碱性磷酸酶与前白蛋白比值对肝细胞癌根治性切除术后患者预后及并发症的预测价值
DOI: 10.3969/j.issn.1001-5256.2023.01.018
Predictive value of preoperative alkaline phosphatase to prealbumin ratio in prognosis and postoperative complications in patients with hepatocellular carcinoma after radical tumor resection
-
摘要:
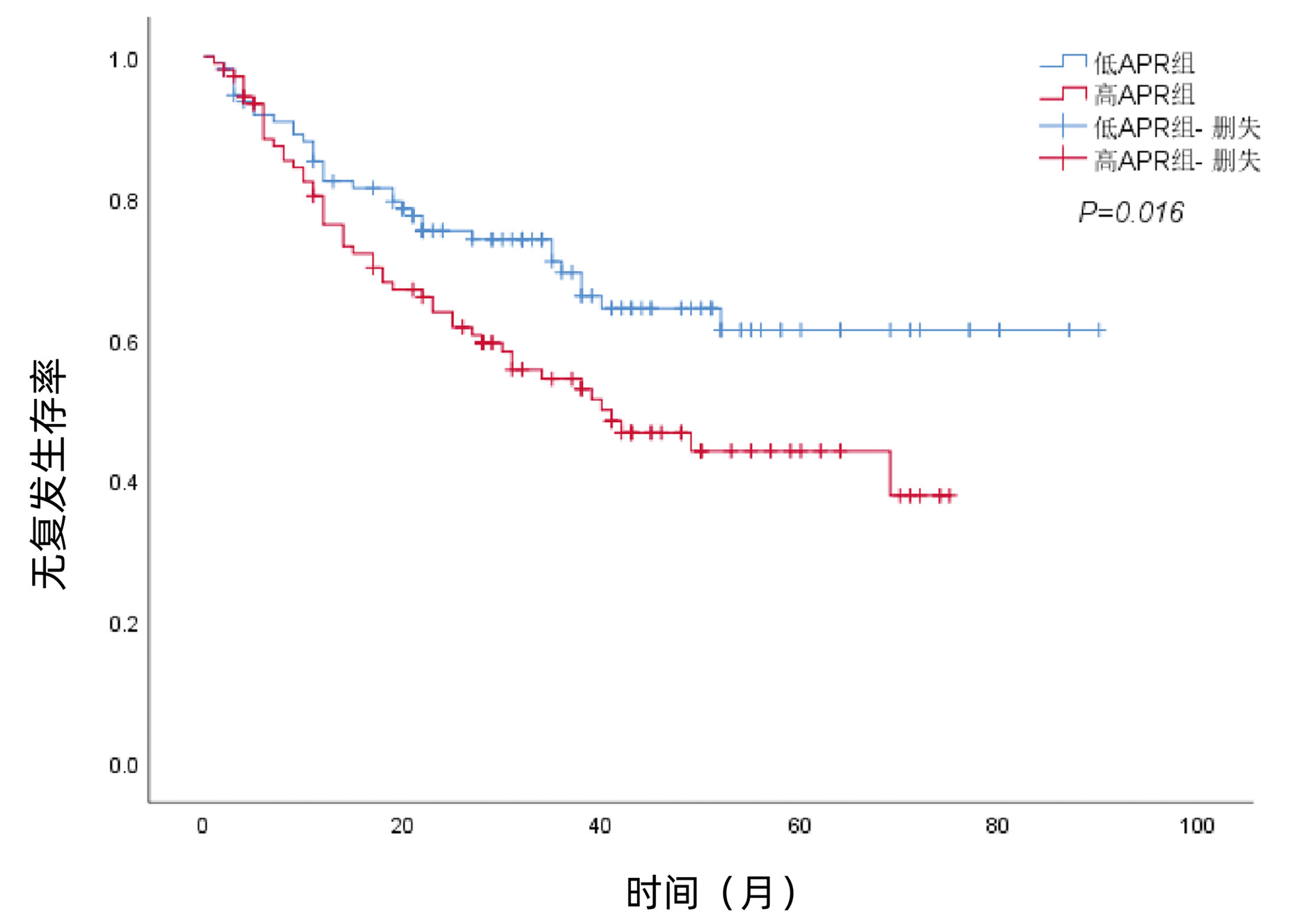
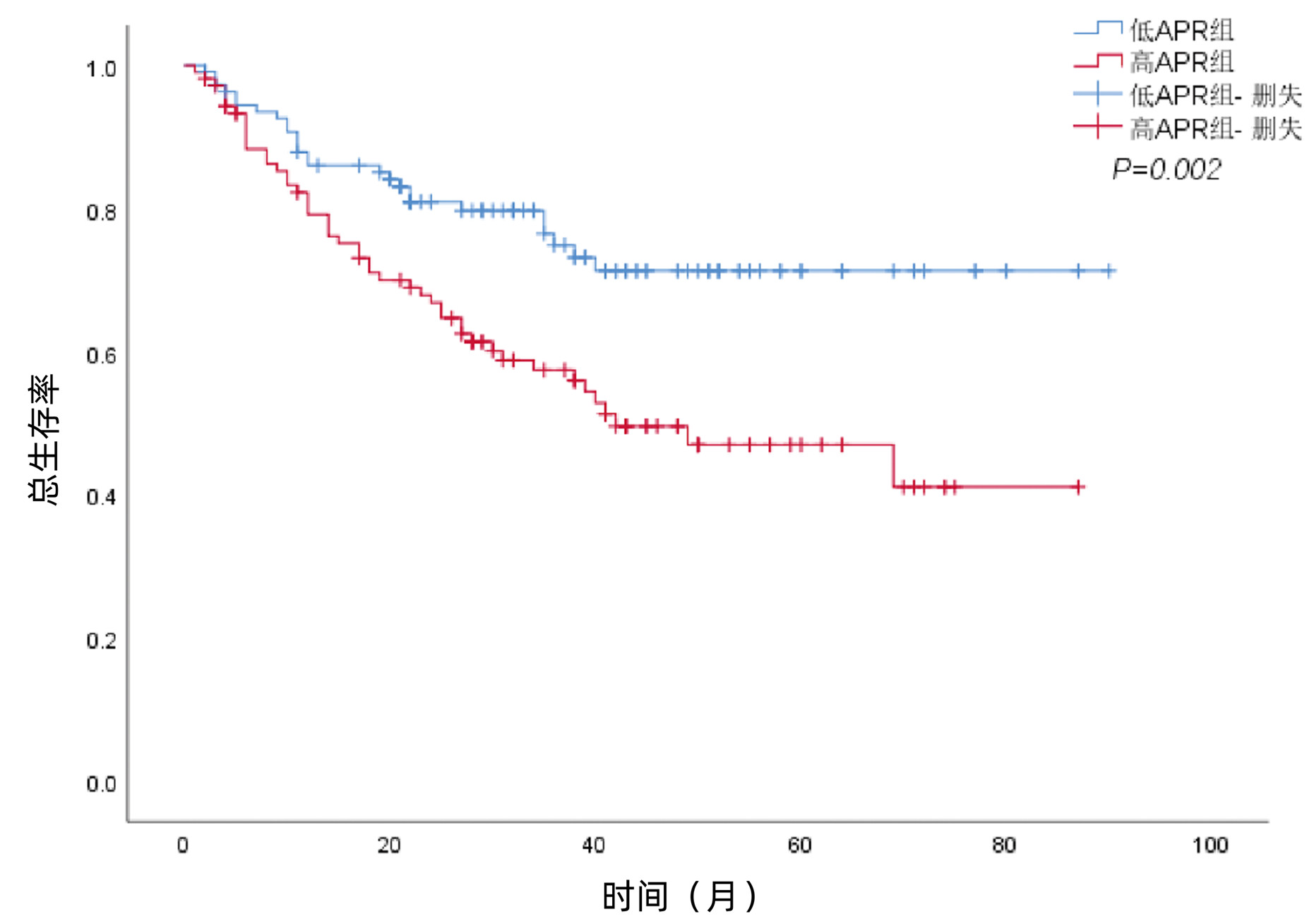
目的 研究术前碱性磷酸酶(ALP)与前白蛋白(PA)比值(APR)对肝细胞癌(HCC)根治性切除术后预后及并发症的预测价值。 方法 回顾性分析2013年1月—2021年8月于西南医科大学附属医院肝胆外科行HCC根治性切除术的217例患者的临床病理资料。采用X-tile软件获取APR最佳截断点。采用χ2检验分析术前APR与患者临床病理特征之间的关系。采用Kaplan-Meier法绘制生存曲线,通过Log-rank检验比较不同组别的差异。采用单因素、多因素Cox回归模型分析影响预后的独立因素。采用单因素、多因素Logistic模型分析影响术后重症并发症的独立因素。通过ROC曲线判断APR对术后并发症的预测价值。ROC曲线下面积的比较采用配对比较的方法。 结果 APR的最佳截断点为0.5,将患者分为低APR组111例,高APR组106例。与低APR组相比,高APR组患者中ALT>50 U/L、Alb<40 g/L、CNLC Ⅲ期、开腹手术途径、肝硬化、多个肿瘤、术后并发症及重症并发症的比例明显增高(P值均<0.05)。低APR组的1、3、5年总生存率为86.0%、74.9%、71.3%,高APR组的1、3、5年总生存率为79.2%、57.5%、47.0%,高APR组的总生存率低于低APR组(χ2=9.825,P=0.002)。多变量Cox回归分析显示,AFP(HR=1.774,95%CI:1.107~2.843,P=0.017)、CNLC分期(HR=2.708,95%CI:1.514~4.844,P=0.001)、肿瘤大小(HR=1.696,95%CI:1.060~2.714,P=0.028)和APR(HR=2.022,95%CI:1.244~3.285,P=0.004)是HCC根治性切除术后患者总生存期的独立危险因素。低APR组的1、3、5年无复发生存率为82.3%、69.4%、61.3%,高APR组的1、3、5年无复发生存率为76.2%、54.4%、44.2%,高APR组的无复发生存率低于APR低组(χ2=5.769,P=0.016)。多变量Cox回归分析显示,CNLC分期(HR=2.509,95%CI:1.423~4.422,P=0.001)、肿瘤大小(HR =1.725,95%CI:1.119~2.660,P=0.014)和APR(HR=1.619,95%CI:1.037~2.527,P=0.034)是HCC根治性切除术后患者无复发生存期的独立危险因素。多因素Logistic回归分析结果显示,高血压(OR=3.090,95%CI:1.385~6.893,P=0.006)、开腹手术(OR=4.198, 95%CI:1.779~9.907,P=0.001)、肝硬化(OR=2.376,95%CI:1.194~4.729,P=0.014)和APR(OR =2.151,95%CI:1.160~3.986,P=0.015)是HCC术后重症并发症发生的独立危险因素。APR、ALP和PA对患者术后重症并发症预测的曲线下面积分别为0.625(95%CI:0.547~0.702)、0.613(95%CI:0.534~0.693)和0.554(95%CI: 0.474~0.634)。 结论 APR可用于预测HCC根治性切除术后患者的预后及重症并发症。 Abstract:Objective To explore the predictive value of preoperative alkaline phosphatase to prealbumin ratio (APR) in prognosis and postoperative complications for patients with hepatocellular carcinoma (HCC) after radical tumor resection. Methods A total of 217 HCC patients who underwent radical tumor resection in the Department of Hepatobiliary Surgery of the Affiliated Hospital of Southwest Medical University from January 2013 to August 2021 were retrospectively recruited and their clinical data were statistically analyzed. The X-tile software was used to obtain the optimal cutoff value of APR. The χ2 test was conducted to analyze association between preoperative APR and other clinicopathological characteristics. The Kaplan-Meier curve was plotted and the Log-rank test was performed to analyze survival of patients. The univariate and multivariate Cox proportional hazards regression models were used to analysis factors affecting the prognosis of HCC patients. The univariate analysis and multivariate Logistic regression were used to identify factors related with postoperative complications. The receiver operating characteristic (ROC) curve was used to determine the predicting value of APR. Results The optimal cutoff value for APR ratio was 0.5 and these 217 patients were divided into the low- and high APR groups (111 vs 106 cases) accordingly. Compared with the low-APR group, the proportion of patients with ALT (> 50 U/L), Alb (< 40 g/L), the CNLC of the III stage, open surgery, liver cirrhosis, multiple tumor lesions, postoperative complication, and major complication were significantly increased in the high-APR patients (all P < 0.05). Moreover, the 1-, 3-, and 5-year OS were 86.0%, 74.9%, and 71.3%, respectively in the low-APR patients, while the numbers were 79.2%, 57.5%, and 47.0%, respectively, in the high-APR patients, indicating that patients in high-APR group had significantly worse OS (P=0.002). AFP (HR=1.774, 95%CI: 1.107-2.843, P=0.017), CNLC stage (HR=2.708, 95%CI: 1.514-4.844, P=0.001), tumor size (HR=1.696, 95%CI: 1.060-2.714, P=0.028), and APR (HR=2.022, 95%CI: 1.244-3.285, P=0.004) were all independent risk predictors for OS. The 1-, 3-, and 5-year RFS were 82.3%, 69.4%, and 61.3%, respectively, in the low-APR patients, whereas the numbers were 76.2%, 54.4%, and 44.2%, respectively in the high-APR patients, suggesting that high-APR patients had significantly worse recurrence-free survival (P=0.016). The CNLC stage (HR=2.509, 95%CI: 1.423-4.422, P=0.001), tumor size (HR=1.725, 95%CI: 1.119-2.660, P=0.014), and APR (HR=1.619: 95%CI: 1.037-2.527, P=0.034) were all independent FRS predictors. Hypertension (OR=3.09, 95%CI: 1.385-6.893, P=0.006), open surgery (OR=4.198, 95%CI: 1.779-9.907, P=0.001), liver cirrhosis (OR=2.376, 95%CI: 1.194-4.729, P=0.014), and APR (OR=2.151, 95%CI: 1.160-3.986, P=0.015) were all independent risk predictors for the postoperative major complications. The AUC for APR, ALP, a nd PA in predicting the major complications was 0.625 (95%CI: 0.547-0.702), 0.613 (95%CI: 0.534-0.693), and 0.554 (0.474-0.634). Conclusion Preoperative APR could be used to predict prognosis and postoperative major complications of HCC patients after radical tumor resection. -
Key words:
- Carcinoma, Hepatocellular /
- Alkaline Phosphatase /
- Prealbumin /
- Postoperative Complications /
- Prognosis
-
表 1 术前APR与HCC患者的临床病理特征的关系
Table 1. Clinical characteristics of patients in the low-APR and high-APR groups
变量 低APR组(n=111) 高APR组(n=106) χ2值 P值 性别[例(%)] 0.885 0.347 男 99 (89.2) 90 (84.9) 女 12 (10.8) 16 (15.1) 年龄[例(%)] 1.598 0.206 ≤60岁 83 (74.8) 71 (67.0) >60岁 28 (25.2) 35 (33.0) 高血压[例(%)] 1.713 0.191 否 89 (80.2) 92 (86.8) 是 22 (19.8) 14 (13.2) 糖尿病[例(%)] 0.306 0.580 否 100 (90.1) 93 (87.7) 是 11 (9.9) 13 (12.3) 乙型肝炎[例(%)] 0.222 0.637 否 2 (1.8) 4 (3.8) 是 109 (98.2) 102 (96.2) ALT[例(%)] 8.803 0.003 ≤50 U/L 91 (82.0) 68 (64.2) >50 U/L 20 (18.0) 38 (35.8) TBil[例(%)] 1.317 0.251 <34.2 μmol/L 109 (98.2) 100 (94.3) ≥34.2 μmol/L 2 (1.8) 6 (5.7) Alb[例(%)] 14.971 <0.001 <40 g/L 32 (28.8) 58 (54.7) ≥40 g/L 79 (71.2) 48 (45.3) AFP[例(%)] 0.450 0.502 <400 ng/mL 77 (69.4) 69 (65.1) ≥400 ng/mL 34 (30.6) 37 (34.9) Child-Pugh分级[例(%)] 0.003 0.958 A 109 (98.2) 103 (97.2) B 2 (1.8) 3 (2.8) CNLC分期[例(%)] 8.337 0.004 Ⅰ/Ⅱ 105 (94.6) 87 (82.1) Ⅲ 6 (5.4) 19 (17.9) 手术途径[例(%)] 6.422 0.011 腹腔镜 34 (30.6) 17 (16.0) 开腹 77 (69.4) 89 (84.0) 术中输血[例(%)] 0.457 0.499 否 90 (81.1) 82 (77.4) 是 21 (18.9) 24 (22.6) 肿瘤大小[例(%)] 0.053 0.817 ≤5 cm 59 (53.2) 58 (54.7) >5 cm 52 (46.8) 48 (45.3) 肿瘤数量[例(%)] 8.800 0.003 单个 104 (93.7) 85 (80.2) 多个 7 (6.3) 21 (19.8) 肝硬化[例(%)] 4.464 0.035 否 42 (37.8) 26 (24.5) 是 69 (62.2) 80 (75.5) 分化程度[例(%)] 0.031 0.859 低/中 89 (80.2) 86 (81.1) 高 22 (19.8) 20 (18.9) 切除范围(肝段)[例(%)] 2.369 0.124 <3 80 (72.1) 66 (62.3) ≥3 31 (27.9) 40 (37.7) 手术时间[例(%)] 1.686 0.194 ≤180 min 59 (53.2) 47 (44.3) >180 min 52 (46.8) 59 (55.7) 术中出血[例(%)] 0.056 0.812 <500 mL 60 (54.1) 59 (55.7) ≥500 mL 51 (45.9) 47 (44.3) 术后并发症[例(%)] 11.532 0.001 否 61 (55.0) 34 (32.1) 是 50 (45.0) 72 (67.9) 重症并发症[例(%)] 9.514 0.002 否 82 (73.9) 57 (53.8) 是 29 (26.1) 49 (46.2) 注:依照本院ALT(9~50 U/L)、Alb(40~55 g/L)的正常参考值范围,分别选取ALT=50 U/L、Alb =40 g/L为各自的阈值。因部分患者AFP为外院检验结果,故依照病程记录中肝癌诊断依据(AFP>400 ng/mL)为分组阈值。 表 2 HCC患者OS的单变量及多变量Cox分析
Table 2. Univariate and multivariate Cox analyses to evaluate the predictors for OS of HCC
变量 单变量分析 多变量分析 HR (95%CI) P值 HR (95%CI) P值 性别(女/男) 0.712 (0.384~1.322) 0.282 年龄(≤60/>60岁) 0.904 (0.541~1.511) 0.701 高血压(否/是) 0.523 (0.240~1.139) 0.102 糖尿病(否/是) 0.409 (0.149~1.120) 0.082 乙型肝炎(否/是) 0.873 (0.214~3.562) 0.849 ALT(≤50/>50 U/L) 1.531 (0.945~2.481) 0.084 TBil(<34.2/≥34.2 μmol/L) 1.514 (0.552~4.150) 0.420 Alb(<40/≥40 g/L) 0.752 (0.477~1.187) 0.221 AFP(<400/≥400 ng/mL) 2.102 (1.327~3.331) 0.002 1.774 (1.107~2.843) 0.017 Child-Pugh分级(A/B级) 2.087 (0.657~6.632) 0.212 CNLC分期(Ⅰ+Ⅱ/Ⅲ期) 3.774 (2.152~6.619) <0.001 2.708 (1.514~4.844) 0.001 手术途径(腔镜/开腹) 1.227 (0.696~2.162) 0.480 肿瘤大小(≤5/>5 cm) 1.798 (1.136~2.844) 0.012 1.696 (1.060~2.714) 0.028 肿瘤数量(单/多个) 1.516 (0.832~2.763) 0.174 肝硬化(否/是) 1.121 (0.681~1.846) 0.654 分化程度(低+中/高) 0.528 (0.270~1.029) 0.061 切除范围(<3/≥3肝段) 1.665 (1.043~2.659) 0.033 术中输血(否/是) 1.451 (0.861~2.446) 0.162 APR(低/高) 2.101 (1.304~3.387) 0.002 2.022 (1.244~3.285) 0.004 手术时间(≤180/>180 min) 1.799 (1.124~2.879) 0.014 出血量(<500/≥500 mL) 1.503 (0.951~2.376) 0.081 术后并发症(否/是) 1.729 (1.072~2.789) 0.025 表 3 HCC患者RFS的单变量及多变量Cox分析
Table 3. Univariate and multivariate Cox analyses to evaluate the predictors for RFS of HCC
变量 单变量分析 多变量分析 HR (95%CI) P值 HR (95%CI) P值 性别(女/男) 0.849 (0.461~1.563) 0.598 年龄(≤60/>60岁) 0.719 (0.435~1.187) 0.197 高血压(否/是) 0.680 (0.352~1.317) 0.253 糖尿病(否/是) 0.447 (0.181~1.104) 0.081 乙型肝炎(否/是) 0.569 (0.179~1.806) 0.339 ALT(≤50/>50 U/L) 1.546 (0.986~2.422) 0.057 TBil(<34.2/≥34.2 μmol/L) 1.711 (0.692~4.229) 0.245 Alb(<40/≥40 g/L) 0.862 (0.562~1.321) 0.495 AFP(<400/≥400 ng/mL) 1.824 (1.183~2.813) 0.007 Child-Pugh分级(A/B级) 1.719 (0.543~5.444) 0.357 CNLC分期(Ⅰ+Ⅱ/Ⅲ期) 3.018 (1.740~5.235) <0.001 2.509 (1.423~4.422) 0.001 手术途径(腔镜/开腹) 1.060 (0.636~1.766) 0.823 肿瘤大小(≤5/>5 cm) 1.728 (1.127~2.649) 0.012 1.725 (1.119~2.660) 0.014 肿瘤数量(单/多个) 1.532 (0.876~2.680) 0.135 肝硬化(否/是) 1.239 (0.772~1.987) 0.375 分化程度(低+中/高) 0.751 (0.429~1.313) 0.315 切除范围(<3/≥3肝段) 1.475 (0.947~2.296) 0.086 术中输血(否/是) 1.235 (0.748~2.041) 0.409 APR(低/高) 1.687 (1.092~2.604) 0.018 1.619 (1.037~2.527) 0.034 手术时间(≤180/>180 min) 1.694 (1.094~2.622) 0.018 出血量(<500/≥500 mL) 1.325 (0.865~2.028) 0.196 术后并发症(否/是) 1.665(1.070~2.591) 0.024 表 4 HCC术后重症并发症发生相关因素的单变量及多变量回归分析
Table 4. Univariate and multivariate analyses of factors associated with major complications after liver resection
变量 单变量分析 多变量分析 HR (95%CI) P值 HR (95%CI) P值 性别(女/男) 1.214 (0.521~2.830) 0.654 年龄(≤60/>60岁) 1.378 (0.755~2.518) 0.297 高血压(否/是) 2.311 (1.120~4.769) 0.023 3.090 (1.385~6.893) 0.006 糖尿病(否/是) 2.327 (0.988~5.481) 0.053 乙型肝炎(否/是) 1.126 (0.202~6.291) 0.893 ALT(≤50/>50 U/L) 2.035 (1.101~3.761) 0.023 TBil(<34.2/≥34.2 μmol/L) 3.105(0.722~13.361) 0.128 Alb(<40/≥40 g/L) 0.741 (0.423~1.299) 0.295 AFP(<400/≥400 ng/mL) 0.870 (0.480~1.578) 0.647 Child-Pugh分级(A/B级) 1.193 (0.195~7.298) 0.849 CNLC分期(Ⅰ+Ⅱ/Ⅲ期) 3.071 (1.306~7.222) 0.010 手术途径(腹腔镜/开腹) 3.919 (1.735~8.855) 0.001 4.198 (1.779~9.907) 0.001 肿瘤大小(≤5/>5 cm) 1.279 (0.733~2.230) 0.386 肿瘤数量(单/多个) 0.824 (0.353~1.921) 0.654 肝硬化(否/是) 2.080 (1.098~3.939) 0.025 2.376 (1.194~4.729) 0.014 分化程度(低+中/高) 1.271 (0.637~2.534) 0.496 切除范围(<3/≥3肝段) 1.367 (0.762~2.453) 0.295 术中输血(否/是) 1.765 (0.907~3.437) 0.094 APR(低/高) 2.431 (1.374~4.299) 0.002 2.151 (1.160~3.986) 0.015 手术时间(≤180/>180 min) 0.931 (0.534~1.620) 0.799 出血量(<500/≥500 mL) 1.470 (0.842~2.567) 0.176 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] ZHOU M, WANG H, ZENG X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394(10204): 1145-1158. DOI: 10.1016/S0140-6736(19)30427-1. [3] CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI: 10.3322/caac.21338. [4] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [5] XU XF, XING H, HAN J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China[J]. JAMA Surg, 2019, 154(3): 209-217. DOI: 10.1001/jamasurg.2018.4334. [6] DHIR M, MELIN AA, DOUAIHER J, et al. A review and update of treatment options and controversies in the management of hepatocellular carcinoma[J]. Ann Surg, 2016, 263(6): 1112-1125. DOI: 10.1097/SLA.0000000000001556. [7] AMISAKI M, SAITO H, TOKUYASU N, et al. Prognostic value of postoperative complication for early recurrence after curative resection of hepatocellular carcinoma[J]. Hepatobiliary Pancreat Dis Int, 2018, 17(4): 323-329. DOI: 10.1016/j.hbpd.2018.03.016. [8] YANG T, ZHANG J, LU JH, et al. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases[J]. World J Surg, 2011, 35(9): 2073-2082. DOI: 10.1007/s00268-011-1161-0. [9] CHOK KS, NG KK, POON RT, et al. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma[J]. Br J Surg, 2009, 96(1): 81-87. DOI: 10.1002/bjs.6358. [10] HARIMOTO N, SHIRABE K, IKEGAMI T, et al. Postoperative complications are predictive of poor prognosis in hepatocellular carcinoma[J]. J Surg Res, 2015, 199(2): 470-477. DOI: 10.1016/j.jss.2015.06.012. [11] THELEN A, BENCKERT C, TAUTENHAHN HM, et al. Liver resection for hepatocellular carcinoma in patients without cirrhosis[J]. Br J Surg, 2013, 100(1): 130-137. DOI: 10.1002/bjs.8962. [12] LI Y, WANG JS, GUO Y, et al. Use of the alkaline phosphatase to prealbumin ratio as an independent predictive factor for the prognosis of gastric cancer[J]. World J Gastroenterol, 2020, 26(44): 6963-6978. DOI: 10.3748/wjg.v26.i44.6963. [13] RAHBARI NN, GARDEN OJ, PADBURY R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS)[J]. Surgery, 2011, 149(5): 713-724. DOI: 10.1016/j.surg.2010.10.001. [14] DINDO D, DEMARTINES N, CLAVIEN PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey[J]. Ann Surg, 2004, 240(2): 205-213. DOI: 10.1097/01.sla.0000133083.54934.ae. [15] CLAVIEN PA, BARKUN J, de OLIVEIRA ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience[J]. Ann Surg, 2009, 250(2): 187-196. DOI: 10.1097/SLA.0b013e3181b13ca2. [16] HAARHAUS M, BRANDENBURG V, KALANTAR-ZADEH K, et al. Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD[J]. Nat Rev Nephrol, 2017, 13(7): 429-442. DOI: 10.1038/nrneph.2017.60. [17] PARK JB, KANG DY, YANG HM, et al. Serum alkaline phosphatase is a predictor of mortality, myocardial infarction, or stent thrombosis after implantation of coronary drug- eluting stent[J]. Eur Heart J, 2013, 34(12): 920-931. DOI: 10.1093/eurheartj/ehs419. [18] LI JP, XU T, YANG FC, et al. Prognostic value of APAR for patients with hepatocellular carcinoma after radical hepatectomy[J/CD]. Chin J Hepat Surg(Electronic Edition), 2021, 10(3): 279-284. DOI: 10.3877/cma.j.issn.2095-3232.2021.03.010.李蒋鹏, 徐婷, 杨发才, 等. APAR对肝细胞癌根治性切除术后患者预后的预测价值[J/CD]. 中华肝脏外科手术学电子杂志, 2021, 10(3): 279-284. DOI: 10.3877/cma.j.issn.2095-3232.2021.03.010. [19] BARGER A, BAKER K, DRISKELL E, et al. The use of alkaline phosphatase and runx2 to distinguish osteosarcoma from other common malignant primary bone tumors in dogs[J]. Vet Pathol, 2022, 59(3): 427-432. DOI: 10.1177/03009858221083035. [20] YANG Y, ZHANG M, ZHANG W, et al. Sensitive sensing of alkaline phosphatase and γ-glutamyltranspeptidase activity for tumor imaging[J]. Analyst, 2022, 147(8): 1544-1550. DOI: 10.1039/d2an00163b. [21] XIA F, NDHLOVU E, LIU Z, et al. Alpha-fetoprotein+alkaline phosphatase (A-A) score can predict the prognosis of patients with ruptured hepatocellular carcinoma underwent hepatectomy[J]. Dis Markers, 2022, 2022: 9934189. DOI: 10.1155/2022/9934189. [22] ZHANG K, DONG S, JING YH, et al. Albumin-to-alkaline phosphatase ratio serves as a prognostic indicator in unresectable pancreatic ductal adenocarcinoma: a propensity score matching analysis[J]. BMC Cancer, 2020, 20(1): 541. DOI: 10.1186/s12885-020-07023-9. [23] ZHOU S, JIANG W, WANG H, et al. Predictive value of pretreatment albumin-to-alkaline phosphatase ratio for overall survival for patients with advanced non-small cell lung cancer[J]. Cancer Med, 2020, 9(17): 6268-6280. DOI: 10.1002/cam4.3244. [24] CHEN XY, LAN M, ZHOU Y, et al. Risk factors for bone metastasis from renal cell cancer[J]. J Bone Oncol, 2017, 9: 29-33. DOI: 10.1016/j.jbo.2017.10.004. [25] SMITH SH. Using albumin and prealbumin to assess nutritional status[J]. Nursing, 2017, 47(4): 65-66. DOI: 10.1097/01.NURSE.0000511805.83334.df. [26] DELLIÈRE S, CYNOBER L. Is transthyretin a good marker of nutritional status?[J]. Clin Nutr, 2017, 36(2): 364-370. DOI: 10.1016/j.clnu.2016.06.004. [27] BECK FK, ROSENTHAL TC. Prealbumin: a marker for nutritional evaluation[J]. Am Fam Physician, 2002, 65(8): 1575-1578. [28] SHEN Z, ZHANG J, ZHAO S, et al. Preoperative biliary drainage of severely obstructive jaundiced patients decreases overall postoperative complications after pancreaticoduodenectomy: A retrospective and propensity score- matched analysis[J]. Pancreatology, 2020, 20(3): 529-536. DOI: 10.1016/j.pan.2020.02.002. [29] LOFTUS TJ, BROWN MP, SLISH JH, et al. Serum levels of prealbumin and albumin for preoperative risk stratification[J]. Nutr Clin Pract, 2019, 34(3): 340-348. DOI: 10.1002/ncp.10271. [30] HUANG L, LI J, YAN JJ, et al. Prealbumin is predictive for postoperative liver insufficiency in patients undergoing liver resection[J]. World J Gastroenterol, 2012, 18(47): 7021-7025. DOI: 10.3748/wjg.v18.i47.7021. [31] LI JD, XU XF, HAN J, et al. Preoperative prealbumin level as an independent predictor of long-term prognosis after liver resection for hepatocellular carcinoma: a multi-institutional study[J]. HPB (Oxford), 2019, 21(2): 157-166. DOI: 10.1016/j.hpb.2018.06.1803. [32] CHRYSOSTOMOU S, STATHAKIS C, PETRIKKOS G, et al. Assessment of prealbumin in hemodialysis and renal-transplant patients[J]. J Ren Nutr, 2010, 20(1): 44-51. DOI: 10.1053/j.jrn.2009.04.001. [33] LIU F, CAI LY, ZHONG L, et al. Model for end-stage liver disease combined with serum prealbumin to predict the prognosis of patients with decompensated liver cirrhosis[J]. J Dig Dis, 2010, 11(6): 352-357. DOI: 10.1111/j.1751-2980.2010.00465.x. [34] ROCHE M, LAW TY, KUROWICKI J, et al. Albumin, prealbumin, and transferrin may be predictive of wound complications following total knee arthroplasty[J]. J Knee Surg, 2018, 31(10): 946-951. DOI: 10.1055/s-0038-1672122. [35] KALANTAR-ZADEH K, ANKER SD, HORWICH TB, et al. Nutritional and anti-inflammatory interventions in chronic heart failure[J]. Am J Cardiol, 2008, 101(11A): 89E-103E. DOI: 10.1016/j.amjcard.2008.03.007. [36] WEN X, YAO M, LU Y, et al. Integration of prealbumin into Child-Pugh classification improves prognosis predicting accuracy in HCC patients considering curative surgery[J]. J Clin Transl Hepatol, 2018, 6(4): 377-384. DOI: 10.14218/JCTH.2018.00004. [37] JIA RR, ZHONG JH, HUO RR, et al. Correlation between serum prealbumin and prognosis of patients with hepatocellular carcinoma after hepatectomy[J]. J Surg Oncol, 2019, 119(6): 794-800. DOI: 10.1002/jso.25378. [38] LI CX, ZHANG H, WU XF, et al. Clinical efficacy and prognostic factors analysis following curative hepatectomy for hepatocellular carcinoma patients with different China Liver Cancer Staging[J]. Chin J Surg, 2021, 59(2): 134-143. DOI: 10.3760/cma.j.cn112139-20200803-00605.李长贤, 张慧, 吴晓峰, 等. 不同中国肝癌分期肝癌根治性切除术后的临床效果及预后因素分析[J]. 中华外科杂志, 2021, 59(2): 134-143. DOI: 10.3760/cma.j.cn112139-20200803-00605. [39] KIM JH, KIM JW, SEO JW, et al. Noninvasive tests for fibrosis predict 5-year mortality and hepatocellular carcinoma in patients with chronic hepatitis B[J]. J Clin Gastroenterol, 2016, 50(10): 882-888. DOI: 10.1097/MCG.0000000000000574. -



 PDF下载 ( 2364 KB)
PDF下载 ( 2364 KB)

 下载:
下载: