丁型肝炎流行病学筛查检测指标及适宜人群
DOI: 10.3969/j.issn.1001-5256.2023.04.006
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:迟秀梅负责查阅文献,收集资料,撰写文稿;牛俊奇负责拟定写作思路,修改审阅文稿及最后定稿。
Epidemiological screening and detection indicators for hepatitis D and suitable populations
-
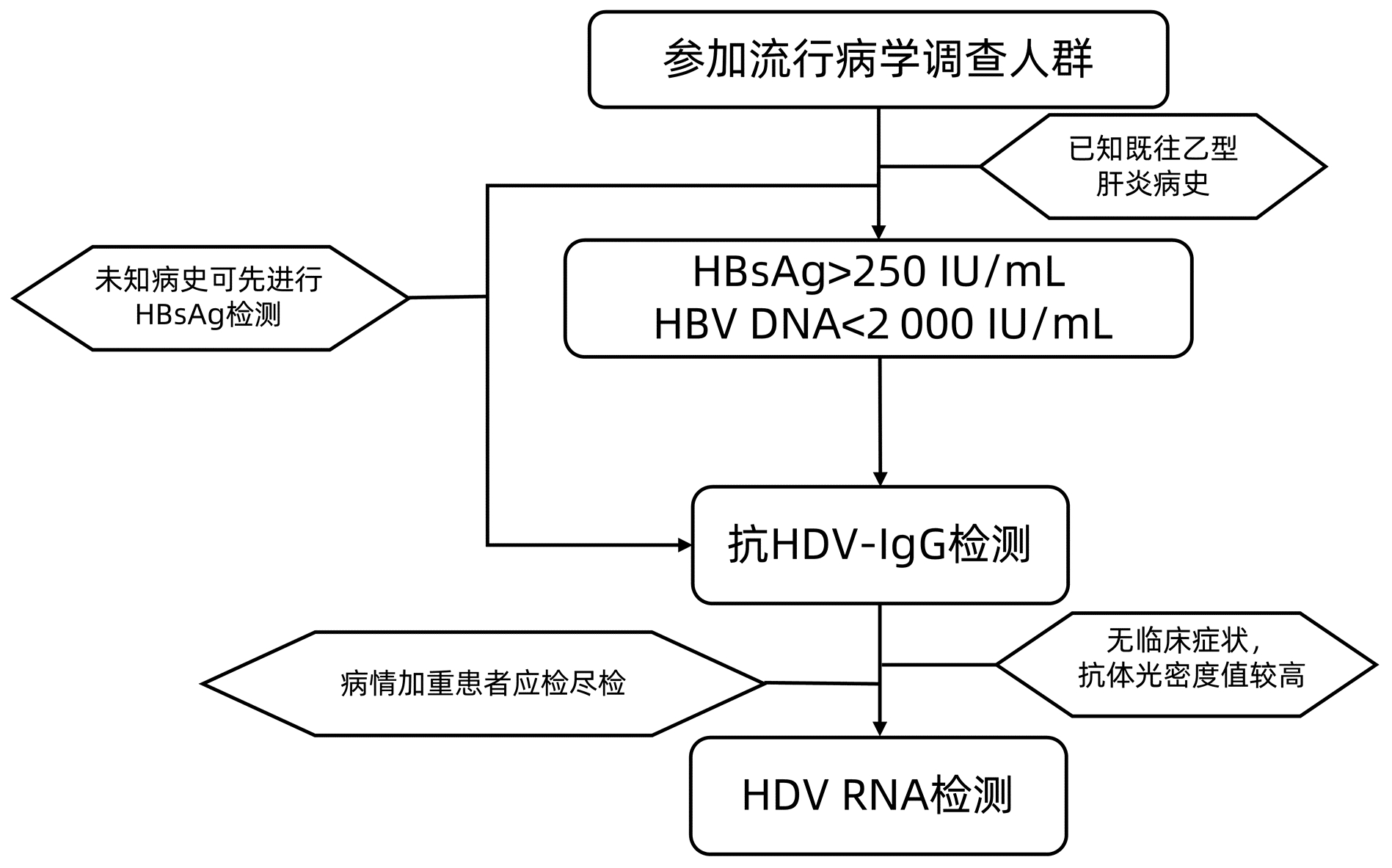
摘要: HDV感染需要借助HBV的参与,感染后会加速疾病的进展,具有很高的发展为肝硬化、肝癌等终末期肝病的风险。近年来全社会对丁型肝炎的认识逐渐增强,一些针对丁型肝炎的治疗药物也成为研究的热点,伴随着临床检验手段的逐步完善,对于丁型肝炎的流行病学调查开始被研究者重视。针对国内HDV感染的具体情况虽已有多项研究,但由于研究队列小、区域性强而数据偏差较大。本文就目前丁型肝炎流行病学调查中的调查人群及调查方法检测指标等进行简要综述,并对其中的关键问题进行讨论,以期未来获取更准确的流行病学资料,能够更有效地筛查HDV感染者,为临床早期的干预和治疗提供帮助。Abstract: Hepatitis D virus (HDV) infection requires the participation of hepatitis B virus (HBV), which accelerates disease progression after infection and induces a high risk of progression to end-stage liver diseases such as liver cirrhosis and liver cancer. With the gradual increase in the understanding of hepatitis D in the whole society, some therapeutic drugs for hepatitis D have become a research hotspot in recent years, and with the further improvement in clinical testing methods, researchers have started to pay attention to the epidemiological investigation of hepatitis D. Although many studies have been conducted for the specific situation of HDV infection in China, large data deviation is observed due to small cohorts with strong regional features. This article briefly reviews the population, methods, and indicators in the current epidemiological investigation of hepatitis D and discusses related key issues, in order to obtain more accurate epidemiological data, effectively screen out HDV infection, and provide help for early clinical intervention and treatment.
-
[1] URBAN S, NEUMANN-HAEFELIN C, LAMPERTICO P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease[J]. Gut, 2021, 70(9): 1782-1794. DOI: 10.1136/gutjnl-2020-323888. [2] MENTHA N, CLÉMENT S, NEGRO F, et al. A review on hepatitis D: From virology to new therapies[J]. J Adv Res, 2019, 17: 3-15. DOI: 10.1016/j.jare.2019.03.009. [3] LEMPP FA, NI Y, URBAN S. Hepatitis delta virus: insights into a peculiar pathogen and novel treatment options[J]. Nat Rev Gastroenterol Hepatol, 2016, 13(10): 580-589. DOI: 10.1038/nrgastro.2016.126. [4] WANG W, LEMPP FA, SCHLUND F, et al. Assembly and infection efficacy of hepatitis B virus surface protein exchanges in 8 hepatitis D virus genotype isolates[J]. J Hepatol, 2021, 75(2): 311-323. DOI: 10.1016/j.jhep.2021.03.025. [5] STOCKDALE AJ, KREUELS B, HENRION M, et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis[J]. J Hepatol, 2020, 73(3): 523-532. DOI: 10.1016/j.jhep.2020.04.008. [6] ZI J, GAO X, DU J, et al. Multiple regions drive hepatitis delta virus proliferation and are therapeutic targets[J]. Front Microbiol, 2022, 13: 838382. DOI: 10.3389/fmicb.2022.838382. [7] GILLICH N, ZHANG Z, BINDER M, et al. Effect of variants in LGP2 on MDA5-mediated activation of interferon response and suppression of hepatitis D virus replication[J]. J Hepatol, 2023, 78(1): 78-89. DOI: 10.1016/j.jhep.2022.08.041. [8] KOHSAR M, LANDAHL J, NEUMANN-HAEFELIN C, et al. Human hepatitis D virus-specific T cell epitopes[J]. JHEP Rep, 2021, 3(4): 100294. DOI: 10.1016/j.jhepr.2021.100294. [9] LUCIFORA J, DELPHIN M. Current knowledge on hepatitis delta virus replication[J]. Antiviral Res, 2020, 179: 104812. DOI: 10.1016/j.antiviral.2020.104812. [10] TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. [11] CHEN LY, PANG XY, GOYAL H, et al. Hepatitis D: challenges in the estimation of true prevalence and laboratory diagnosis[J]. Gut Pathog, 2021, 13(1): 66. DOI: 10.1186/s13099-021-00462-0. [12] LAZARUS JV, AL-RIFAI A, SANAI FM, et al. Hepatitis delta virus infection prevalence, diagnosis and treatment in the Middle East: A scoping review[J]. Liver Int, 2022. DOI: 10.1111/liv.15338.[Online ahead of print] [13] VLACHOGIANNAKOS J, PAPATHEODORIDIS GV. New epidemiology of hepatitis delta[J]. Liver Int, 2020, 40 Suppl 1: 48-53. DOI: 10.1111/liv.14357. [14] WRANKE A, HEIDRICH B, ERNST S, et al. Anti-HDV IgM as a marker of disease activity in hepatitis delta[J]. PLoS One, 2014, 9(7): e101002. DOI: 10.1371/journal.pone.0101002. [15] CHEN X, OIDOVSAMBUU O, LIU P, et al. A novel quantitative microarray antibody capture assay identifies an extremely high hepatitis delta virus prevalence among hepatitis B virus-infected mongolians[J]. Hepatology, 2017, 66(6): 1739-1749. DOI: 10.1002/hep.28957. [16] LEMPP FA, ROGGENBACH I, NKONGOLO S, et al. A rapid point-of-care test for the serodiagnosis of hepatitis delta virus infection[J]. Viruses, 2021, 13(12): 2371. DOI: 10.3390/v13122371. [17] HOBLOS R, KEFALAKES H. Immunology of hepatitis D virus infection: General concepts and present evidence[J]. Liver Int, 2022. DOI: 10.1111/liv.15424.[Online ahead of print] [18] DANDRI M, BERTOLETTI A, LVTGEHETMANN M. Innate immunity in hepatitis B and D virus infection: consequences for viral persistence, inflammation, and T cell recognition[J]. Semin Immunopathol, 2021, 43(4): 535-548. DOI: 10.1007/s00281-021-00864-x. [19] YAO T, LV M, MA S, et al. Ubiquitinated hepatitis D antigen-loaded microvesicles induce a potent specific cellular immune response to inhibit HDV replication in vivo[J]. Microbiol Spectr, 2021, 9(3): e0102421. DOI: 10.1128/Spectrum.01024-21. [20] XU L, ZHANG X, CAO Y, et al. Digital droplet PCR for detection and quantitation of hepatitis delta virus[J]. Clin Transl Gastroenterol, 2022, 13(7): e00509. DOI: 10.14309/ctg.0000000000000509. [21] PFLVGER LS, NÖRZ D, VOLZ T, et al. Clinical establishment of a laboratory developed quantitative HDV PCR assay on the cobas6800 high-throughput system[J]. JHEP Rep, 2021, 3(6): 100356. DOI: 10.1016/j.jhepr.2021.100356. [22] PALOM A, SOPENA S, RIVEIRO-BARCIELA M, et al. One-quarter of chronic hepatitis D patients reach HDV-RNA decline or undetectability during the natural course of the disease[J]. Aliment Pharmacol Ther, 2021, 54(4): 462-469. DOI: 10.1111/apt.16485. [23] ZHANG Z, NI Y, LEMPP FA, et al. Hepatitis D virus-induced interferon response and administered interferons control cell division-mediated virus spread[J]. J Hepatol, 2022, 77(4): 957-966. DOI: 10.1016/j.jhep.2022.05.023. [24] ROULOT D, BRICHLER S, LAYESE R, et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta[J]. J Hepatol, 2020, 73(5): 1046-1062. DOI: 10.1016/j.jhep.2020.06.038. [25] SCHWARZ C, CHROMY D, BANGERT C, et al. Immediate-type hypersensitivity reaction to bulevirtide and successful desensitization in a patient with HBV/HDV-associated compensated cirrhosis[J]. J Hepatol, 2022, 77(1): 254-255. DOI: 10.1016/j.jhep.2022.03.004. [26] LIN YX, QIN YL, ZHANG JM. Clinical significance and methods of hepatitis D virus RNA detection[J]. J Clin Hepatol, 2022, 38(12): 2830-2835. DOI: 10.3969/j.issn.1001-5256.2022.12.028.林妍雪, 秦艳丽, 张继明. HDV RNA检测的临床意义与方法[J]. 临床肝胆病杂志, 2022, 38(12): 2830-2835. DOI: 10.3969/j.issn.1001-5256.2022.12.028. [27] KATSOULIDOU A, MANESIS E, ROKKA C, et al. Development and assessment of a novel real-time PCR assay for quantitation of hepatitis D virus RNA to study viral kinetics in chronic hepatitis D[J]. J Viral Hepat, 2013, 20(4): 256-262. DOI: 10.1111/jvh.12000. [28] SCHOLTES C, ICARD V, AMIRI M, et al. Standardized one-step real-time reverse transcription-PCR assay for universal detection and quantification of hepatitis delta virus from clinical samples in the presence of a heterologous internal-control RNA[J]. J Clin Microbiol, 2012, 50(6): 2126-2128. DOI: 10.1128/JCM.06829-11. [29] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. [30] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. [31] RIZZETTO M, HAMID S, NEGRO F. The changing context of hepatitis D[J]. J Hepatol, 2021, 74(5): 1200-1211. DOI: 10.1016/j.jhep.2021.01.014. [32] BÉGUELIN C, ATKINSON A, BOYD A, et al. Hepatitis delta infection among persons living with HIV in Europe[J]. Liver Int, 2023. DOI: 10.1111/liv.15519.[Online ahead of print] -



 PDF下载 ( 1899 KB)
PDF下载 ( 1899 KB)

 下载:
下载:


