多房棘球蚴源性外泌体对巨噬细胞极化的影响
DOI: 10.3969/j.issn.1001-5256.2023.04.019
Effect of exosomes derived from Echinococcus multilocularis on macrophage polarization: A preliminary study
-
摘要:
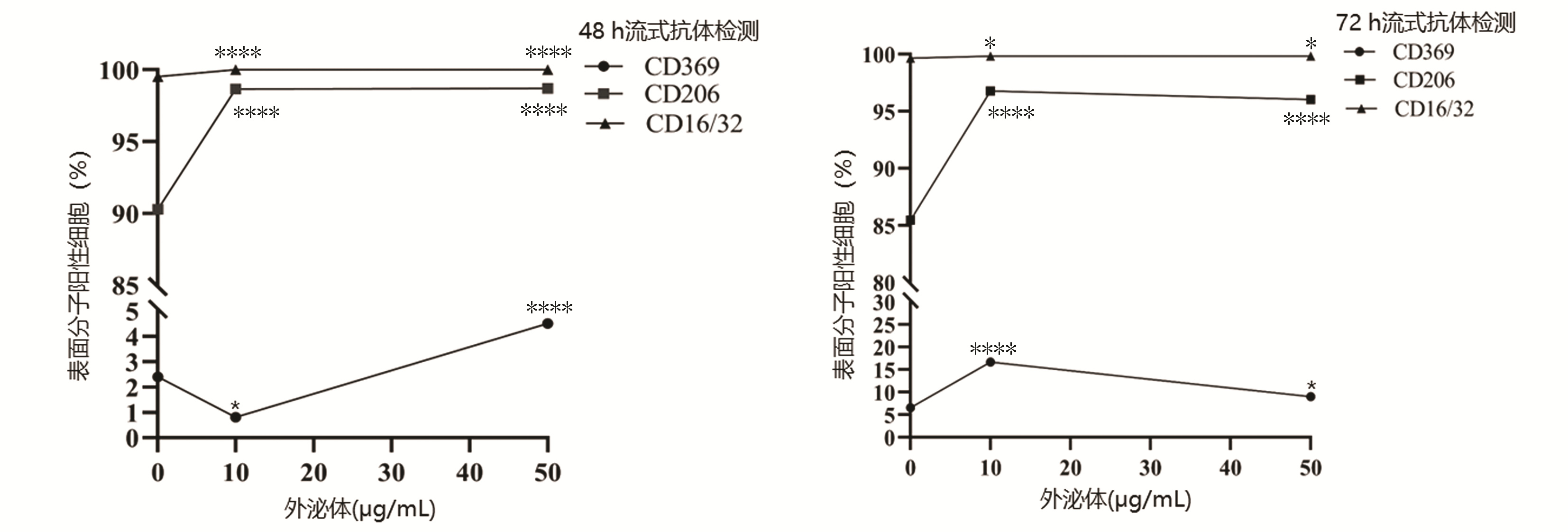
目的 探究不同时间及浓度多房棘球蚴源性外泌体对巨噬细胞极化的影响。 方法 从60只造模BALB/c小鼠中随机选取4只,应用7.0T MRI观察腹腔病灶生长情况;解剖造模小鼠,通过腹腔病灶提取原头节进行体外培养,超速离心法从培养上清中提取外泌体,透射电镜及蛋白质免疫印迹法鉴定外泌体表征。将未加外泌体处理的巨噬细胞单独培养组设为对照组,不同浓度多房棘球蚴来源外泌体与巨噬细胞共培养组设为实验组(10 μg/mL组、50 μg/mL组),分别共培养48 h和72 h,显微镜下观察巨噬细胞形态变化。通过流式细胞术和酶联免疫吸附实验(ELISA)检测极化状态。符合正态分布的计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 7.0T MRI显示小鼠腹腔内弥漫分布、大小不等的病灶形成;多房棘球蚴源性外泌体直径100 nm左右,呈杯型或茶托型,其表面标志物CD9、TSG101和CD63表达阳性。共培养后,实验组大部分细胞拉长,形态不规则,主要呈多角形;流式细胞术检测发现,共培养48 h,对照组CD16/32、CD206、CD369阳性率分别为(99.53±0.06)%、(90.27±0.21)%、(2.40±0.20)%;与对照组相比,除10 μg/mL外泌体组CD369阳性率[(0.80±0.00)%]降低(P<0.05),其余组别CD16/32、CD206、CD369阳性率均明显升高(P值均<0.000 1);共培养72 h,对照组CD16/32、CD206、CD369阳性率分别为(99.67±0.06)%、(85.47±0.55)%、(6.60±0.20)%,实验组CD16/32、CD206、CD369阳性率较对照组均明显升高(P值均<0.05)。ELISA结果示:共培养48 h,对照组IL-6及TNFα水平分别为(58.53±15.52) pg/mL、(320.70±5.30)pg/mL,实验组外泌体浓度为50 μg/mL时IL-6[(98.81±15.55) pg/mL]较对照组升高(P<0.05);共培养72 h,对照组IL-6及TNFα水平分别为(76.22±9.68)pg/mL、(323.90±87.37)pg/mL,当外泌体浓度为10 μg/mL时TNFα水平[(164.20±14.17)pg/mL]较对照组明显下降(P<0.05);当外泌体浓度为50 μg/mL时IL-6水平[(99.52±8.35)pg/mL]较对照组升高(P<0.05)。 结论 多房棘球蚴源性外泌体可调控巨噬细胞极化,且在浓度为10 μg/mL,共培养72 h后,可导致巨噬细胞M2样极化,具体方式有待进一步研究。 -
关键词:
- 棘球蚴病, 肝 /
- 外泌体 /
- 巨噬细胞 /
- 小鼠,近交BALB C
Abstract:Objective To investigate the effect of exosomes derived from Echinococcus multilocularis on macrophage polarization after treatment for different durations and concentrations. Methods A total of 60 BALB/c mice were used for modeling, among which 4 mice were selected to observe the growth of abdominal lesions on 7.0T MRI. The mice for modeling were dissected, and the protoscoleces was taken from the abdominal lesion and cultured in vitro; ultracentrifugation was used to extract the exosomes from the supernatant, and transmission electron microscopy and Western blotting were used for the characterization of exosomes. The macrophages without exosome treatment were established as control group, and the macrophages co-cultured with different concentrations of exosomes derived from Echinococcus multilocularis were established as experimental group (10 μg/mL group and 50 μg/mL group) and were cultured for 48 and 72 hours. The morphological changes of macrophages were observed under a microscope, and flow cytometry and ELISA were used to observe polarization state. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results The results of 7.0T MRI showed the formation of diffuse lesions with different sizes in the abdominal cavity of mice, and the exosomes derived from Echinococcus multilocularis were approximately 100 nm in diameter and were cup-shaped or saucer-shaped, with the positive expression of the surface markers CD9, TSG101, and CD63. After co-culture, most of the cells in the experimental group were elongated with an irregular and polygonal shape. Flow cytometry showed that after 48 hours of co-culture, the positive rates of CD16/32, CD206, and CD369 in the control group were 99.53%±0.06%, 90.27%±0.21%, and 2.40%±0.20%, respectively; compared with the control group, except that the 10 μg/mL exosome group had a significant reduction in the positive rate of CD369 (0.80%±0.00%) (P < 0.05), all the other groups had a significant increase in the positive rates of CD16/32, CD206, and CD369 (all P < 0.000 1); after 72 hours of co-culture, the positive rates of CD16/32, CD206, and CD369 in the control group were 99.67%±0.06%, 85.47%±0.55%, and 6.60%±0.20%, respectively, and compared with the control group, the experimental group had significant increases in the positive rates of CD16/32, CD206, and CD369 (all P < 0.05). ELISA showed that after 48 hours of co-culture, the levels of IL-6 and TNFα in the control group were 58.53±15.52 pg/mL and 320.70±5.30 pg/mL, respectively, and when the exosome concentration was 50 μg/mL, the level of IL-6 in the experimental group was 98.81±15.55 pg/mL, which was higher than that in the control group (P < 0.05); after 72 hours of co-culture, the levels of IL-6 and TNFα in the control group were 76.22±9.68 pg/mL and 323.90±87.37 pg/mL, respectively, and when the exosome concentration was 10 μg/mL, the level of TNFα was 164.20±14.17 pg/mL, which was significantly lower than that in the control group (P < 0.05); when the exosome concentration was 50 μg/mL, the level of IL-6 was 99.52±8.35 pg/mL, which was significantly higher than that in the control group (P < 0.05). Conclusion Exosomes derived from Echinococcus multilocularis can regulate macrophage polarization and induce M2-like polarization of macrophages after co-culture at a concentration of 10 μg /mL for 72 hours, and further studies are needed to clarify the specific method. -
Key words:
- Echinococcosis, Hepatic /
- Exosomes /
- Macrophages /
- Mice, Inbred BALB C
-
肝硬化是临床常见的慢性进行性肝病,引起肝硬化的病因有很多,欧美国家以酒精性肝硬化为主,而我国是以乙型肝炎引发的肝硬化为主,肝硬化患者的HBsAg阳性率高达76.7%[1],其形成机制主要是由于肝炎病毒感染导致广泛性肝细胞变性坏死、肝小叶结构破坏、肝脏弥漫性纤维化、结节性再生同时伴有假小叶形成,肝脏逐渐变形、变硬而发展为肝硬化。常选用保肝、降酶、退黄等基础治疗,而终末期肝硬化临床治疗效果差,缺乏广泛可行的治疗手段。干细胞移植的出现, 以其独特的生物学特性,为肝病治疗带来新的治疗思路。
脐带血中造血干细胞含量丰富,其增殖能力是成人骨髓造血干细胞的数倍[2],近年来国内外多项研究证实人脐带血单个核细胞(human umbilical cord blood mononuclear cells, hUCB-MNCs)移植对肝硬化也有显著的辅助治疗效果[3]。
过去认为,骨髓间充质干细胞(bone marrow mesenchymal stem cells,BMSCs)治疗肝脏疾病趋向于受损的肝脏区域而后分化成肝细胞、修复受损肝细胞或通过免疫调节抑制炎性反应,促进肝功能恢复,但采集大量的骨髓多数患者很难接受,从而限制了临床的广泛应用。而人脐带组织来源的间充质干细胞(human umbilical cord msenchymal stem cells, hUC-MSCs),取材方便,较BMSCs有更高的增殖、分化潜能和免疫调节功能,其免疫原性远低于BMSCs[4],具有更加广泛的临床应用与研究价值[5]。为了提高肝硬化疗效,本研究采用了hUCB-MNCs联合hUC- MSCs治疗肝硬化,笔者前期临床研究发现,hUCB-MNCs具有极其良好的提高患者免疫的功效[6],因此先行输注hUCB-MNCs,改善患者整体状况,提高其免疫功能。而后输入hUC-MSCs,使其归巢到肝脏,分化为肝细胞或进入肝脏后修复受损的肝细胞[7-9],并可通过某些细胞因子的改变, 来调节免疫、减轻炎症反应、抗肝细胞凋亡、促进血管增生、抗肝纤维化等修复损伤肝组织,同时促进hUC-MSCs的多向分化[10-12]。两种细胞联合治疗,以更好的发挥其疗效。本研究为单中心、回顾性、非随机、自体对照临床研究。通过观察联合输注治疗肝硬化后各项指标的改变,对其治疗肝硬化的免疫调节及安全性进行评价,为肝硬化的治疗探索新的治疗方法。
1. 材料与方法
1.1 实验材料
脐带血全部来源于山东省齐鲁干细胞库;脐带采集于内蒙古医科大学附属医院妇产科,选取经剖宫产足月健康胎儿的脐带,并征得父母授权同意,4 ℃预冷生理盐水密闭容器保存。MSCs所用无血清培养基购自Invitrogen, USA、流式细胞分析仪所用单克隆抗体购自美国BD公司;ELISA试剂盒购自上海润裕生物公司。
1.2 方法
1.2.1 研究对象
选取2016年11月—2019年6月内蒙古医科大学附属医院收治的失代偿性肝硬化患者,纳入标准:(1)诊断符合2000年中华医学会肝病学分会修订的《病毒性肝炎防治方案》[13]、2005年中华医学会肝病学分会修订的《慢性乙型肝炎防治指南》的肝硬化临床诊断标准[14],诊断为乙型肝炎肝硬化,且Child-Pugh分级为B、C级(失代偿期肝硬化);(2)年龄40~60岁;(3)在加入本研究之前,理解并签署知情同意书,并且遵守研究方案的要求;同意不参加其他任何临床研究。排除标准:(1)除肝衰竭或肝硬化失代偿以外的任何严重的全身性疾病,研究者认为可能会干扰受试者的治疗或干扰受试者的依从性,包括任何未被控制的具有临床意义的泌尿、循环、呼吸、神经、精神、消化、内分泌、免疫等系统疾病及肿瘤等;(2)肝静脉或门静脉血栓形成;(3)HIV抗体阳性者;(4)筛选前3个月内接受过其他药物的临床研究;(5)1年内有酗酒或有静脉药瘾者;(6)HBV DNA>200 IU/ml;(7) 肾衰竭,血肌酐>451 mol/L;(8)肺功能指标,FEV1<35%,DLCO-SB<39%;(9)根据AHA 1994年心功能标准D级;(10)其他可能妨碍临床研究的严重情况。最终纳入肝硬化患者11例,根据患者整体状况进行Child- Pugh分级[15](表 1)。入组的肝硬化患者经常规治疗(保肝、降黄疸、降血氨、抗病毒、抗感染、必要时输白蛋白等对症支持处理,同时静脉注射促肝细胞生长素80 mg/d),治疗4周后效果不佳(即肝功能、血氨、凝血因子、血清细胞因子浓度及淋巴细胞亚群的无变化,且神经、精神症状未改善者),患者自愿申请参加本研究。
表 1 入组患者的信息和条件患者 年龄(岁) 性别 腹水 TBil(μmol/L) Alb(g/L) PT(s) Child-Pugh分级 No.1 50 男 - 48.1 26.5 19.3 B级 No.2 48 男 - 47.9 22.9 19.9 B级 No.3 55 男 Ⅰ 47.4 24.8 22.5 B级 No.4 56 男 - 34.9 26.0 22.9 B级 No.5 60 男 Ⅰ 54.4 22.8 20.5 C级 No.6 52 女 - 48.5 31.9 20.2 B级 No.7 41 男 - 43.8 40.8 19.4 B级 No.8 49 男 - 50.2 23.9 17.2 B级 No.9 51 男 Ⅰ 34.8 27.4 16.2 B级 No.10 53 男 Ⅱ 67.6 22.6 19.4 C级 No.11 51 男 Ⅰ 48.7 21.9 19.4 B级 1.2.2 hUCB-MNCs制备
采集患者外周血,送山东省齐鲁干细胞库进行HLA血清和基因分型,选择至少4个位点相合的脐带血作为供者,然后将从脐带血分离获取的hUCB-MNCs(山东省齐鲁干细胞库操作制备)悬浮于含有2%人血白蛋白的0.9%生理盐水(150 ml)备用。
1.2.3 hUC-MSCs制备
脐带标本采集后至于4 ℃预冷生理盐水密闭容器中,6 h内完成分离脐带中华通氏胶,用MSCs无血清培养基培养制备hUC-MSCs,连续传代培养,取P3代hUC-MSCs,应用流式细胞仪检测细胞表面标记CD34、CD44、CD90、CD105和HLA-DR的表达情况。台盼蓝染色活性检测>95%,细胞HIV、EB病毒、HBV、HCV核酸分别用PCR和逆转录PCR(RT-PCR)检测,检测无任何微生物污染。最后制备的hUC-MSCs, 用含有2%人血白蛋白的0.9%生理盐水(150 ml)来悬细胞备用。
1.2.4 hUCB-MNCs和hUC-MSCs输注方案
患者经常规治疗后,临床检测肝肾功能、生化指标、肿瘤标志物、临床评估心肺功能、神经、精神状况达到入组标准。本研究方案为:第1周输注1次hUCB-MNCs(>18×109/次),第2、3、4周每周1次,每次输入hUC-MSCs 1×106/kg,每个患者3次输注的hUC-MSCs为同一来源的脐带所分离培养。为了防止可能发生的过敏反应,输注前5 min静脉输入5 mg地塞米松。hUCB-MNCs和hUC-MSCs回输结束第4、8、12周后跟踪随访。
1.2.5 hUCB-MNCs和hUC-MSCs输注的不良反应、实验室检查及疗效评估
一个治疗方案结束后,有1例患者(No.5)腋下体温38.1 ℃,持续时间为2 h,患者自行服用布洛芬,大量饮用温开水发汗,排尿后体温降到正常。在之后的随访过程中,所有患者均未出现明显副作用;hUCB-MNCs和hUC-MSCs回输结束第4、8、12周后采集静脉血,检测肝功能、血氨、凝血功能等各项指标,比较输注前后及输注不同时间后指标变化;同时对比观察治疗前后患者神经、精神状况,根据患者整体状况进行Child-Pugh肝功能分级。
1.2.6 细胞输注后免疫细胞及细胞因子定量检测
患者在细胞治疗疗程结束4周后开始检测淋巴细胞亚群和血清细胞因子。流式细胞仪测定CD3+T淋巴细胞、CD3+CD4+T淋巴细胞、CD3+CD8+T淋巴细胞、CD4+CD25+调节性T淋巴细胞(Treg)、CD19+B淋巴细胞百分率;ELISA试剂盒量检测血清IL-6、TNF、IL-10、TGFβ。
1.3 伦理学审查
本研究获得内蒙古医科大学附属医院伦理委员会批准,批号:2017医第(028)号。所纳入患者均签署知情同意书。
1.4 统计学方法
采用SPSS 23.0统计学软件进行数据分析。计量资料以x±s表示,不同时间点比较采用单因素重复测量方差分析。P<0.05为差异有统计学意义。
2. 结果
2.1 hUC-MSCs的鉴定
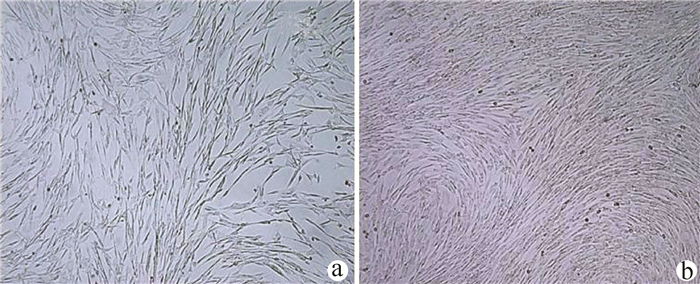
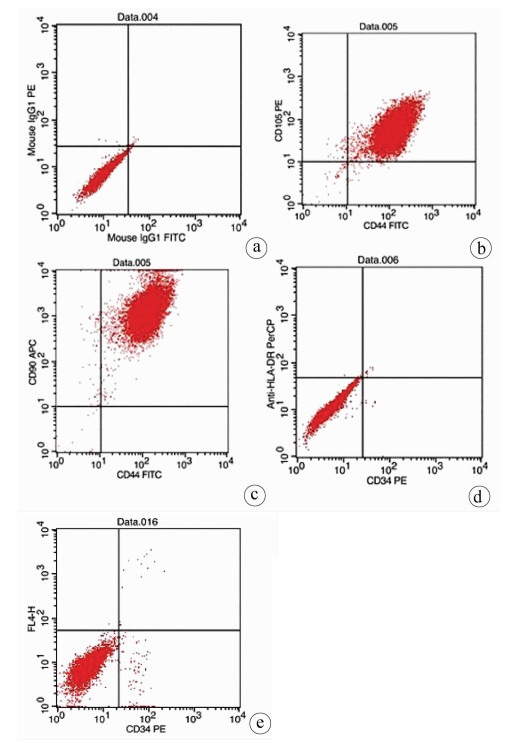
实验室制备的hUC-MSCs呈贴壁生长,外形为均匀梭形(图 1),流式细胞分析检测,CD44、CD90和CD105表达均>98%(阳性)(图 2b、2c),而CD34和HLA-DR表达为阴性(图 2d);山东省齐鲁干细胞库提供的hUCB-MNCs总数>1.8×109;台盼蓝染色显示活性>95%;血清学和基因HLA分型,供体与受体至少有4个位点相合(山东省齐鲁干细胞库提供),无任何可检测到的微生物污染,对山东脐血库提供的细胞再次进行了流式细胞分析,检测显示其中CD34+细胞>1.5%(图 2e);制备的hUC-MSCs可以被诱导成肝细胞、神经细胞(图 3);常规和核酸PCR/RT-PCR检测无任何微生物污染;裸鼠实验证实无致瘤性;hUC-MSCs活性>95%。
2.2 hUCB-MNCs联合hUC-MSCs输注前后及不同时间对肝功能、血氨及其他状况的影响
在hUCB-MNCs联合hUC-MSCs输注结束后,对PT和PTA影响最快,细胞输注结束后第4周即可显著改善(P值均<0.05),而患者的肝功能酶学指标在细胞输注后第8周逐渐恢复到正常水平(P值均<0.05),在输注结束第12周时,肝功能指标较之前明显好转(P值均<0.01)(表 2)。
表 2 细胞输注前后肝功能变化输注时间 ALT(U/L) AST(U/L) ALP(U/L) GGT(U/L) TBil(μmol/L) Alb(g/L) PTA(%) PT(s) 输注前 88.4±15.31) 99.8±25.01) 123.8±39.41) 305.1±83.41) 47.8±8.11) 26.5±6.41) 45.8±7.91) 16.2±1.31) 输注(4周) 77.9±22.7 85.5±31.3 97.5±40.1 189.1±66.4 23.2±9.8 30.5±3.6 66.0±6.0 12.6±0.5 输注(8周) 59.1±21.5 26.8±7.9 58.1±17.6 86.6±39.9 9.9±5.3 36.0±4.2 74.9±6.9 12.2±1.8 输注(12周) 42.7±12.3 24.2±8.3 59.5±28.0 43.2±19.8 6.7±1.8 40.8±2.4 87.6±3.0 11.3±0.2 F值 14.496 41.163 10.941 45.740 79.730 20.496 86.289 1 029.600 P值 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 注:与输注后的第4、8、12周比较, 1)P<0.05。 细胞输注4周后,患者血清中血氨浓度明显降低(P<0.05),随着时间的延长,血氨水平持续下降,除患者No. 7、10、11外,其他患者在hUCB-MNCs和HUC-MSCs输注12周后均降低到正常水平(P值均<0.05)(表 3)。
表 3 hUCB-MNCs联合hUC-MSCs输注前后血氨值变化输注时间 血氨(μmol/L) 输注前 166.6±47.71) 输注(4周) 115.6±32.8 输注(8周) 87.2±36.3 输注(12周) 66.2±32.5 F值 104.6 P值 P<0.05 注:与输注后的第4、8、12周比较, 1)P<0.05。 细胞输注后第4、8周,5例肝硬化腹水患者的腹水均有所减少,除却No.10患者仍有少量腹水,且Child-Pugh分级仍然处于C级。10例患者的乏力、食欲、睡眠、精神、腹胀、下肢水肿等明显改善,血清胆红素及PT基本恢复正常,血清Alb也明显升高,Child-Pugh评分随之降低,差异有统计学意义(P值均<0.05)(表 4)。
表 4 hUCB-MNCs联合hUC-MSCs输注前后Child-Pugh评分变化输注时间 Child-Pugh评分 输注前 10.10±2.431) 输注(4周) 8.36±2.94 输注(8周) 6.82±2.27 输注(12周) 6.09±1.58 F值 68.4 P值 P<0.05 注:与输注后的第4、8、12周比较, 1)P<0.05。 2.3 hUCB-MNCs联合hUC-MSCs输注对血清细胞因子和淋巴细胞亚群的影响
采集患者静脉血,ELISA定量检测hUCB-MNCs和hUC-MSCs输注前后血清炎性和抗炎细胞因子的含量,结果显示输注4周后患者血清炎性细胞因子IL-6、TNFα水平均明显降低(P值均<0.01),从8到12周,其血清中含量持续维持在正常水平;患者血清抗炎细胞因子IL-10、TGFβ在治疗后4周显著上升(P值均<0.01),4周后基本恢复到正常值,到治疗后的12周,其表达水平一直维持在正常值(表 5)。
表 5 hUCB-MNCs联合HUC-MSCs输注前后细胞因子和淋巴细胞亚群变化输注时间 IL-6(ng/L) TNFα(ng/L) IL-10(pg/L) TGFβ(pg/L) CD3+ CD3+CD4+ CD3+CD8+ CD4+CD25+ CD19+ 细胞输注前 198.8±54.01) 300.6±73.51) 18.3±7.81) 25.9±4.31) 75.8±11.01) 23.3±4.21) 48.3±9.31) 1.3±1.21) 32.1±4.21) 输注后(4周) 96.2±19.6 198.9±106.4 48.9±15.7 49.3±7.1 74.8±8.9 53.1±10.9 20.4±6.8 18.3±9.5 12.7±3.6 输注后(8周) 58.9±16.9 92.2±26.1 47.3±8.7 43.9±4.4 76.9±12.4 54.0±7.7 20.4±3.4 18.0±5.0 11.7±5.3 输注后(12周) 59.8±16.4 92.8±13.7 49.6±15.9 35.5±6.4 80.1±10.9 55.9±6.4 16.7±6.0 19.1±8.3 6.1±2.4 F值 49.497 37.071 15.918 35.843 0.493 44.755 52.242 17.337 89.097 P值 <0.01 <0.01 <0.01 <0.01 >0.05 <0.01 <0.01 <0.01 <0.01 注:与输注后的第4、8、12周比较, 1)P<0.05。 采用流式细胞仪分析淋巴细胞亚群比例,细胞输注对CD3+T淋巴细胞无明显影响,输注4周后CD3+CD4+T淋巴细胞和CD4+CD25+Treg显著升高(P值均<0.01);而对CD3+CD8+T淋巴细胞和CD19+B淋巴细胞具有较明显的抑制作用,此状态一直维持至12周(表 5)。
3. 讨论
我国HBV感染率高,最终导致肝硬化、肝功能失代偿、门静脉高压、肝硬化,甚至肝癌等,死亡率呈逐年上升的趋势。内蒙古地区是少数民族聚集地,人群感染HBV发生率更高[16]。加之内蒙古地区冬天比较寒冷和传统习惯,多数男性喜欢饮酒,更加重了肝细胞的损伤,加速了肝硬化、门静脉高压以及肝癌的发生,因此很多患者在感染肝炎病毒后,发生肝硬化失代偿的年龄相对较轻,大多数在60岁之前,而且以男性居多。
肝功能失代偿并发肝硬化时,多数患者一般状况较差,免疫功能低,易并发感染,特别是肠道感染,再加肝功能失代偿年久,多数患者伴有腹水。所以应用干细胞治疗此类患者前需要评估患者整体状况,本临床研究参与的患者,Child-Pugh分级多在B级和C级。
由于输注细胞需要反复多次操作,而且肝硬化患者凝血功能差,为了尽量减少对患者的创伤,本研究未采用肝动脉介入注射技术,而是应用多次静脉回输的方法,更简便、安全。
在输注细胞前,除了对患者整体状态进行评估外,还应用流式细胞检测患者的免疫状态。多数肝硬化患者的免疫功能普遍较低,特别是辅助性T淋巴细胞(CD3+CD4+),明显低于正常参考值,因此在hUC-MSCs治疗前必须调整患者一般状况,提高其免疫功能。笔者团队前期临床研究[6]发现,hUCB-MNCs具有良好的提高患者免疫的功效,本研究与患者及其家属沟通,经患者本人及家属同意,先输注hUCB-MNCs,以便先改善患者整体状况,提高其免疫功能。临床观察发现部分患者在输注hUCB-MNCs后,甚至在1周之内,全身不适、食欲、精神状况明显改善,流式细胞分析静脉血中辅助性T淋巴细胞有所上升,此效果与笔者团队前期研究[6]结果相同,说明hUCB-MNCs也可以改善肝硬化患者的一般状况和免疫功能。
研究提示hUC-MSCs能够向多方向分化,如神经、骨骼、肌肉、心肌、肝细胞等[17],同时具有强大的修复功能[18]。已知肝细胞感染HBV感染后,肝细胞损伤、坏死,释放大量的肝细胞酶,经常规治疗后门静脉压降低,腹水形成也相应减少,但是胃肠道的有毒物质未经肝脏处理,直接进入体循环,部分患者血氨明显升高。而hUC-MSCs输入后,可以归巢到肝脏,分化为肝细胞或进入肝脏后修复受损的肝细胞,血液内的有毒物质可以被代谢并排除体外,从而起到治疗肝硬化的作用,本研究结果证实,hUCB-MNCs和hUC-MSCs联合输入4周后,部分患者的血氨下降,肝硬化症状逐步得以控制;除此之外,hUC-MSCs还可以分化为神经细胞,同时具有强大的修复神经细胞损伤的功能[19-20],所以hUC-MSCs输入后肝硬化患者精神、神经状态不佳的情况相应减轻,笔者推断可能与此也有一定的关系,有待进一步验证。
由于肝细胞受损,静脉血中肝细胞酶类升高、胆红素释放增加、合成Alb功能降低,输注hUC-MSCs归巢到肝脏,受微环境的影响分化为肝细胞,同时发挥hUC-MSCs的修复功能,使损伤的肝细胞得以恢复,酶释放减少,凝血酶原和Alb合成增加,腹水减轻,患者的一般临床症状改善, Child-Pugh评分随之降低, 而Child-Pugh评分又可预测失代偿期肝硬化患者的短期预后,对选择适当的临床治疗方案有一定指导作用[21]。研究发现细胞治疗对于门静脉高压导致的腹水疗效较差,如本研究的No.10患者腹水减轻并不明显。
众所周知,HBV感染后,肝细胞及其周围组织同时发生持续和反复慢性炎症反应,由于参与炎性反应的细胞主要是CD3+CD8+T淋巴细胞和产生抗体的CD19+B淋巴细胞,此类细胞在肝内的聚集,释放大量的炎性细胞因子,刺激肝内纤维细胞过度增生,导致肝硬化,同时释放的炎性细胞因子和抗体也可以进一步杀伤和破坏肝细胞。已知hUC-MSCs本身及其释放的外泌体具有强大的免疫调节功能[22-23],可以抑制过度活跃、聚集于肝内的炎性细胞。本研究中心经长期的临床试验发现,hUC-MSCs输注后,随着时间的延长,炎性细胞及其炎性因子,如IL-6、TNFα逐渐降低[24]。而CD4+CD25+Treg逐渐上升,说明输注hUC-MSCs可上调肝硬化患者CD4+CD25+ Treg水平,这一结果与文献研究结果[25-26]相吻合;抗炎细胞因子IL-10、TGFβ逐渐上升,抑制了炎性反应的进一步加重,防止肝细胞持续损伤、坏死,同时间接地调控肝组织的进一步纤维化[24]。提示hUCB-MNCs联合hUC-MSCs治疗可通过影响机体的免疫状况来调节免疫性疾病的转归,可能产生的副作用目前还在随访观察中。截至目前,11例入组治疗患者均未发现明显的不良反应。这些研究结果为hUCB-MNCs和hUC-MSCs联合疗法可以优化肝功能且抑制病情发展提供了有力的依据。
hUCB-MNCs和hUC-MSCs联合输注可以分化为肝细胞或进入肝脏后修复受损的肝细胞,抑制炎性因子释放,上调Treg水平,从而调节肝硬化患者免疫功能,改善患者一般状态。针对检测指标的改变,对其治疗肝硬化的免疫调节机制进行评价,为终末期肝病的治疗探索新方法。
-
表 1 外泌体与巨噬细胞共培养48 h流式结果
Table 1. Flow cytometry results of exosomes co-cultured with macrophages for 48 hours
分组 CD16/32(%) CD206(%) CD369(%) 48 h 72 h 48 h 72 h 48 h 72 h 对照组 99.53±0.06 99.67±0.06 90.27±0.21 85.47±0.55 2.40±0.20 6.60±0.20 10 μg/mL组 100.00±0.002) 99.83±0.061) 98.67±0.212) 96.77±0.152) 0.80±0.001) 16.67±0.452) 50 μg/mL组 100.00±0.002) 99.83±0.061) 98.70±0.202) 96.03±0.232) 4.50±0.302) 9.03±0.231) F值 196.00 8.33 1 678.00 946.90 238.40 836.90 P值 <0.000 1 <0.05 <0.000 1 <0.000 1 <0.000 1 <0.000 1 注:与对照组比较,1)P<0.05, 2)P<0.000 1。 表 2 外泌体与巨噬细胞共培养ELISA结果
Table 2. ELISA results of exosomes co-cultured with macrophages
组别 IL-6(pg/mL) TNFα(pg/mL) 48 h 72 h 48 h 72 h 对照组 58.53±15.52 76.22±9.68 320.70±5.30 323.90±87.37 10 μg/mL组 52.19±13.16 76.46±6.03 324.70±33.48 164.20±14.171) 50 μg/mL组 98.81±15.551) 99.52±8.351) 319.60±15.91 342.70±52.79 F值 8.77 8.07 0.05 8.15 P值 <0.05 <0.05 0.95 <0.05 注:与对照组比较,1)P<0.05。 表 3 M2/M1型巨噬细胞相关分子比值
Table 3. The ratio of M2/M1 macrophage-associated molecules
组别 CD206/(CD16/32) CD369/(CD16/32) 48 h 72 h 48 h 72 h 对照组 0.907±0.002 0.858±0.005 0.024±0.002 0.066±0.002 10 μg/mL组 0.987±0.0021) 0.969±0.0021) 0.008±0.0001) 0.169±0.0081) 50 μg/mL组 0.987±0.0021) 0.962±0.0031) 0.045±0.0031) 0.090±0.0022) F值 1 767.0 953.6 237.5 378.9 P值 <0.000 1 <0.000 1 <0.000 1 <0.000 1 注:与对照组比较,1)P<0.000 1,2)P<0.001。 -
[1] Sichuan Hydatid Disease Clinical Medical Research Center, Hydatid Disease Professional Committee of Sichuan Medical Association. Expert consensus on diagnosis and treatment of alveolar hepatic echinococcosis(2020 version)[J]. Chin J Bases Clin Gen Surg, 2020, 27(1): 13-17. DOI: 10.7507/1007-9424.201911105.四川省包虫病临床医学研究中心, 四川省医师协会包虫病专业委员会. 泡型肝包虫病诊疗专家共识(2020版)[J]. 中国普外基础与临床杂志, 2020, 27(1): 13-17. DOI: 10.7507/1007-9424.201911105. [2] SCHULTZE JL, SCHMIDT SV. Molecular features of macrophage activation[J]. Semin Immunol, 2015, 27(6): 416-423. DOI: 10.1016/j.smim.2016.03.009. [3] SCHULTZE JL, FREEMAN T, HUME DA, et al. A transcriptional perspective on human macrophage biology[J]. Semin Immunol, 2015, 27(1): 44-50. DOI: 10.1016/j.smim.2015.02.001. [4] LI WH, ZHANG YX, ZHAO D, et al. Dectin-1 affects heart remodeling after myocardial infarction by regulating macrophage polarization[J]. Immunol J, 2021, 37(8): 692-697. DOI: 10.13431/j.cnki.immunol.j.20210096.李文华, 张一馨, 赵迪, 等. Dectin-1通过调节巨噬细胞极化影响心肌梗死后的心脏重塑[J]. 免疫学杂志, 2021, 37(8): 692-697. DOI: 10.13431/j.cnki.immunol.j.20210096. [5] MOSSER DM, EDWARDS JP. Exploring the full spectrum of macrophage activation[J]. Nat Rev Immunol, 2008, 8(12): 958-969. DOI: 10.1038/nri2448. [6] YAO T, XU ZH, YAO JY, et al. Effect of hepatocellular carcinoma cell-derived exosomes on M2 polarization of tumor-associated macrophages[J]. J Clin Hepatol, 2022, 38(3): 558-562. DOI: 10.3969/j.issn.1001-5256.2022.03.013.姚涛, 徐植红, 姚纪友, 等. 肝癌细胞来源外泌体对肿瘤相关M2型巨噬细胞极化的影响[J]. 临床肝胆病杂志, 2022, 38(3): 558-562. DOI: 10.3969/j.issn.1001-5256.2022.03.013. [7] van NIEL G, D'ANGELO G, RAPOSO G. Shedding light on the cell biology of extracellular vesicles[J]. Nat Rev Mol Cell Biol, 2018, 19(4): 213-228. DOI: 10.1038/nrm.2017.125. [8] GRUBOR NM, JOVANOVA-NESIC KD, SHOENFELD Y. Liver cystic echinococcosis and human host immune and autoimmune follow-up: A review[J]. World J Hepatol, 2017, 9(30): 1176-1189. DOI: 10.4254/wjh.v9.i30.1176. [9] WU Z, WANG L, LI J, et al. Extracellular vesicle-mediated communication within host-parasite interactions[J]. Front Immunol, 2018, 9: 3066. DOI: 10.3389/fimmu.2018.03066. [10] WANG L, LI Z, SHEN J, et al. Exosome-like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune- activity of macrophage[J]. Parasitol Res, 2015, 114(5): 1865-1873. DOI: 10.1007/s00436-015-4373-7. [11] ZAMANIAN M, FRASER LM, AGBEDANU PN, et al. Release of small RNA-containing exosome-like vesicles from the human filarial parasite brugia malayi[J]. PLoS Negl Trop Dis, 2015, 9(9): e0004069. DOI: 10.1371/journal.pntd.0004069. [12] LI Y, LIU Y, XIU F, et al. Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses[J]. Int J Nanomedicine, 2018, 13: 467-477. DOI: 10.2147/IJN.S151110. [13] PAN BT, JOHNSTONE RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor[J]. Cell, 1983, 33(3): 967-978. DOI: 10.1016/0092-8674(83)90040-5. [14] HESSVIK NP, LLORENTE A. Current knowledge on exosome biogenesis and release[J]. Cell Mol Life Sci, 2018, 75(2): 193-208. DOI: 10.1007/s00018-017-2595-9. [15] RAPOSO G, NIJMAN HW, STOORVOGEL W, et al. B lymphocytes secrete antigen-presenting vesicles[J]. J Exp Med, 1996, 183(3): 1161-1172. DOI: 10.1084/jem.183.3.1161. [16] COAKLEY G, MAIZELS RM, BUCK AH. Exosomes and other extracellular vesicles: The new communicators in parasite infections[J]. Trends Parasitol, 2015, 31(10): 477-489. DOI: 10.1016/j.pt.2015.06.009. [17] COAKLEY G, BUCK AH, MAIZELS RM. Host parasite communications-Messages from helminths for the immune system: Parasite communication and cell-cell interactions[J]. Mol Biochem Parasitol, 2016, 208(1): 33-40. DOI: 10.1016/j.molbiopara.2016.06.003. [18] SHAPOURI-MOGHADDAM A, MOHAMMADIAN S, VAZINI H, et al. Macrophage plasticity, polarization, and function in health and disease[J]. J Cell Physiol, 2018, 233(9): 6425-6440. DOI: 10.1002/jcp.26429. [19] ZHANG C, LIN R, LI Z, et al. Immune exhaustion of T cells in alveolar echinococcosis patients and its reversal by blocking checkpoint receptor TIGIT in a murine model[J]. Hepatology, 2020, 71(4): 1297-1315. DOI: 10.1002/hep.30896. [20] GAO YS, ZHU MB, GUO YZ, et al. Clinical analysis on hepatic hydatid disease in Yili River Valley[J]. Chin J Parasitol Parasitic Dis, 2005, 23(1): 3-13. DOI: 10.3969/j.issn.1000-7423.2005.01.003.高永盛, 朱马拜, 郭永忠, 等. 新疆伊犁河谷肝棘球蚴病临床资料分析[J]. 中国寄生虫学与寄生虫病杂志, 2005, 23(1): 3-13. DOI: 10.3969/j.issn.1000-7423.2005.01.003. [21] WANG DX, WANG H, FAN HN, et al. Study on the role of macrophage polarization during E. multilocularis-infection in mice[J]. Chin High Altitude Med Biology, 2018, 39(2): 118-122. DOI: 10.13452/j.cnki.jqmc.2018.02.010.王东旭, 王虎, 樊海宁, 等. 巨噬细胞极化在小鼠泡型包虫病中的作用[J]. 中国高原医学与生物学杂志, 2018, 39(2): 118-122. DOI: 10.13452/j.cnki.jqmc.2018.02.010. 期刊类型引用(8)
1. 刘晓敏,祁冉,郑玉峰,蒋珍,张腊梅. 影响肝硬化失代偿患者人脐带间充质干细胞治疗效果的影响因素探究. 罕少疾病杂志. 2024(04): 48-50 .  百度学术
百度学术2. 袁雪青,朱梅. 间充质干细胞修复大鼠子宫切口瘢痕缺陷的实验研究. 国际医药卫生导报. 2024(14): 2343-2347 .  百度学术
百度学术3. 汤艳,沈爱春. 恩替卡韦联合复方鳖甲软肝片在慢性乙型肝炎肝硬化患者中的治疗效果. 中国医学创新. 2024(29): 64-68 .  百度学术
百度学术4. 陈刚,谷光丽,何映雪,唐尧. 血清表皮生长因子对肝硬化食管胃静脉曲张患者再出血的诊断价值分析. 转化医学杂志. 2024(10): 1698-1702 .  百度学术
百度学术5. 杨明霞. 产前乙肝病毒检查对母婴结局的临床意义分析. 中国现代药物应用. 2023(09): 76-79 .  百度学术
百度学术6. 杨玉伟,李万里,陈继冰,丰丙政,徐之然,吴玲玲,武桢,顾新伟,高宏君. 间充质干细胞包裹人胰岛减轻即刻经血液介导的炎症反应的体外研究. 器官移植. 2023(04): 562-569 .  百度学术
百度学术7. 梅小利,杜伟,吴思澜,杨雪,于冰,李聪,黄崇刚. 人脐带间充质干细胞连续静脉注射对大鼠的安全性及肝脏的影响. 西部医学. 2023(07): 959-963 .  百度学术
百度学术8. 李丹丹,王艳南,王品鸽. 舒肝宁联合富马酸替诺福韦二吡呋酯对慢性乙肝肝硬化患者免疫功能及肝功能的影响. 内蒙古医学杂志. 2022(10): 1177-1180 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 5713 KB)
PDF下载 ( 5713 KB)

 下载:
下载:




 下载:
下载:





 百度学术
百度学术