肝细胞癌患者外周血CD100的表达变化及其对T淋巴细胞的功能调控
DOI: 10.3969/j.issn.1001-5256.2023.01.019
Expression of peripheral CD100 and its regulation to T lymphocytes function in patients with hepatocellular carcinoma
-
摘要:
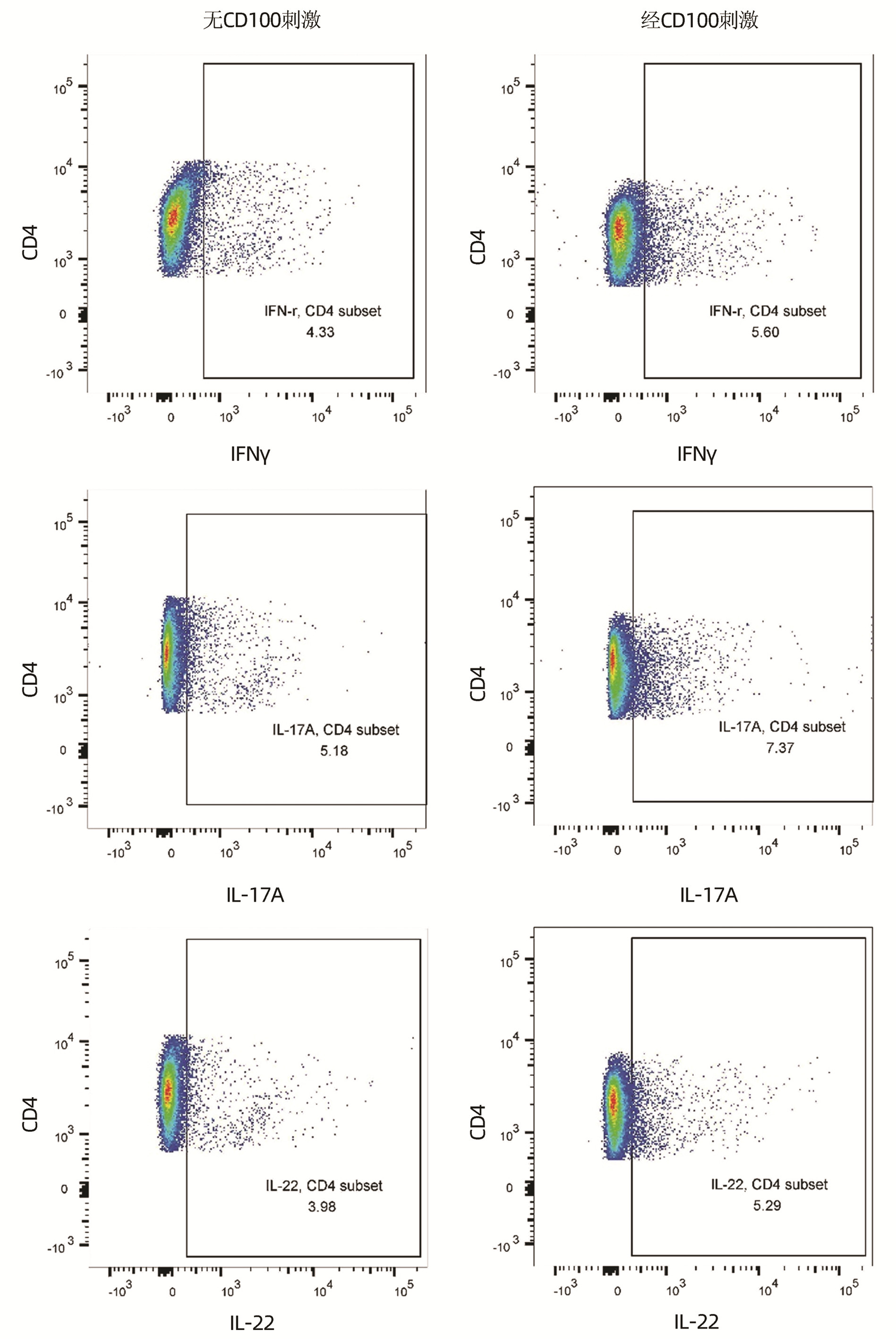
目的 观察肝细胞癌(HCC)患者外周血CD100的变化,探讨CD100对HCC患者T淋巴细胞功能的调控作用。 方法 前瞻性选择2020年4月—2021年7月在空军军医大学第二附属医院就诊的HCC患者57例和对照者22例。采集抗凝外周血,分离血浆和外周血单个核细胞(PBMC),酶联免疫吸附试验检测血浆可溶型CD100(sCD100)水平,流式细胞术检测CD4+和CD8+T淋巴细胞表面膜型CD100(mCD100)表达。使用重组人CD100刺激HCC患者PBMC,细胞计数试剂盒-8法检测细胞增殖,流式细胞术检测不同类型辅助性T淋巴细胞(Th细胞)和杀伤性T淋巴细胞(Tc细胞)比例,酶联斑点吸附试验检测甲胎蛋白(AFP)特异性CD8+T淋巴细胞分泌穿孔素和颗粒酶B水平。纯化HCC患者CD8+T淋巴细胞,使用重组人CD100刺激后与HepG2细胞共培养,检测AFP特异性CD8+T淋巴细胞诱导HepG2细胞死亡水平。符合正态分布的计量资料两组间比较采用t检验或配对t检验;不符合正态分布的计量资料两组间比较采用Mann-Whitney U秩和检验。计数资料两组间比较采用χ2检验。 结果 HCC组血浆sCD100水平低于对照组[(2.73±0.58)ng/mL vs(3.33±0.84)ng/mL,t=3.584,P<0.001]。HCC组CD4+T淋巴细胞中mCD100阳性细胞比例(55.57%±11.33% vs 43.67%±6.40%,t=4.636,P<0.001)和mCD100平均荧光强度(294.80±74.01 vs 255.00±74.01,t=2.126,P=0.037)均高于对照组。HCC组CD8+T淋巴细胞中mCD100阳性细胞比例(48.65%±7.71% vs 41.74%±4.77%,t=3.914,P<0.001)和mCD100平均荧光强度(289.20±89.30 vs 246.10±60.73,t=2.082,P=0.041)亦均高于对照组。HCC患者PBMC增殖、T淋巴细胞比例在经CD100刺激和无CD100刺激之间差异无统计学意义(P值均>0.05)。经CD100刺激后,HCC患者PBMC中CD4+IFNγ+Th1细胞、CD4+IL-17A+Th17细胞、CD4+IL-22+Th22细胞、CD8+IFNγ+Tc1细胞比例均高于无CD100刺激(t值分别为2.608、5.663、4.113、4605,P值均<0.05),CD8+IL-17A+Tc17细胞和CD8+IL-22+Tc22细胞比例在经CD100刺激和无CD100刺激之间的差异无统计学意义(P值均>0.05)。HLA-A02限制性HCC患者的PBMC经CD100刺激后,AFP特异性CD8+T淋巴细胞分泌穿孔素和颗粒酶B的细胞数量均高于无CD100刺激(t值分别为6.794、2.308,P值均<0.05)。CD100刺激后,AFP特异性CD8+T淋巴细胞诱导HepG2细胞死亡的比例亦升高(P<0.05)。 结论 HCC患者中存在sCD100和T淋巴细胞中mCD100表达失衡,sCD100水平降低可能无法维持T淋巴细胞功能活性,造成HCC免疫耐受。 Abstract:Objective To investigate the changes of peripheral CD100 in patients with hepatocellular carcinoma (HCC), and to assess the regulatory function of CD100 to T lymphocytes in HCC patients. Methods A prospective study was conducted. Fifty-seven HCC patients and twenty-two controls who were hospitalized in our hospital between April 2020 and July 2021 were enrolled. Anti-coagulant peripheral blood was collected. Plasma and peripheral blood mononuclear cells (PBMC) were isolated. Plasma soluble CD100 (sCD100) level was measured by enzyme-linked immunosorbent assay. Membrane-bound CD100 (mCD100) expression on CD4+ and CD8+ T lymphocytes was measured by flow cytometry. PBMC from HCC patients were stimulated with recombinant human CD100. Cellular proliferation was measured by cell counting kit-8. Different types of T helper cells (Th cells) and cytotoxic T cells (Tc cells) were assessed by flow cytometry. Perforin and granzyme B secretion by alpha fetoprotein (AFP) specific CD8+ T lymphocytes was assessed by enzyme-linked immunospot assay. CD8+ T lymphocytes were purified from HCC patients, and were stimulated with recombinant human CD100. Stimulated CD8+ T lymphocytes were co-cultured with HepG2 cells. AFP specific CD8+ T lymphocytes-induced HepG2 cell death was investigated. Student's t test or paired t test was used for comparison of normally distributed continuous data between two groups. Mann-Whitney U test was used for comparison of abnormally distributed continuous data between two groups. Chi square test was used for comparison of categorial data between two groups. Results Plasma sCD100 level was lower in HCC group when compared with control group ((2.73±0.58)ng/mL vs(3.33±0.84)ng/mL, t=3.584, P < 0.001). HCC group had higher percentage of mCD100+ cells (55.57%±11.33% vs 43.67%±6.40%, t=4.636, P < 0.001) and elevated mCD100 mean fluorescent intensity (MFI) (294.80±74.01 vs 255.00±74.01, t=2.126, P=0.037) within CD4+ T lymphocytes when compared with control group. Similarly, HCC group also had higher percentage of mCD100+ cells (48.65%±7.71% vs 41.74%±4.77%, t=3.914, P < 0.001) and elevated mCD100 MFI (289.20±89.30 vs 246.10±60.73, t=2.082, P=0.041) within CD8+ T lymphocytes when compared with control group. There were no significant differences of either cellular proliferation or T lymphocytes percentage between PBMC with and without recombinant human CD100 stimulation in HCC patients (all P > 0.05). The percentages of CD4+IFNγ+Th1 cells, CD4+IL-17A+Th17 cells, CD4+IL-22+Th22 cells, and CD8+IFNγ+Tc1 cells were notably increased in response to CD100 stimulation when compared with no CD100 stimulation (t=2.608、5.663、4.113、4605, all P < 0.05). There were no remarkably differences of either CD8+IL-17A+Tc17 or CD8+IL-22+Tc22 cell frequency between PBMCs with and without recombinant human CD100 stimulation in HCC patients (all P > 0.05). Perforin and granzyme B secretion by AFP specific CD8+ T lymphocytes were significantly elevated in response to CD100 stimulation when compared with no CD100 stimulation in HLA-A02 restricted HCC patients (P < 0.05). AFP specific CD8+ T lymphocytes-induced HepG2 cell death was also increased in response to CD100 stimulation (t=6.794、2.308, both P < 0.05). Conclusion There was an imbalance between sCD100 and mCD100 on T lymphocytes in HCC patients. Reduced sCD100 level might be insufficient for maintenance of T lymphocytes activity, leading to the immunotolerance in HCC. -
Key words:
- Liver Neoplasms /
- CD100 /
- T-Lymphocytes
-
表 1 患者的一般临床资料
Table 1. General clinical data for enrolled subjects
项目 对照组(n=22) HCC组(n=57) 统计值 P值 男/女(例) 17/5 44/13 χ2=0.739 0.690 年龄(岁) 47.0±10.7 48.4±12.6 t=0.461 0.646 AFP(ng/mL) 4(2~5) 1227(719~>65 000) U=9.088 <0.001 BCLC分期(A/B/C/D,例) 17/21/11/8 注:BCLC,巴塞罗那临床肝癌分期。 表 2 对照组和HCC组中血浆sCD100、CD4+和CD8+T淋巴细胞中mCD100表达比较
Table 2. Comparison of plasma sCD100 and mCD100 expression on CD4+ and CD8+ T cells between control group and HCC group
项目 对照组(n=22) HCC组(n=57) t值 P值 血浆sCD100(ng/mL) 3.33±0.84 2.73±0.58 3.584 <0.001 CD4+mCD100+比例(%) 43.67±6.40 55.57±11.33 4.636 <0.001 CD4+mCD100 MFI 255.00±74.01 294.80±74.01 2.126 0.037 CD8+mCD100+比例(%) 41.74±4.77 48.65±7.71 3.914 <0.001 CD8+mCD100 MFI 246.10±60.73 289.20±89.30 2.082 0.041 表 3 重组人CD100对HCC患者PBMC细胞增殖和T淋巴细胞比例的影响
Table 3. The influence of recombinant human CD100 to PBMC cellular stimulation and T lymphocytes percentage in HCC patients
项目 无CD100刺激 经CD100刺激 t值 P值 细胞增殖(×106个) 0.75±0.10 0.72±0.12 0.850 0.399 CD4+T淋巴细胞比例(%) 33.34±7.86 36.00±7.52 1.271 0.210 CD8+T淋巴细胞比例(%) 29.54±6.51 30.81±6.45 0.725 0.472 表 4 重组人CD100对HCC患者PBMC中Th细胞和Tc细胞亚群的影响
Table 4. The influence of recombinant human CD100 to Th cells and Tc cell subsets in PBMC from HCC patients
项目 无CD100刺激 经CD100刺激 t值 P值 CD4+IFNγ+Th1(%) 4.85±1.28 6.47±2.97 2.608 0.012 CD4+IL-17A+Th17(%) 5.62±1.04 7.28±1.12 5.663 <0.001 CD4+IL-22+Th22(%) 3.70±0.90 4.84±1.13 4.113 <0.001 CD8+IFNγ+Tc1(%) 2.84±1.02 3.91±0.65 4.605 <0.001 CD8+IL-17A+Tc17(%) 2.69±0.88 2.57±0.90 0.492 0.625 CD8+IL-22+Tc22(%) 1.13±0.20 1.15±0.15 0.406 0.687 表 5 重组人CD100对HCC患者AFP特异性CD8+T淋巴细胞分泌穿孔素和颗粒酶B的影响
Table 5. The influence of recombinant human CD100 to perforin and granzyme B by AFP specific CD8+ T cells in HCC patients
项目 无CD100刺激 经CD100刺激 t值 P值 穿孔素+(SFC/106个) 52.45±15.09 132.50±36.07 6.794 <0.001 颗粒酶B+(SFC/106个) 161.10±60.84 240.50±96.64 2.308 0.032 注:SFC,斑点形成细胞。 -
[1] Chinese Society of Hepatology, Chinese Medical Association. Consensus on the secondary prevention for primary liver cancer (2021 edition)[J]. J Clin Hepatol, 2021, 37(3): 532-542. DOI: 10.3969/j.issn.1001-5256.2021.03.008.中华医学会肝病学分会. 原发性肝癌二级预防共识(2021年版)[J]. 临床肝胆病杂志, 2021, 37(3): 532-542. DOI: 10.3969/j.issn.1001-5256.2021.03.008. [2] KALATHIL SG, THANAVALA Y. Natural killer cells and T cells in hepatocellular carcinoma and viral hepatitis: current status and perspectives for future immunotherapeutic approaches[J]. Cells, 2021, 10(6): 1332. DOI: 10.3390/cells10061332. [3] YANG S, WANG L, PAN W, et al. MMP2/MMP9-mediated CD100 shedding is crucial for inducing intrahepatic anti-HBV CD8 T cell responses and HBV clearance[J]. J Hepatol, 2019, 71(4): 685-698. DOI: 10.1016/j.jhep.2019.05.013. [4] LI BJ, HE Y, ZHANG Y, et al. Interferon-α-induced CD100 on naïve CD8+ T cells enhances antiviral responses to hepatitis C infection through CD72 signal transduction[J]. J Int Med Res, 2017, 45(1): 89-100. DOI: 10.1177/0300060516676136. [5] ZHANG DN, LIU Y, LI X, et al. Imbalance between soluble and membrane-bound CD100 regulates monocytes activity in hepatitis B virus-associated acute-on-chronic liver failure[J]. Viral Immunol, 2021, 34(4): 273-283. DOI: 10.1089/vim.2020.0311. [6] WANG HM, ZHANG XH, YE LQ, et al. Insufficient CD100 shedding contributes to suppression of CD8+ T-cell activity in non-small cell lung cancer[J]. Immunology, 2020, 160(2): 209-219. DOI: 10.1111/imm.13189. [7] Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [8] YANG L, SHAO X, JIA S, et al. Interleukin-35 dampens CD8+ T cells activity in patients with non-viral hepatitis-related hepatocellular carcinoma[J]. Front Immunol, 2019, 10: 1032. DOI: 10.3389/fimmu.2019.01032. [9] LIU H, YANG Y, XIAO J, et al. Semaphorin 4D expression is associated with a poor clinical outcome in cervical cancer patients[J]. Microvasc Res, 2014, 93: 1-8. DOI: 10.1016/j.mvr.2014.02.007. [10] CHEN Y, ZHANG L, LV R, et al. Overexpression of Semaphorin4D indicates poor prognosis and prompts monocyte differentiation toward M2 macrophages in epithelial ovarian cancer[J]. Asian Pac J Cancer Prev, 2013, 14(10): 5883-5890. DOI: 10.7314/apjcp.2013.14.10.5883. [11] LU JJ, SU YW, WANG CJ, et al. Semaphorin 4D promotes the proliferation and metastasis of bladder cancer by activating the PI3K/AKT pathway[J]. Tumori, 2019, 105(3): 231-242. DOI: 10.1177/0300891618811280. [12] CHEN WG, SUN J, SHEN WW, et al. Sema4D expression and secretion are increased by HIF-1α and inhibit osteogenesis in bone metastases of lung cancer[J]. Clin Exp Metastasis, 2019, 36(1): 39-56. DOI: 10.1007/s10585-018-9951-5. [13] LIU B, MA Y, ZHANG Y, et al. CD8low CD100- T cells identify a novel CD8 T cell subset associated with viral control during human hantaan virus infection[J]. J Virol, 2015, 89(23): 11834-11844. DOI: 10.1128/JVI.01610-15. [14] YOSHIDA Y, OGATA A, KANG S, et al. Semaphorin 4D contributes to rheumatoid arthritis by inducing inflammatory cytokine production: pathogenic and therapeutic implications[J]. Arthritis Rheumatol, 2015, 67(6): 1481-1490. DOI: 10.1002/art.39086. [15] GANG HS, PENG DF, HU YJ, et al. Regulation of CD100 on T lymphocytes function in septic cardiomyopathy patients[J]. Clin J Emerg Med, 2020, 29(11): 1432-1438. DOI: 10.3760/cma.j.issn.1671-0282.2020.11.009.冮洪生, 彭定凤, 胡勇钧, 等. CD100对脓毒性心肌病患者T淋巴细胞功能的调控作用[J]. 中华急诊医学杂志, 2020, 29(11): 1432-1438. DOI: 10.3760/cma.j.issn.1671-0282.2020.11.009. [16] FRANCO F, JACCARD A, ROMERO P, et al. Metabolic and epigenetic regulation of T-cell exhaustion[J]. Nat Metab, 2020, 2(10): 1001-1012. DOI: 10.1038/s42255-020-00280-9. [17] DONISI C, PUZZONI M, ZIRANU P, et al. Immune checkpoint inhibitors in the treatment of HCC[J]. Front Oncol, 2020, 10: 601240. DOI: 10.3389/fonc.2020.601240. [18] LI M, O'SULLIVAN KM, JONES LK, et al. CD100 enhances dendritic cell and CD4+ cell activation leading to pathogenetic humoral responses and immune complex glomerulonephritis[J]. J Immunol, 2006, 177(5): 3406-3412. DOI: 10.4049/jimmunol.177.5.3406. [19] ST PAUL M, OHASHI PS. The roles of CD8+ T cell subsets in antitumor immunity[J]. Trends Cell Biol, 2020, 30(9): 695-704. DOI: 10.1016/j.tcb.2020.06.003. [20] IWAHORI K. Cytotoxic CD8+ lymphocytes in the tumor microenvironment[J]. Adv Exp Med Biol, 2020, 1224: 53-62. DOI: 10.1007/978-3-030-35723-8_4. [21] LI Y, QIN L, BAI Q, et al. CD100 modulates cytotoxicity of CD8+ T cells in patients with acute myocardial infarction[J]. BMC Immunol, 2021, 22(1): 13. DOI: 10.1186/s12865-021-00406-y. -



 PDF下载 ( 3538 KB)
PDF下载 ( 3538 KB)


 下载:
下载: