肝癌肝切除术后感染风险预测模型的建立与评价
DOI: 10.3969/j.issn.1001-5256.2023.01.017
Establishment and validation of a nomogram risk prediction model for infection complications in patients after hepatectomy for liver cancer
-
摘要:
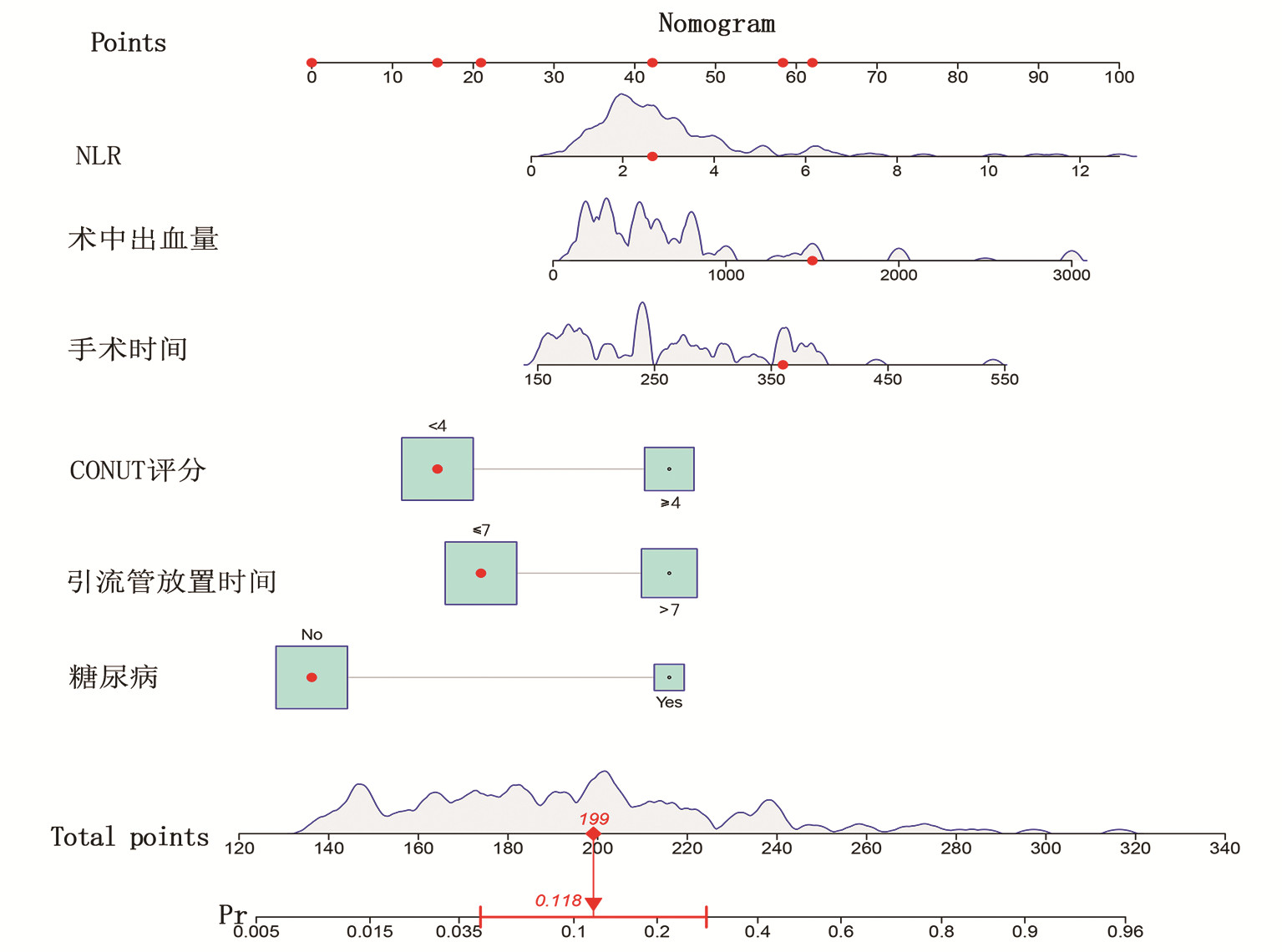
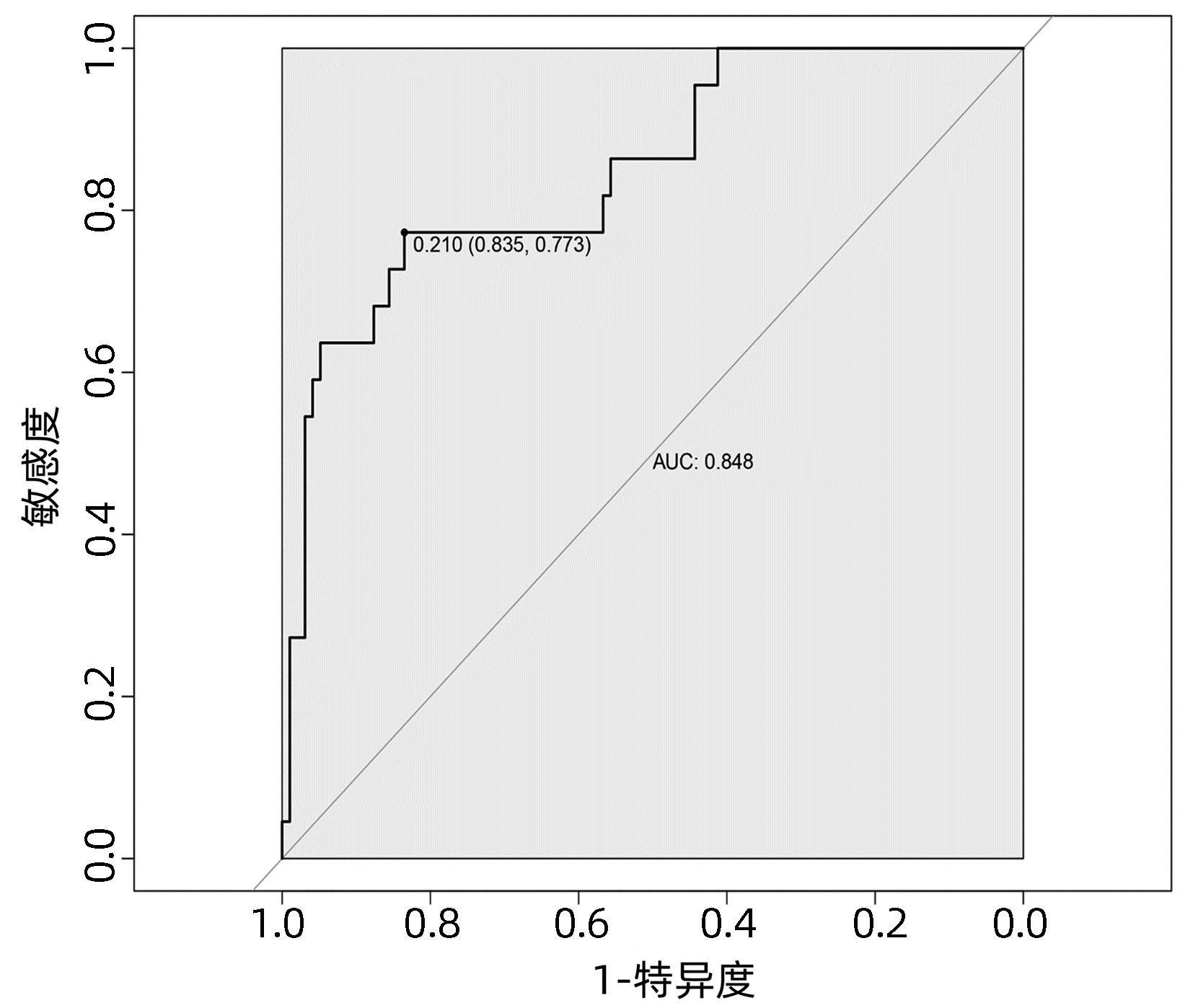
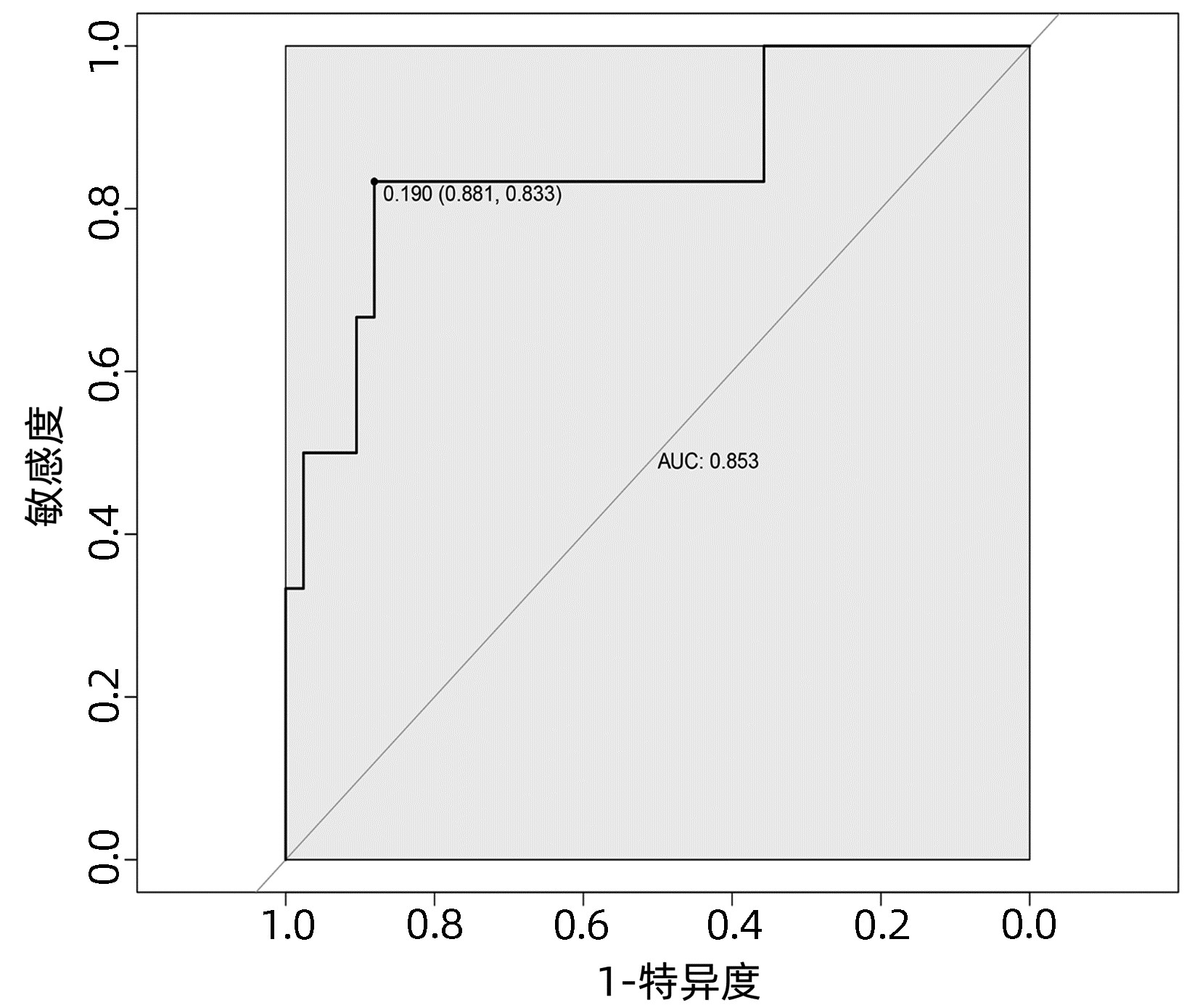
目的 探讨肝癌肝切除术后发生感染的危险因素,建立并验证风险预测模型。 方法 收集2020年1月—2022年4月于武汉大学人民医院行肝切除术的167例原发性肝癌患者的临床资料。根据术后早期是否发生感染,将所有患者分为术后感染组(n=28) 和非感染组(n=139)。计量资料两组间比较采用t检验或Mann- Whitney U检验,计数资料两组间比较采用χ2检验。采用单因素分析与Logistic回归分析筛选肝癌肝切除术后感染的影响因素,并建立术后发生感染的列线图风险预测模型,将所有患者按7∶ 3随机分为训练集(n=119)和验证集(n=48),采用Bootstrap法对模型进行内部验证,应用模型校准曲线和受试者工作特征曲线(ROC曲线)来评价列线图模型的校准度和区分度。 结果 167例患者中28例(16.8%)发生术后感染。Logistc多因素分析结果显示,糖尿病、CONUT评分≥4分、术前NLR、手术时间、术中失血量、引流管放置时间>7 d是肝癌肝切除术后发生感染的独立危险因素(P值均<0.05)。基于上述6个危险因素构建的列线图,训练集和验证集的ROC曲线下面积分别为0.848、0.853。列线图模型校准曲线显示预测值与实际观测值基本一致,表明列线图模型预测的准确度较好。 结论 基于糖尿病、CONUT评分≥4分、术前NLR、手术时间、术中失血量、引流管放置时间>7 d建立的个体化列线图风险预测模型预测效能良好,对高风险患者具有较高的预测价值。 Abstract:Objective To investigate the risk factors of infection after hepatectomy for liver cancer, and to establish and validate a risk prediction model. Methods The clinical data of 167 patients with primary liver cancer who underwent hepatectomy in People's Hospital of Wuhan University from January 2020 to March 2022 were retrospectively collected. All patients were divided into postoperative infection group (n=28) and non-infection group (n=139) according to whether postoperative infection complications occurred. The t-test or Mann-Whitney U test was used for comparison of continuous data between two groups and the chi-square test was used for comparison of categorical data between two groups. Univariate analysis and logistic regression analysis were used to screen the risk factors of infection after hepatectomy for hepatocellular carcinoma, and a nomogram risk prediction model for postoperative infection was established. All patients were randomly divided into training cohort (n=119) and the validation cohort (n=48) according to the ratio of 7∶ 3, the Bootstrap method was used for internal validation of the model, and the model calibration curve and ROC curve were used to evaluate the calibration and discrimination of the nomogram model. Results Postoperative infection occurred in 28 of 167 patients (16.8%). Logistic regression analysis showed that diabetes, CONUT score ≥4 points, preoperative NLR, operation time, intraoperative blood loss, and drainage tube placement time > 7 d were independent risk factors for infection after hepatectomy for liver cancer (all P < 0.05). Based on the nomogram constructed from the above six risk factors, the area under the ROC curve of the training cohort and the validation cohort was 0.848, and 0.853, respectively. The calibration curve of the nomogram model shows that the predicted value is basically consistent with the actual observed value, indicating that the accuracy of the nomogram model prediction is better. Conclusion The individualized nomogram risk prediction model based on diabetes, CONUT score ≥4 points, preoperative NLR, operation time, intraoperative blood loss, and drainage tube placement time > 7 d has good predictive performance and has high predictive value for high-risk patients. -
Key words:
- Carcinoma, Hepatocellular /
- Hepatectomy /
- Infection /
- Risk Factors /
- Nomogram
-
表 1 肝切除术后感染影响因素的单因素分析
Table 1. Univariate analysis of the influencing factors of infection after hepatectomy
变量 感染组(n=28) 非感染组(n=139) 统计值 P值 年龄(岁) 63.3±8.2 59.3±11.1 t=1.783 0.076 男/女(例) 22/6 117/22 χ2=0.524 0.469 高血压(是/否,例) 11/15 47/90 χ2=0.610 0.435 糖尿病(是/否,例) 9/19 16/123 χ2=7.794 0.005 乙型肝炎(是/否,例) 18/10 99/40 χ2=0.535 0.465 肝硬化(是/否,例) 11/17 72/67 χ2=1.60 0.227 肿瘤数目[例(%)] χ2=3.571 0.059 单发 17(60.7) 108(77.7) 多发 11(39.3) 31(22.3) 肿瘤大小[例(%)] χ2=2.056 0.152 <5 cm 8(28.6) 60(43.2) ≥5 cm 20(71.4) 79(65.8) Edmonson分级[例(%)] χ2=0.004 0.952 Ⅰ~Ⅱ级 7(25.0) 34(24.5) Ⅲ~Ⅳ级 21(75.0) 105(75.5) 手术部位[例(%)] χ2=0.307 0.858 左半肝 8(28.6) 44(31.6) 右半肝 18(64.3) 82(59.0) 双侧半肝 2(7.1) 13(9.4) 肝段切除[例(%)] χ2=0.874 0.350 <3段 19(67.9) 106(76.3) ≥3段 9(32.1) 33(23.7) 手术方式[例(%)] χ2=8.224 0.004 开腹 24(85.7) 79(56.8) 腹腔镜 4(14.3) 60(43.2) AFP[例(%)] χ2=0.204 0.652 ≥20 ng/mL 10(35.7) 56(40.3) <20 ng/mL 18(64.3) 83(59.7) ALT(U/L) 28.5(18.3~53.3) 27.0(17.0~40.0) Z=-0.791 0.429 AST(U/L) 29.5(24.3~42.3) 29.0(22.0~47.0) Z=-0.079 0.937 TBil(μmol/L) 18.9(12.8~34.5) 16.0(11.7~21.4) Z=-1.545 0.122 Alb (g/L) 40.0(37.0~42.9) 40.1(37.3~43.0) Z=-0.261 0.794 PT (s) 11.5(10.8~12.2) 11.5(10.9~12.2) Z=-0.718 0.473 PLT(×109/L) 164.0(122.0~221.0) 171.5(119.0~213.3) Z=-0.287 0.774 血肌酐(μmol/L) 60.0(52.3~80.8) 65.5(56.8~74.0) Z=-0.600 0.548 总胆固醇(mmol/L) 4.0(3.1~4.5) 3.9(3.4~4.5) Z=-0.006 0.995 乳酸脱氢酶(U/L) 199.5(181.5~249.2) 212.0(182.0~257.0) Z=-0.651 0.515 碱性磷酸酶(U/L) 97.3(73.5~139.9) 84.0(69.0~114.7) Z=-1.294 0.196 术前NLR 3.4(2.2~4.6) 2.4(1.9~3.2) Z=-2.609 0.009 ALBI评分 -2.6(-2.8~-2.2) -2.6(-2.9~-2.4) Z=-0.782 0.434 CONUT评分[例(%)] χ2=6.487 0.011 ≥4分 15(53.6) 40(28.8) <4分 13(46.4) 99(71.2) 引流管放置时间[例(%)] χ2=10.102 0.001 >7 d 18(64.3) 45(32.4) ≤7 d 10(35.7) 94(67.6) ASA分级[例(%)] χ2=0.157 0.692 1~2级 16(57.1) 85(61.2) 3~4级 12(42.9) 54(38.8) 手术时间(min) 365(228~380) 240(190~300) Z=-3.451 0.001 术中出血量(mL) 800(600~1300) 500(300~700) Z=-4.992 0.001 术中补液量(mL) 2500(2100~2800) 2150(1800~2700) Z=-2.542 0.011 肝门阻断时间(min) 40(30~49) 30(19~40) Z=-2.134 0.033 血浆输注(有/无,例) 17/11 52/87 χ2=5.220 0.022 红细胞输注(有/无,例) 18/10 55/84 χ2=5.787 0.016 术后胆漏(有/无,例) 7/21 10/129 χ2=8.081 0.004 表 2 肝切除术后感染影响因素的多因素分析
Table 2. Multivariate analysis of the influencing factors of infection after hepatectomy
变量 β值 SE Wald OR 95%CI P值 糖尿病 -1.934 0.618 9.780 0.145 0.043~0.486 0.002 CONUT≥4分 1.254 0.525 5.695 3.504 1.251~9.814 0.017 术前NLR -0.247 0.114 4.736 0.781 0.625~0.976 0.030 手术时间 -0.006 0.003 4.533 0.994 0.988~0.999 0.033 术中失血量 -0.001 0.001 5.870 0.999 0.998~1.000 0.015 引流管放置时间>7 d 1.019 0.516 3.891 2.770 1.006~7.621 0.049 -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. [2] VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380(15): 1450-1462. DOI: 10.1056/NEJMra1713263. [3] LI YM, CHEN H, FENG ZD. Surgical treatments of primary liver cancer[J/CD]. Chin J Hepat Surg(Electronic Edition), 2021, 10(4): 343-347. DOI: 10.3877/cma.j.issn.2095-3232.2021.04.001.李玉民, 陈昊, 冯泽东. 原发性肝癌外科治疗[J/CD]. 中华肝脏外科手术学电子杂志, 2021, 10(4): 343-347. DOI: 10.3877/cma.j.issn.2095-3232.2021.04.001. [4] YUAN SX, ZHOU WP. Progress and hot spots of comprehensive treatment for primary liver cancer[J]. Chin J Dig Surg, 2021, 20(2): 163-170. DOI: 10.3760/cma.j.cn115610-20201211-00776.袁声贤, 周伟平. 原发性肝癌综合治疗的进展和热点[J]. 中华消化外科杂志, 2021, 20(2): 163-170. DOI: 10.3760/cma.j.cn115610-20201211-00776. [5] RAHBARI NN, MEHRABI A, MOLLBERG NM, et al. Hepatocellular carcinoma: current management and perspectives for the future[J]. Ann Surg, 2011, 253(3): 453-469. DOI: 10.1097/SLA.0b013e31820d944f. [6] SADAMORI H, YAGI T, SHINOURA S, et al. Risk factors for major morbidity after liver resection for hepatocellular carcinoma[J]. Br J Surg, 2013, 100(1): 122-129. DOI: 10.1002/bjs.8957. [7] WU KT, WANG CC, LU LG, et al. Hepatocellular carcinoma: clinical study of long-term survival and choice of treatment modalities[J]. World J Gastroenterol, 2013, 19(23): 3649-3657. DOI: 10.3748/wjg.v19.i23.3649. [8] KAIBORI M, ISHIZAKI M, MATSUI K, et al. Postoperative infectious and non-infectious complications after hepatectomy for hepatocellular carcinoma[J]. Hepatogastroenterology, 2011, 58(110-111): 1747-1756. DOI: 10.5754/hge10533. [9] LIU HJ, BAO CM, BAI WL, et al. Risk factors for infectious complications in primary liver cancer[J]. J Clin Hepatol, 2018, 34(5): 1033-1037. DOI: 10.3969/j.issn.1001-5256.2018.05.021.刘洪金, 鲍春梅, 白文林, 等. 原发性肝癌发生感染性并发症的危险因素分析[J]. 临床肝胆病杂志, 2018, 34(5): 1033-1037. DOI: 10.3969/j.issn.1001-5256.2018.05.021. [10] TANG H, LU W, YANG Z, et al. Risk factors and long-term outcome for postoperative intra-abdominal infection after hepatectomy for hepatocellular carcinoma[J]. Medicine (Baltimore), 2017, 96(17): e6795. DOI: 10.1097/MD.0000000000006795. [11] QIU JJ, MO XS, TENG YJ, et al. Establishment and evaluation of a nomogram risk prediction model for severe complications in patients after hepatectomy for hepatocellular carcinoma[J]. Chin J Gen Surg, 2021, 30(1): 24-31. DOI: 10.7659/j.issn.1005-6947.2021.01.004.邱洁净, 莫新少, 滕艳娟, 等. 肝细胞癌患者肝切除术后严重并发症列线图风险预测模型的建立与评价[J]. 中国普通外科杂志, 2021, 30(1): 24-31. DOI: 10.7659/j.issn.1005-6947. 2021.01.004. [12] YANG T, LIU K, LIU CF, et al. Impact of postoperative infective complications on long-term survival after liver resection for hepatocellular carcinoma[J]. Br J Surg, 2019, 106(9): 1228-1236. DOI: 10.1002/bjs.11231. [13] HAVERKAMP L, WEIJS TJ, van der SLUIS PC, et al. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis[J]. Surg Endosc, 2013, 27(5): 1509-1520. DOI: 10.1007/s00464-012-2661-1. [14] WANG XH, LIU FB, ZHAO YJ, et al. Influence of hepatic steatosis on postoperative infections in patients undergoing liver resection[J]. Chin J Nosocomiol, 2015, 25(21): 4944-4946. DOI: 10.11816/cn.ni.2015-142708.汪学华, 刘付宝, 赵义军, 等. 肝脏脂肪变性对肝脏切除术后感染的影响分析[J]. 中华医院感染学杂志, 2015, 25(21): 4944-4946. DOI: 10.11816/cn.ni.2015-142708. [15] GAO F, ZHANG XN, CHEN MZ, et al. Prophylactic use of antibiotics after interventional procedures for primary hepatocellular carcinoma: its clinical significance[J]. J Intervent Radiol, 2013, 22(2): 151-153. DOI: 10.3969/j.issn.1008-794X.2013.02.016.高峰, 张雪娜, 陈茂振, 等. 原发性肝癌TACE术后预防性抗生素应用价值研究[J]. 介入放射学杂志, 2013, 22(2): 151-153. DOI: 10.3969/j.issn.1008-794X.2013.02.016. [16] WANG H, YANG J, YANG J, et al. Development and validation of a prediction score for complications after hepatectomy in hepatitis B-related hepatocellular carcinoma patients[J]. PLoS One, 2014, 9(8): e105114. DOI: 10.1371/journal.pone.0105114. [17] MA KW, CHEUNG TT, SHE WH, et al. Risk prediction model for major complication after hepatectomy for malignant tumour - A validated scoring system from a university center[J]. Surg Oncol, 2017, 26(4): 446-452. DOI: 10.1016/j.suronc.2017.08.007. [18] FU XB, HE JY, ZHANG YH, et al. Perioperative management of liver cancer patients with concomitant hyperglycemia. [J]. Chin J Gen Surg, 2011, 20(7): 680-682. https://www.cnki.com.cn/Article/CJFDTOTAL-ZPWZ201107006.htm傅熙博, 贺金云, 张玉会, 等. 并存高血糖肝癌患者的围手术期处理[J]. 中国普通外科杂志, 2011, 20(7): 680-682. https://www.cnki.com.cn/Article/CJFDTOTAL-ZPWZ201107006.htm [19] OKABAYASHI T, ICHIKAWA K, NAMIKAWA T, et al. Effect of perioperative intensive insulin therapy for liver dysfunction after hepatic resection[J]. World J Surg, 2011, 35(12): 2773-2778. DOI: 10.1007/s00268-011-1299-9. [20] DAN J, ZHANG Y, PENG Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation[J]. PLoS One, 2013, 8(3): e58184. DOI: 10.1371/journal.pone.0058184. [21] JUNG MR, PARK YK, JEONG O, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer[J]. J Surg Oncol, 2011, 104(5): 504-510. DOI: 10.1002/jso.21986. [22] RUAN DY, LIN ZX, LI Y, et al. Poor oncologic outcomes of hepatocellular carcinoma patients with intra-abdominal infection after hepatectomy[J]. World J Gastroenterol, 2015, 21(18): 5598-5606. DOI: 10.3748/wjg.v21.i18.5598. [23] MAO YS, HAO SJ, ZOU CF, et al. Controlling Nutritional Status score is superior to Prognostic Nutritional Index score in predicting survival and complications in pancreatic ductal adenocarcinoma: a Chinese propensity score matching study[J]. Br J Nutr, 2020, 124(11): 1190-1197. DOI: 10.1017/S0007114520002299. [24] HARIMOTO N, YOSHIZUMI T, SAKATA K, et al. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma[J]. World J Surg, 2017, 41(11): 2805-2812. DOI: 10.1007/s00268-017-4097-1. [25] ZHAO Y, JIN Y, WU Y. Postoperative infectious complications after liver resection for hepatocellular carcinoma[J]. J Cancer Res Ther, 2016, 12(Suppl): C268-C270. DOI: 10.4103/0973-1482.200754. [26] CHACON E, EMAN P, DUGAN A, et al. Effect of operative duration on infectious complications and mortality following hepatectomy[J]. HPB (Oxford), 2019, 21(12): 1727-1733. DOI: 10.1016/j.hpb.2019.05.001. [27] NANASHIMA A, ARAI J, OYAMA S, et al. Associated factors with surgical site infections after hepatectomy: predictions and countermeasures by a retrospective cohort study[J]. Int J Surg, 2014, 12(4): 310-314. DOI: 10.1016/j.ijsu.2014.01.018. [28] LI X, YUAN LY, LIU SS, et al. Influencing factors of postoperative infections in primary liver cancer patients underwent partial hepatectomy. [J]. J Prac Hepatol, 2018, 21(1): 127-128. DOI: 10.3969/j.issn.1672-5069.2018.01.035.李霞, 袁玲雁, 刘双双, 等. 肝部分切除术患者术后并发感染的相关影响因素分析[J]. 实用肝脏病杂志, 2018, 21(1): 127-128. DOI: 10.3969/j.issn.1672-5069.2018.01.035. [29] OKABAYASHI T, NISHIMORI I, YAMASHITA K, et al. Risk factors and predictors for surgical site infection after hepatic resection[J]. J Hosp Infect, 2009, 73(1): 47-53. DOI: 10.1016/j.jhin.2009.04.022. [30] ZHOU WY, LI L, ZHONG JT, et al. Laparotomy and cool-tip radiofrequency ablation for large liver tumors: short-term results. [J]. Chin J Hepatobiliary Surg, 2013, 19(9): 677-680. DOI: 10.3760/cma.j.issn.1007-8118.2013.09.010.周武元, 李磊, 钟敬涛, 等. 开腹射频消融治疗较大肝肿瘤近期疗效分析[J]. 中华肝胆外科杂志, 2013, 19(9): 677-680. DOI: 10.3760/cma.j.issn.1007-8118.2013.09.010. 期刊类型引用(1)
1. 孔钦香,董金玲. 慢性丙型肝炎合并脂肪肝的临床特征及危险因素分析. 中国基层医药. 2022(10): 1495-1500 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2267 KB)
PDF下载 ( 2267 KB)

 下载:
下载:


 百度学术
百度学术

