细胞焦亡在非酒精性脂肪性肝病中的作用
DOI: 10.3969/j.issn.1001-5256.2023.01.027
-
摘要: 细胞焦亡作为一种新型的细胞死亡方式,在非酒精性脂肪性肝病(NAFLD)中扮演着重要角色,对细胞焦亡的研究有助于NAFLD治疗新靶点的挖掘。本文从细胞焦亡的研究背景及机制和细胞焦亡在NAFLD中的作用两方面对细胞焦亡的研究进展进行综述,尤其对GSDME、caspase-11等细胞焦亡执行分子,以及AIM2等既往关注较少的炎性小体的功能作用进行了阐释。Abstract: As a novel mode of cell death, pyroptosis plays an important role in nonalcoholic fatty liver disease (NAFLD), and the research on pyroptosis may help to explore new therapeutic targets for NAFLD. This article reviews the advances in pyroptosis from the research background and mechanism of pyroptosis and the role of pyroptosis in NAFLD and elaborates on the pyroptosis execution molecules such as GSDME and caspase-11 and the function of inflammasomes including AIM2.
-
Key words:
- Non-alcoholic Fatty Liver Disease /
- Pyroptosis /
- Inflammasomes
-
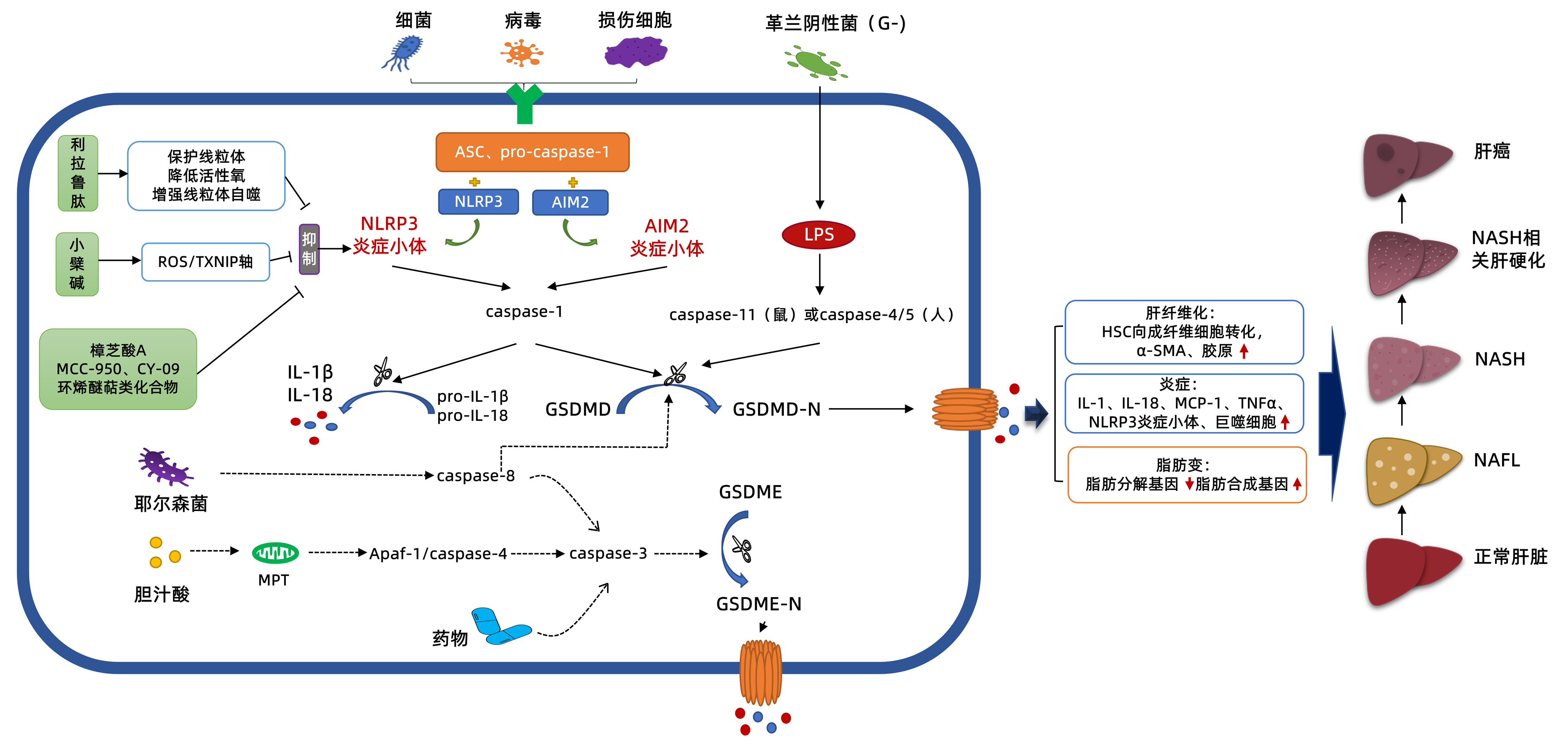
图 1 细胞焦亡及其阻断剂在NAFLD中的调控机制示意图
注:Apaf-1,凋亡酶激活因子-1;AIM2,一种DNA感受器;ASC,凋亡相关斑点样蛋白;DAMP,损伤相关分子模式;dsDNA,双链DNA;GSDMD-N,GSDMD的N端结构域;GSDME-N,GSDME的N端结构域;LPS,细菌脂多糖;MPT,线粒体通透性转换;MCP-1,单核细胞趋化蛋白-1;PAMP,病原相关分子模式;pro-caspase-1,前半胱天冬酶-1;TXNIP,硫氧还蛋白结合蛋白。虚线箭头:表示尚未有证据显示参与NAFLD的过程。
Figure 1. The regulatory mechanism diagram of pyroptosis and its blocker in NAFLD
表 1 程序性死亡之间的区别
Table 1. The difference between procedural deaths
类别 诱因 主要机制 细胞形态 细胞膜 细胞器 细胞核 细胞焦亡 病理性或损伤性因素 caspase-1/4/5/11切割GSDMD在质膜成孔,导致细胞裂解 肿胀变形 破裂 变形 变化不明显 细胞凋亡 生理条件下基因调控 由caspase-3/6/7/8/9/10等激活所介导细胞死亡 皱缩,可见凋亡小体 完好 完整 固缩 细胞自噬 营养缺乏或应激 细胞形成自噬体包裹细胞内成分转移至溶酶体进行消化,使细胞死亡 内可见自噬泡 完好 被自噬体包裹后在溶酶体中消化 变化不明显 坏死性凋亡 病理性或损伤性因素 外来刺激激活RIPK1-PIPK3-MLKL通路或PIPK3-MLKL通路,在质膜打孔导致细胞裂解 肿胀变形 破裂 肿胀 被分解 铁死亡 铁和活性氧(ROS)蓄积 谷胱甘肽过氧化酶4受抑制或在二价铁和酯氧合酶作用下,脂质发生过氧化,诱导细胞死亡 内可见气球样
表型破裂 线粒体体积减小、双层膜密度增加、外膜破裂、线粒体嵴消失 变化不明显 NETosis 细菌、真菌感染 外界刺激诱导中性粒细胞内NE、MPO、PAD4等蛋白表达,促进胞质和细胞核成分融合,并随着细胞破裂在细胞外形成NET捕获网,以捕获和杀死病原体 有NET捕获网形成 破裂 被挤压成网状结构 核膜溶解,染色质和组蛋白形成NET捕获网 -
[1] POWELL EE, WONG VW, RINELLA M. Non-alcoholic fatty liver disease[J]. Lancet, 2021, 397(10290): 2212-2224. DOI: 10.1016/S0140-6736(20)32511-3. [2] LI J, ZOU B, YEO YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2019, 4(5): 389-398. DOI: 10.1016/S2468-1253(19)30039-1. [3] YAN J, XIE W, OU WN, et al. Epidemiological survey and risk factor analysis of fatty liver disease of adult residents, Beijing, China[J]. J Gastroenterol Hepatol, 2013, 28(10): 1654-1659. DOI: 10.1111/jgh.12290. [4] KUANG S, ZHENG J, YANG H, et al. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis[J]. Proc Natl Acad Sci U S A, 2017, 114(40): 10642-10647. DOI: 10.1073/pnas.1708194114. [5] XIAO WS, LE YY, ZENG SL, et al. Role of pyroptosis in liver diseases[J]. J Clin Hepatol, 2020, 36(12): 2847-2850. DOI: 10.3969/j.issn.1001-5256.2020.12.044.肖伟松, 乐滢玉, 曾胜澜, 等. 细胞焦亡在肝脏疾病中的作用[J]. 临床肝胆病杂志, 2020, 36(12): 2847-2850. DOI: 10.3969/j.issn.1001-5256.2020.12.044. [6] PUGLIESE N, PLAZ TORRES MC, PETTA S, et al. Is there an 'ideal' diet for patients with NAFLD?[J]. Eur J Clin Invest, 2022, 52(3): e13659. DOI: 10.1111/eci.13659. [7] BOISE LH, COLLINS CM. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death?[J]. Trends Microbiol, 2001, 9(2): 64-67. DOI: 10.1016/s0966-842x(00)01937-5. [8] SHI J, ZHAO Y, WANG K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death[J]. Nature, 2015, 526(7575): 660-665. DOI: 10.1038/nature15514. [9] KAYAGAKI N, STOWE IB, LEE BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling[J]. Nature, 2015, 526(7575): 666-671. DOI: 10.1038/nature15541. [10] HIRSOVA P, GORES GJ. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis[J]. Cell Mol Gastroenterol Hepatol, 2015, 1(1): 17-27. DOI: 10.1016/j.jcmgh.2014.11.005. [11] D'ARCY MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy[J]. Cell Biol Int, 2019, 43(6): 582-592. DOI: 10.1002/cbin.11137. [12] YAN J, WAN P, CHOKSI S, et al. Necroptosis and tumor progression[J]. Trends Cancer, 2022, 8(1): 21-27. DOI: 10.1016/j.trecan.2021.09.003. [13] BATTAGLIA AM, CHIRILLO R, AVERSA I, et al. Ferroptosis and cancer: Mitochondria meet the "iron maiden" cell death[J]. Cells, 2020, 9(6): 1505. DOI: 10.3390/cells9061505. [14] THIAM HR, WONG SL, WAGNER DD, et al. Cellular mechanisms of NETosis[J]. Annu Rev Cell Dev Biol, 2020, 36: 191-218. DOI: 10.1146/annurev-cellbio-020520-111016. [15] SARHAN J, LIU BC, MUENDLEIN HI, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection[J]. Proc Natl Acad Sci U S A, 2018, 115(46): E10888-E10897. DOI: 10.1073/pnas.1809548115. [16] TANG R, XU J, ZHANG B, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity[J]. J Hematol Oncol, 2020, 13(1): 110. DOI: 10.1186/s13045-020-00946-7. [17] GAUTHERON J, GORES GJ, RODRIGUES C. Lytic cell death in metabolic liver disease[J]. J Hepatol, 2020, 73(2): 394-408. DOI: 10.1016/j.jhep.2020.04.001. [18] COLAK Y, HASAN B, ERKALMA B, et al. Pathogenetic mechanisms of nonalcoholic fatty liver disease and inhibition of the inflammasome as a new therapeutic target[J]. Clin Res Hepatol Gastroenterol, 2021, 45(4): 101710. DOI: 10.1016/j.clinre.2021.101710. [19] LOZANO-RUIZ B, GONZÁLEZ-NAVAJAS JM. The emerging relevance of AIM2 in liver disease[J]. Int J Mol Sci, 2020, 21(18): 6535. DOI: 10.3390/ijms21186535. [20] AL MAMUN A, WU Y, JIA C, et al. Role of pyroptosis in liver diseases[J]. Int Immunopharmacol, 2020, 84: 106489. DOI: 10.1016/j.intimp.2020.106489. [21] WANG Y, GAO W, SHI X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin[J]. Nature, 2017, 547(7661): 99-103. DOI: 10.1038/nature22393. [22] XU W, CHE Y, ZHANG Q, et al. Apaf-1 pyroptosome senses mitochondrial permeability transition[J]. Cell Metab, 2021, 33(2): 424-436. e10. DOI: 10.1016/j.cmet.2020.11.018. [23] XU B, JIANG M, CHU Y, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice[J]. J Hepatol, 2018, 68(4): 773-782. DOI: 10.1016/j.jhep.2017.11.040. [24] ZHU Y, ZHAO H, LU J, et al. Caspase-11-mediated hepatocytic pyroptosis promotes the progression of nonalcoholic steatohepatitis[J]. Cell Mol Gastroenterol Hepatol, 2021, 12(2): 653-664. DOI: 10.1016/j.jcmgh.2021.04.009. [25] MITSUYOSHI H, YASUI K, HARA T, et al. Hepatic nucleotide binding oligomerization domain-like receptors pyrin domain-containing 3 inflammasomes are associated with the histologic severity of non-alcoholic fatty liver disease[J]. Hepatol Res, 2017, 47(13): 1459-1468. DOI: 10.1111/hepr.12883. [26] CSAK T, PILLAI A, GANZ M, et al. Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88-dependent manner in dietary steatohepatitis[J]. Liver Int, 2014, 34(9): 1402-1413. DOI: 10.1111/liv.12537. [27] GONG Z, ZHANG X, SU K, et al. Deficiency in AIM2 induces inflammation and adipogenesis in white adipose tissue leading to obesity and insulin resistance[J]. Diabetologia, 2019, 62(12): 2325-2339. DOI: 10.1007/s00125-019-04983-x. [28] YU X, HAO M, LIU Y, et al. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy[J]. Eur J Pharmacol, 2019, 864: 172715. DOI: 10.1016/j.ejphar.2019.172715. [29] MAI W, XU Y, XU J, et al. Berberine inhibits nod-like receptor family pyrin domain containing 3 inflammasome activation and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP axis[J]. Front Pharmacol, 2020, 11: 185. DOI: 10.3389/fphar.2020.00185. [30] RUAN S, HAN C, SHENG Y, et al. Antcin A alleviates pyroptosis and inflammatory response in Kupffercells of non-alcoholic fatty liver disease by targeting NLRP3[J]. Int Immunopharmacol, 2021, 100: 108126. DOI: 10.1016/j.intimp.2021.108126. [31] ZHU P, PENG Y, WU LL, et al. Research progress on the involvement of pyroptosis in nonalcoholic fatty liver disease[J]. Chin Hepatol, 2021, 26(11): 1290-1293. DOI: 10.14000/j.cnki.issn.1008-1704.2021.11.026.朱鹏, 彭旸, 吴莉莉, 等. 细胞焦亡参与非酒精性脂肪性肝病的相关研究进展[J]. 肝脏, 2021, 26(11): 1290-1293. DOI: 10.14000/j.cnki.issn.1008-1704.2021.11.026. [32] DEWIDAR B, MEYER C, DOOLEY S, et al. TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019[J]. Cells, 2019, 8(11): 1419. DOI: 10.3390/cells8111419. [33] WU J, LIN S, WAN B, et al. Pyroptosis in liver disease: New insights into disease mechanisms[J]. Aging Dis, 2019, 10(5): 1094-1108. DOI: 10.14336/AD.2019.0116. [34] INZAUGARAT ME, JOHNSON CD, HOLTMANN TM, et al. NLR family pyrin domain-containing 3 inflammasome activation in hepatic stellate cells induces liver fibrosis in mice[J]. Hepatology, 2019, 69(2): 845-859. DOI: 10.1002/hep.30252. [35] GAUL S, LESZCZYNSKA A, ALEGRE F, et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis[J]. J Hepatol, 2021, 74(1): 156-167. DOI: 10.1016/j.jhep.2020.07.041. [36] GUO B, FU S, ZHANG J, et al. Targeting inflammasome/IL-1 pathways for cancer immunotherapy[J]. Sci Rep, 2016, 6: 36107. DOI: 10.1038/srep36107. [37] ZHANG X, LI C, CHEN D, et al. H. pylori CagA activates the NLRP3 inflammasome to promote gastric cancer cell migration and invasion[J]. Inflamm Res, 2022, 71(1): 141-155. DOI: 10.1007/s00011-021-01522-6. [38] GARCÍA-PRAS E, FERNÁNDEZ-IGLESIAS A, GRACIA-SANCHO J, et al. Cell death in hepatocellular carcinoma: Pathogenesis and therapeutic opportunities[J]. Cancers (Basel), 2021, 14(1): 48. DOI: 10.3390/cancers14010048. [39] YAN H, LUO B, WU X, et al. Cisplatin induces pyroptosis via activation of MEG3/NLRP3/caspase-1/GSDMD pathway in triple-negative breast cancer[J]. Int J Biol Sci, 2021, 17(10): 2606-2621. DOI: 10.7150/ijbs.60292. [40] ZHOU CB, FANG JY. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection[J]. Biochim Biophys Acta Rev Cancer, 2019, 1872(1): 1-10. DOI: 10.1016/j.bbcan.2019.05.001. [41] HUANG DQ, EL-SERAG HB, LOOMBA R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(4): 223-238. DOI: 10.1038/s41575-020-00381-6. [42] CHEN YF, QI HY, WU FL. Euxanthone exhibits anti-proliferative and anti-invasive activities in hepatocellular carcinoma by inducing pyroptosis: preliminary results[J]. Eur Rev Med Pharmacol Sci, 2018, 22(23): 8186-8196. DOI: 10.26355/eurrev_201812_16511. [43] WEI Q, MU K, LI T, et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression[J]. Lab Invest, 2014, 94(1): 52-62. DOI: 10.1038/labinvest.2013.126. [44] WEI Q, ZHU R, ZHU J, et al. E2-induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells[J]. Oncol Res, 2019, 27(7): 827-834. DOI: 10.3727/096504018X15462920753012. [45] ZHANG Y, YANG H, SUN M, et al. Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis[J]. Pharmacol Rep, 2020, 72(5): 1370-1382. DOI: 10.1007/s43440-020-00064-8. -



 PDF下载 ( 2197 KB)
PDF下载 ( 2197 KB)

 下载:
下载:


