0.05 were defined as differentially expressed genes; after differentially expressed genes were screened out between the control group and the model group, GO and KEGG enrichment analyses were performed, and qRT-PCR was used to validate the expression of the differentially expressed genes. The independent samples t-test was used for comparison of normally distributed continuous data between two groups. Results There were no significant differences between the two groups in body weight and the serum levels of ALT and AST (all P > 0.05). Compared with the control group, the model group had a significantly higher serum level of TG (2.02±0.50 mmol/L vs 1.00±0.29 mmol/L, t=-4.45, P=0.001). HE staining showed diffuse steatosis and ballooning degeneration in the model group, and oil red staining showed that the model group had a significant increase in orange-red lipid droplets in the cytoplasm of hepatocytes and a significantly higher grade of hepatocyte steatosis than the control group (1.88±0.64 vs 1.00±0.00, t=-3.86, P=0.006). RNA-seq results showed a total of 1367 differentially expressed genes between the two groups, among which there were 608 upregulated genes and 759 downregulated genes, and there were 17 differentially expressed GST genes between the two groups. The top 10 GST genes in terms of fold change were validated, and compared with the control group, the model group had downregulated expression of GSTa2, GSTa3, GSTa4, GSTm1, GSTm2, GSTm3, GSTm4, GSTp1, and GSTo1 and upregulated expression of GSTk1. The results of qRT-PCR were consistent with the results of sequencing. Conclusion GST affects lipid metabolism by participating in various biological processes such as steroid metabolism, fatty acid metabolism, and cholesterol metabolism and is closely associated with the pathogenesis of NAFLD. -
表 1 RT-PCR引物序列表
Table 1. RT-PCR primer sequence list
基因 引物 序列 产物长度(bp) GSTp1 上游 5′-ATGCCACCATACACCATTGTC-3′ 161 下游 5′-GGGAGCTGCCCATACAGAC-3′ GSTm1 上游 5′-ATACTGGGATACTGGAACGTCC-3′ 349 下游 5′-AGTCAGGGTTGTAACAGAGCAT-3′ GSTa3 上游 5′-AAGAATGGAGCCTATCCGGTG-3′ 81 下游 5′-AGGTCATCCCGAGTTTTCAGAA-3′ GSTm3 上游 5′-GCGGACTGACTCACTCCATC-3′ 77 下游 5′-CCCCATGACATATCTCTTCTCCT-3′ GSTm2 上游 5′-ACACCCGCATACAGTTGGC-3′ 118 下游 5′-TGCTTGCCCAGAAACTCAGAG-3′ GSTa4 上游 5′-TACCTCGCTGCCAAGTACAAC-3′ 109 下游 5′-GAGCCACGGCAATCATCATCA-3′ GSTk1 上游 5′-GGTCCTATGCAGATACCAACAC-3′ 124 下游 5′-GTACTGGCCTTTTCGGGGAA-3′ GSTo1 上游 5′-ATCCGGCACGAAGTCATCAAT-3′ 124 下游 5′-TGACAGATTCGGTGACCAAGT-3′ GSTm4 上游 5′-CTGAAGGTGGAATACTTGGAGC-3′ 112 下游 5′-GCCCAGGAACTGTGAGAAGA-3′ GSTa2 上游 5′-ACATGAAGGAGAGAGCCCTGAT-3′ 63 下游 5′-GCAGTCTTGGCTTCTCTTTGGT-3′ GAPDH 上游 5′-AGGAGTAAGAAACCCTGGAC-3′ 110 下游 5′-CTGGGATGGAATTGTGAG-3′ 表 2 GST差异表达基因GO和KEGG富集分析
Table 2. GO and KEGG enrichment analysis of GST differentially expressed genes
分类 注释 P值 基因 GO 谷胱甘肽代谢过程 5.97×10-18 GSTa2/GSTa4/GSTp1/Gsr/GSTa1/Park7/GSTt3/Gclc/Nat8/
GSTk1/Cth/Ethe1/GSTm1/Gclm/GSTm4/GSTp2/GSTa3/Sod2/
GSTz1/GSTt1/Nfe2l2/Sod1/GSTt2/Gpx3/GSTm2/GSTm3KEGG 谷胱甘肽代谢通路 2.84×10-10 GSTa2/GSTa4/GSTp1/GSTo1/Gsr/GSTa1/Nat8f2/GSTt3/Gclc/
Nat8f5/Nat8/GSTk1/GSTm1/Gclm/Txndc12/GSTm4/GSTp2/
GSTa3/Anpep/GSTt1/Idh2/GSTt2/Gpx3/GSTm2/GSTm3 -
[1] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [2] LAN T, HU Y, HU F, et al. Hepatocyte glutathione S-transferase mu 2 prevents non-alcoholic steatohepatitis by suppressing ASK1 signaling[J]. J Hepatol, 2022, 76(2): 407-419. DOI: 10.1016/j.jhep.2021.09.040. [3] HAYES PC, BOUCHIER IA, BECKETT GJ. Glutathione S-transferase in humans in health and disease[J]. Gut, 1991, 32(7): 813-818. DOI: 10.1136/gut.32.7.813. [4] CAI QF, WU Q. Research progress of glutathione transferase[J]. J Hanan Med Coll, 2011, 17(12): 1735-1738. DOI: 46-1049/R.20111011.1408.007.蔡群芳, 邬强. 谷胱甘肽转移酶的研究进展[J]. 海南医学院学报, 2011, 17(12): 1735-1738. DOI: 46-1049/R.20111011.1408.007. [5] MORRIS MJ, LIU D, WEAVER LM, et al. A structural basis for cellular uptake of GST-fold proteins[J]. PLoS One, 2011, 6(3): e17864. DOI: 10.1371/journal.pone.0017864. [6] MOREL F, ANINAT C. The glutathione transferase kappa family[J]. Drug Metab Rev, 2011, 43(2): 281-291. DOI: 10.3109/03602532.2011.556122. [7] National Workshop on Fatty Liver and Alcoholic Liver Disease. Guidelines for diagnosis and treatment of nonalcoholic fatty liver disease (revised in February 2006)[J]. Mod Digest Interv, 2007, 12(4): 266-268. DOI: 10.3969/j.issn.1672-2159.2007.04.020.中华医学会肝脏病学分会脂肪肝和酒精性肝病学组. 非酒精性脂肪性肝病诊疗指南(2006年2月修订)[J]. 现代消化及介入诊疗, 2007, 12(4): 266-268. DOI: 10.3969/j.issn.1672-2159.2007.04.020. [8] ZHU GR, WANG J, HUANG K, et al. A transcriptomic analysis of acute hepatotoxicity induced by aristolochic acidⅠin mice[J]. J Clin Hepatol, 2021, 37(10): 2389-2394. DOI: 10.3969/j.issn.1001-5256.2021.10.026.朱哿瑞, 王静, 黄恺, 等. 马兜铃酸Ⅰ致小鼠急性肝毒性的转录组学分析[J]. 临床肝胆病杂志, 2021, 37(10): 2389-2394. DOI: 10.3969/j.issn.1001-5256.2021.10.026. [9] PRYSYAZHNYUK V, SYDORCHUK L, SYDORCHUK R, et al. Glutathione-S-transferases genes-promising predictors of hepatic dysfunction[J]. World J Hepatol, 2021, 13(6): 620-633. DOI: 10.4254/wjh.v13.i6.620. [10] HAYES JD, FLANAGAN JU, JOWSEY IR. Glutathione transferases[J]. Annu Rev Pharmacol Toxicol, 2005, 45: 51-88. DOI: 10.1146/annurev.pharmtox.45.120403.095857. [11] NEBERT DW, VASILIOU V. Analysis of the glutathione S-transferase (GST) gene family[J]. Hum Genomics, 2004, 1(6): 460-464. DOI: 10.1186/1479-7364-1-6-460. [12] PRYSYAZHNYUK VP, ROSSOKHA ZI, GOROVENKO NG. Variation in particular biochemical indicators, cytokine and adipokine profiles of the blood, and the structural and functional parameters of the liver in patients with nonalcoholic fatty liver disease and different genotypes by the polymorphic locus A313G of the GSTP1 gene[J]. Cytol Genet, 2017, 6: 50-57. DOI: 10.3103/S0095452717060111. [13] HASHEMI M, ESKANDARI-NASAB E, FAZAELI A, et al. Association of genetic polymorphisms of glutathione-S-transferase genes (GSTT1, GSTM1, and GSTP1) and susceptibility to nonalcoholic fatty liver disease in Zahedan, Southeast Iran[J]. DNA Cell Biol, 2012, 31(5): 672-677. DOI: 10.1089/dna.2011.1343. [14] KASER S, MOSCHEN A, KASER A, et al. Circulating adiponectin reflects severity of liver disease but not insulin sensitivity in liver cirrhosis[J]. J Intern Med, 2005, 258(3): 274-280. DOI: 10.1111/j.1365-2796.2005.01543.x. [15] PRYSYAZHNYUK V, VOLOSHYN O, PRYSIAZHNIUK I, et al. Glutathione S-transferase T1 and M1 null genotype distribution among non-alcoholic fatty liver disease patients and its association with cytokine and adipokine profiles[J]. Clin Exp Hepatol, 2020, 6(2): 142-149. DOI: 10.5114/ceh.2020.95678. [16] RONIS M, MERCER K, ENGI B, et al. Global deletion of glutathione S-transferase A4 exacerbates developmental nonalcoholic steatohepatitis[J]. Am J Pathol, 2017, 187(2): 418-430. DOI: 10.1016/j.ajpath.2016.10.022. [17] LI N, SUN YR, HE LB, et al. Amelioration by idesia polycarpa maxim. var. vestita diels. of oleic acid-induced nonalcoholic fatty liver in HepG2 cells through antioxidant and modulation of lipid metabolism[J]. Oxid Med Cell Longev, 2020, 2020: 1208726. DOI: 10.1155/2020/1208726. [18] ZHU YL, ZHANG SJ, YANG C, et al. Quantitative analysis of differential proteins in liver tissues of patients with non-alcoholic steatohepatitis using iTRAQ technology[J]. J South Med Univ, 2021, 41(9): 1381-1387. DOI: 10.12122/j.issn.1673-4254.2021.09.13.朱雅莉, 章述军, 阳成, 等. 非酒精性脂肪性肝病患者的肝组织差异蛋白的定量分析: 基于iTRAQ技术[J]. 南方医科大学学报, 2021, 41(9): 1381-1387. DOI: 10.12122/j.issn.1673-4254.2021.09.13. 期刊类型引用(5)
1. 康志龙,周益民,宋燕州,张昆,苏一男,魏文平,赵新,李志伟. 靶向免疫治疗联合经肝动脉灌注化疗治疗不可切除肝细胞癌的临床效果及转化治疗的影响因素. 中国医学创新. 2025(01): 47-51 .  百度学术
百度学术2. 许海明,胡永奎,梁平,钟腾猛,陆炳站. 中晚期肝癌患者经动脉化疗栓塞治疗后短期效果及长期预后的影响因素分析. 大医生. 2025(06): 27-31 .  百度学术
百度学术3. 彭林辉,陈涛,徐云修修,王捷,陈捷,李永,黄拼搏,钟国平,陈茜,叶聪婷,陈亚进. mFOLFOX7方案全身化疗联合卡瑞利珠单克隆抗体和阿帕替尼治疗肝细胞癌合并Vp4型门静脉癌栓的疗效. 中华消化外科杂志. 2024(02): 265-271 .  百度学术
百度学术4. 李洁,李瑞娟,鲁志兵,曾筱怡. 肝动脉灌注化疗联合程序性死亡受体1抗体及仑伐替尼治疗晚期肝癌合并门静脉癌栓的效果. 中国当代医药. 2024(26): 43-46+51 .  百度学术
百度学术5. 黄乾鑫,神斌,肖晋昌,高志康,吕墩涛,李艳,徐浩,张庆桥. 载药微球经导管动脉化疗栓塞术联合肠系膜上动脉灌注化疗治疗肝细胞癌合并门静脉癌栓的效果分析. 临床肝胆病杂志. 2024(12): 2457-2463 .  本站查看
本站查看其他类型引用(0)
-




 PDF下载 ( 4657 KB)
PDF下载 ( 4657 KB)

 下载:
下载:

 百度学术
百度学术

