富马酸丙酚替诺福韦治疗青岛地区60岁及以上慢性乙型肝炎患者的有效性和安全性
DOI: 10.3969/j.issn.1001-5256.2023.05.010
Efficacy and safety of tenofovir alafenamide fumarate in treatment of chronic hepatitis B patients aged ≥60 years in Qingdao, China
-
摘要:
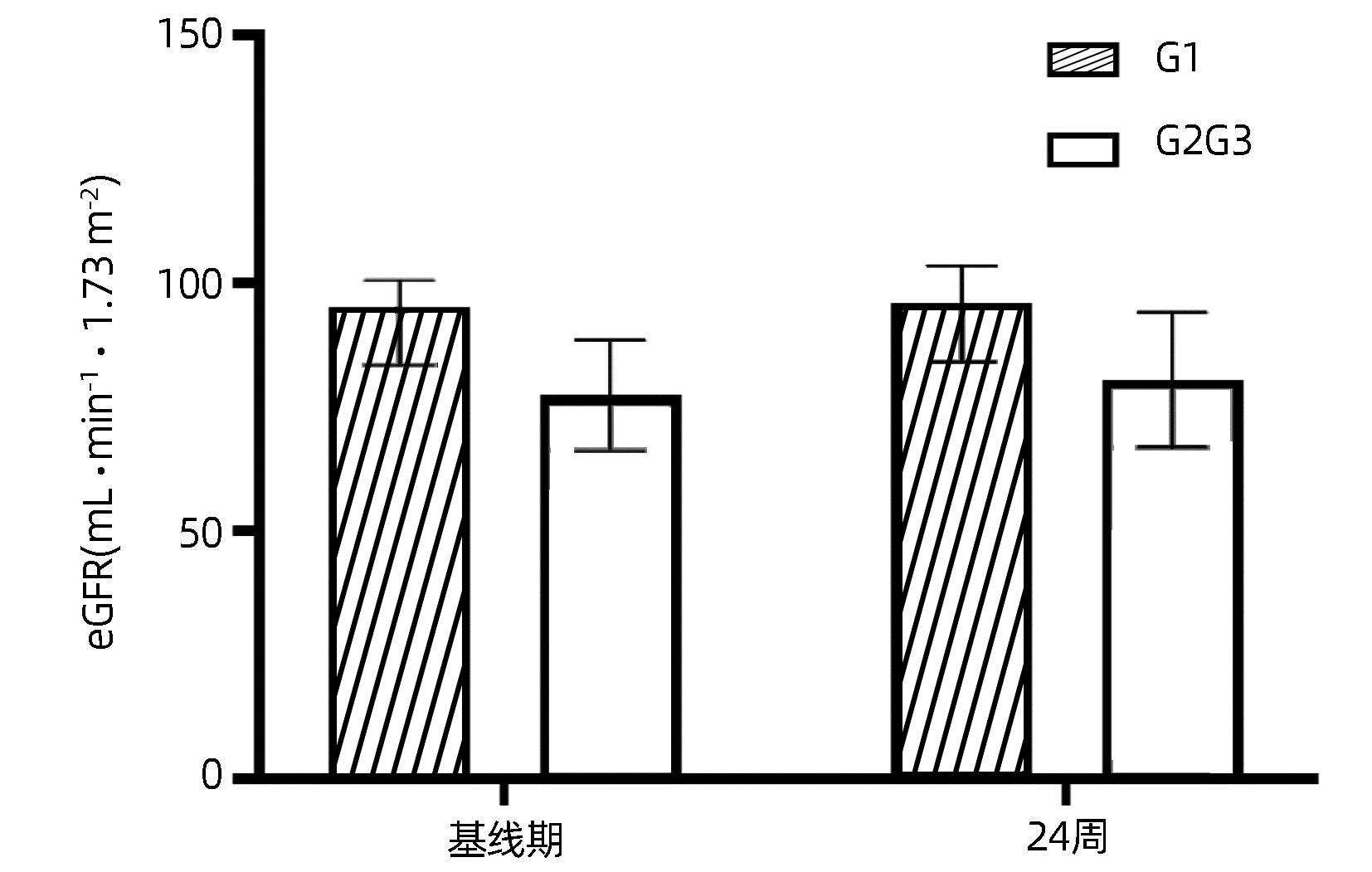
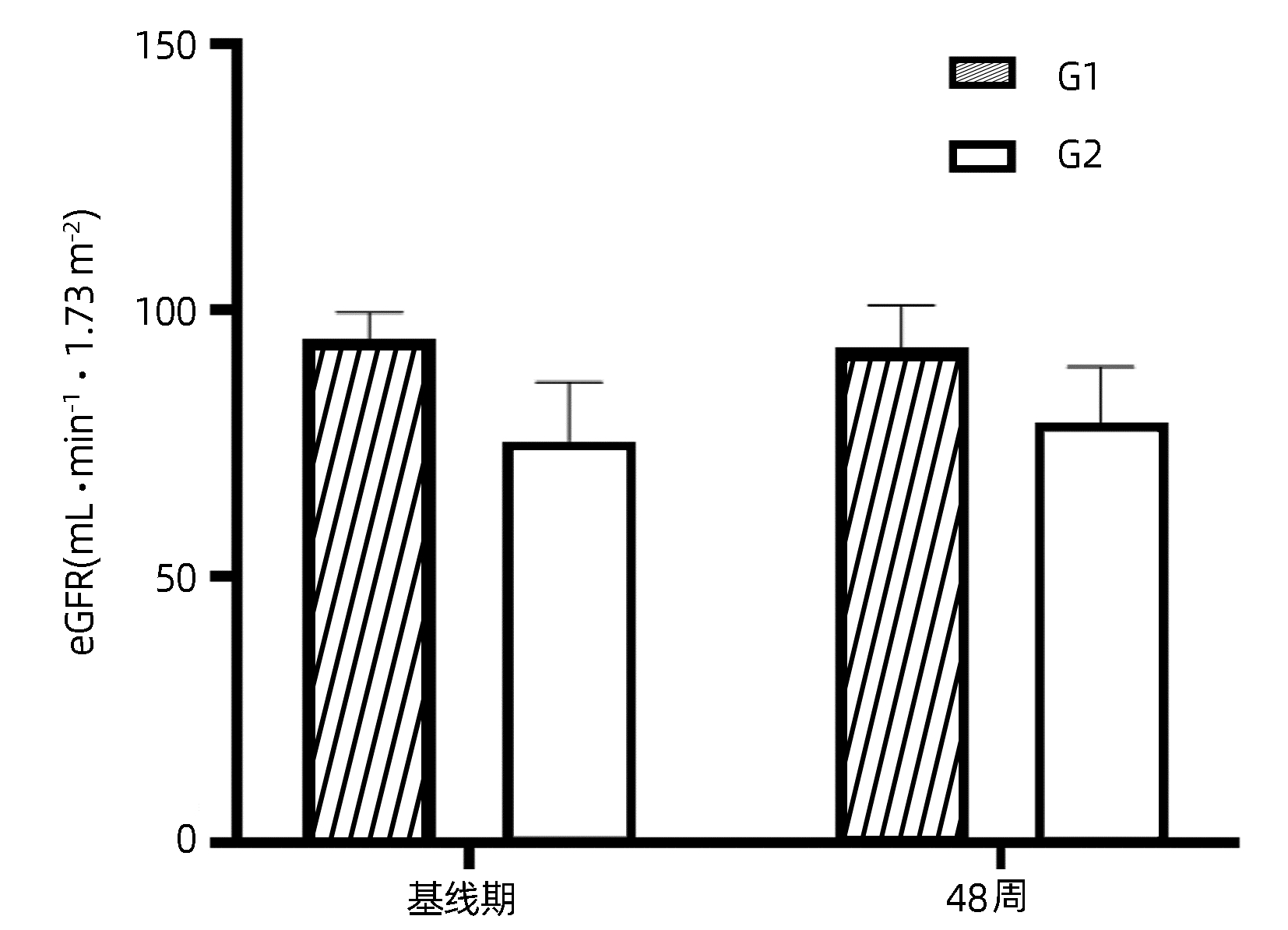
目的 探讨富马酸丙酚替诺福韦(TAF)在老年慢性乙型肝炎(CHB)患者中的应用价值,明确其对骨骼和肾脏的影响。 方法 选取2021年6月—2022年10月在青岛市市立医院、青岛大学附属医院、青岛市第六人民医院、青岛市城阳人民医院、青岛市即墨人民医院接受TAF抗病毒治疗的60岁及以上CHB患者36例。所有患者接受TAF(25 mg /d)抗病毒治疗。收集患者基线期、48周病毒学指标、生化指标、尿蛋白电泳指标以及甲胎蛋白(AFP)、肝脏超声瞬时弹性成像测定(FibroScan)、骨密度测定数据。收集24周病毒学指标,生化指标,尿蛋白电泳指标。正态分布计量资料治疗前后进行配对t检验,非正态分布计量资料治疗前后比较采用Wilcoxon符号秩和检验。计数资料的比较采用χ2检验或Fisher确切概率法。 结果 36例CHB患者完成了24周随访。TAF治疗24周后,完全病毒学应答率为83.3%(30/36),与基线期77.8%(28/36)比较,差异无统计学意义(χ2=0.36, P=0.55)。24周DBil(t=-2.42, P=0.02)、Cys C(t=-4.34, P<0.001) 显著下降,与基线比较差异均有统计学意义。18例CHB患者完成了48周随访。治疗48周后,完全病毒学应答率为94.4%(17/18),与基线期77.8%(14/18)比较,差异无统计学意义(χ2=2.22, P=0.34);IBil(t=2.43,P=0.03)和TBA(Z=-2.24,P=0.03)均升高,腰椎(t=2.92,P=0.01)及股骨颈骨密度(t=2.42, P=0.03)T评分均升高,LSM水平降低(t=-2.31, P=0.03),与基线比较差异均有统计学意义。β2-MG、URBP、α1-MG在治疗前后差异均无统计学意义(P值均>0.05)。 结论 TAF在60岁及以上CHB患者中有良好的抗病毒效果,使更多CHB患者达到完全病毒学应答,未发现对肾脏造成损害,能够改善骨密度及肝纤维化程度。 Abstract:Objective To investigate the application value of tenofovir alafenamide fumarate (TAF) in elderly patients with chronic hepatitis B (CHB) and its influence on bones and kidneys. Methods A total of 36 CHB patients, aged ≥60 years, who received TAF antiviral therapy in Qingdao Municipal Hospital, The Affiliated Hospital of Qingdao University, Qingdao Sixth People's Hospital, Chengyang People's Hospital, and Jimo People's Hospital from June 2021 to October 2022 were enrolled in this study, and all patients received TAF (25 mg/d) antiviral therapy. Related data were collected at baseline and weeks 24 and 48 of treatment, including virological indicators, biochemical parameters, urinary protein electrophoresis indices, transient elastography (FibroScan), and bone mineral density. Virological indicators included high-sensitivity HBV DNA quantification; biochemical parameters included total bilirubin, direct bilirubin (DBil), indirect bilirubin (IBil), alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, total bile acid (TBA), glucose, blood urea nitrogen, creatinine, estimated glomerular filtration rate, and cystatin C (Cys C); urinary protein electrophoresis indices included urinary β2 microglobulin (β2-MG), urinary retinol (URBP), and urinary α1 microspherin (α1-MG). The paired t-test was used for comparison of normally distributed continuous data before and after treatment, and the Wilcoxon signed-rank test was used for comparison of non-normally distributed continuous data before and after treatment; the chi-square test or the Fisher's exact test was used for comparison of categorical data. Results A total of 36 CHB patients completed 24 weeks of follow-up. The complete virological response rate after 24 weeks of treatment was higher than that at baseline [83.3% (30/36) vs 77.8% (28/36), χ2=0.36, P=0.55], and there were significant reductions in DBil (t=-2.42, P=0.02) and Cys C (t=-4.34, P < 0.001) from baseline to week 24. A total of 18 CHB patients completed 48 weeks of follow-up. The complete virological response rate after 48 weeks of treatment was higher than that at baseline (94.4% vs 77.8%, χ2=2.22, P=0.34), and there were significant increases in IBil (t=2.43, P=0.03), TBA (Z=-2.24, P=0.03), and bone mineral density T score of lumbar vertebra (t=2.92, P= 0.01) and femoral neck (t=2.42, P=0.03) and a significant reduction in liver stiffness measurement (t=-2.31, P=0.03). There were no significant changes in β2-MG, URBP, and α1-MG after treatment (all P > 0.05). Conclusion TAF has a good antiviral effect in CHB patients aged ≥60 years and can help more CHB patients achieve complete virological response, without causing damage to the kidney, and it can also improve bone mineral density and liver fibrosis degree. -
Key words:
- Hepatitis B, Chronic /
- Tenofovir Alafenemide Fumarate /
- Treatment Outcome /
- Aged
-
表 1 受试者基线临床特征
Table 1. Baseline clinical characteristics of subjects
指标 结果 年龄(岁) 64.67±3.82 男性[例(%)] 19(52.80) BMI(kg/m2) 24.81±3.67 BMI分级[例(%)] 低体质量 3(8.30) 正常 13(36.10) 超重 14(38.90) 肥胖 6(16.70) 完全病毒学应答[例(%)] 28(77.80) 合并疾病[例(%)] 高血压病 11(30.60) 糖尿病 4(11.10) 冠心病 3(8.30) HBeAg阳性[例(%)] 11(30.60) HBsAg(IU/mL) 752.35(212.14~2 908.23) TBil(μmol/L) 14.10(12.30~20.40) DBil(μmol/L) 4.08±1.68 IBil(μmol/L) 10.80(8.60~15.20) ALT(U/L) 22.18(16.90~30.52) AST(U/L) 23.71(20.02~32.53) ALP(U/L) 79.57±26.12 GGT(U/L) 23.46(17.72~33.25) TBA(μmol/L) 2.40(1.90~4.20) GLu(mmol/L) 5.68(5.11~6.94) BUN(mmol/L) 5.92±1.07 eGFR(mL·min-1·1.73 m-2) 77.30(62.07~84.65) Cr(μmol/L) 74.97±16.21 Cys C(mg/L) 0.95±0.22 AFP(IU/mL) 1.88(1.40~2.62) CAP(dB/m) 240.08±43.94 CAP分级[例(%)] 正常 15(41.70) 轻度脂肪肝 13(36.10) 中度脂肪肝 5(13.90) 重度脂肪肝 3(8.30) LSM(kPa) 6.50(5.65~8.75) LSM分级[例(%)] S0 25(69.40) S1 3(8.30) S2 4(11.10) S3 4(11.10) 骨密度[例(%)] 正常 12(34.30) 骨量减少 13(37.10) 骨质疏松 10(28.60) 表 2 TAF治疗基线期与24周数据对比
Table 2. Comparison of data at baseline and 24 weeks of TAF treatment
指标 基线期 24周 统计值 P值 TBil(μmol/L) 14.05(12.30~20.38) 16.45(12.35~19.58) Z=0.36 0.73 DBil(μmol/L) 4.08±1.68 3.53±1.41 t=-2.42 0.02 IBil(μmol/L) 10.80(8.63~15.20) 12.90(9.55~15.61) Z=1.35 0.19 ALT(U/L) 22.18(16.90~30.52) 22.74(15.87~33.13) Z=-1.34 0.18 AST(U/L) 23.71(20.02~32.53) 22.93(19.33~31.45) Z=-1.23 0.22 ALP(U/L) 79.57±26.12 79.45±21.28 t=-0.05 0.97 GGT(U/L) 23.46(17.72~33.25) 22(16.32~27.96) Z=-0.85 0.39 TBA(μmol/L) 2.50(1.89~4.70) 2.60(1.20~4.40) Z=-0.69 0.49 GLu(mmol/L) 5.70(5.10~6.96) 5.64(5.12~6.36) Z=-1.01 0.32 BUN(mmol/L) 5.92±1.07 5.99±1.06 t=0.30 0.77 eGFR(mL·min-1·1.73 m-2) 84.25±12.78 86.53±13.85 t=1.84 0.08 Cr(μmol/L) 74.97±16.21 72.64±19.28 t=-1.65 0.11 Cys C(mg/L) 0.94±0.22 0.86±0.24 t=-4.34 <0.001 表 3 TAF治疗基线期与48周数据对比
Table 3. Comparison of data at baseline and 48 weeks of TAF treatment
指标 基线期 48周 统计值 P值 HBsAg(IU/mL) 500.43(161.33~2 209.89) 387.29(134.69~2 064.71) Z=-1.93 0.05 TBil(μmol/L) 14.45(12.30~21.33) 16.75(13.03~22.08) Z=1.44 0.17 DBil(μmol/L) 4.20(3.25~5.69) 3.38(2.70~5.05) Z=-1.59 0.11 IBil(μmol/L) 11.92±4.88 14.53±5.30 t=2.43 0.03 ALT (U/L) 27.36(16.97~35.05) 23.83(16.00~32.37) Z=-0.98 0.33 AST(U/L) 24.71(20.54~40.65) 22.65(19.89~30.47) Z=-1.07 0.29 ALP(U/L) 75.14±24.65 81.66±20.61 t=1.57 0.14 GGT(U/L) 21.04(17.80~34.06) 24.43(15.72~34.46) Z=-0.07 0.94 TBA(μmol/L) 2.30(1.90~2.67) 3.00(1.31~4.55) Z=-2.24 0.03 GLu(mmol/L) 5.66(5.15~6.56) 5.41(4.90~5.97) t=-1.18 0.26 BUN(mmol/L) 5.96±1.28 5.65±1.41 t=-0.87 0.40 eGFR(mL·min-1·1.73 m-2) 85.06±12.89 85.89±11.62 t=0.44 0.67 Cr(μmol/L) 72.76±17.19 70.82±17.08 t=-1.06 0.30 Cys C(mg/L) 0.90±0.15 0.86±0.18 t=-2.02 0.07 AFP(IU/mL) 1.82(1.37~2.26) 1.67(1.04~2.40) Z=-1.94 0.05 CAP(dB/m) 241.00±39.35 269.00±52.62 t=1.84 0.08 LSM(kPa) 7.57±2.62 6.20±2.05 t=-2.31 0.03 腰椎骨密度(SD) -0.99±1.80 -0.68±1.75 t=2.92 0.01 股骨颈骨密度(SD) -1.34±0.98 -1.14±0.90 t=2.42 0.03 -
[1] WANG H, MEN P, XIAO Y, et al. Hepatitis B infection in the general population of China: a systematic review and meta-analysis[J]. BMC Infect Dis, 2019, 19(1): 811. DOI: 10.1186/s12879-019-4428-y. [2] LIU J, ZHANG S, WANG Q, et al. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: a population-based, cross-sectional study[J]. Lancet Infect Dis, 2016, 16(1): 80-86. DOI: 10.1016/S1473-3099(15)00218-2. [3] LIU Z, LI M, HUTTON DW, et al. Impact of the national hepatitis B immunization program in China: a modeling study[J]. Infect Dis Poverty, 2022, 11(1): 106. DOI: 10.1186/s40249-022-01032-5. [4] ZHU JY, GAO M, SONG QY, et al. Prevalence of osteoporosis in Chinese elderly people: a Meta-analysis[J]. Chin Gen Pract, 2022, 25(3): 346-353. DOI: 10.12114/j.issn.1007-9572.2021.02.083朱洁云, 高敏, 宋秋韵, 等. 中国老年人骨质疏松症患病率的Meta分析[J]. 中国全科医学, 2022, 25(3): 346-353. DOI: 10.12114/j.issn.1007-9572.2021.02.083 [5] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [6] AGARWAL K, BRUNETTO M, SETO WK, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection[J]. J Hepatol, 2018, 68(4): 672-681. DOI: 10.1016/j.jhep.2017.11.039. [7] Compilation committee of Chinese expert consensus on medical nutritional therapy for overweight/obesity. Chinese expert consensus on medical nutritional therapy for overweight/obesity (2016)[J]. Chin J Diabetes Mellitus, 2016, 8(9): 525-540. DOI: 10.3760/cma.j.issn.1674-5809.2016.09.004.中国超重肥胖医学营养治疗专家共识编写委员会. 中国超重/肥胖医学营养治疗专家共识(2016年版)[J]. 中华糖尿病杂志, 2016, 8(9): 525-540. DOI: 10.3760/cma.j.issn.1674-5809.2016.09.004. [8] NGUYEN MH, ATSUKAWA M, ISHIKAWA T, et al. Outcomes of sequential therapy with tenofovir alafenamide after long-term entecavir[J]. Am J Gastroenterol, 2021, 116(6): 1264-1273. DOI: 10.14309/ajg.0000000000001157. [9] FONG TL, LEE BT, TIEN A, et al. Improvement of bone mineral density and markers of proximal renal tubular function in chronic hepatitis B patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide[J]. J Viral Hepat, 2019, 26(5): 561-567. DOI: 10.1111/jvh.13053. [10] LEE BT, CHANG M, LIM C, et al. Bone and renal safety profile at 72 weeks after switching to tenofovir alafenamide in chronic hepatitis B patients[J]. JGH Open, 2021, 5(2): 258-263. DOI: 10.1002/jgh3.12481. [11] BUTI M, GANE E, SETO WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial[J]. Lancet Gastroenterol Hepatol, 2016, 1(3): 196-206. DOI: 10.1016/S2468-1253(16)30107-8. [12] CHAN HL, FUNG S, SETO WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial[J]. Lancet Gastroenterol Hepatol, 2016, 1(3): 185-195. DOI: 10.1016/S2468-1253(16)30024-3. [13] PAPATHEODORIDIS GV, MIMIDIS K, MANOLAKOPOULOS S, et al. HERACLIS-TAF: a multi-centre prospective cohort study on 2-year safety and efficacy of tenofovir alafenamide in patients with chronic hepatitis B with renal and/or bone disorders or risks[J]. Aliment Pharmacol Ther, 2022, 56(4): 702-712. DOI: 10.1111/apt.17093. [14] SURIAL B, BEGUELIN C, CHAVE JP, et al. Brief report: switching from TDF to TAF in HIV/HBV-coinfected individuals with renal dysfunction-a prospective cohort study[J]. J Acquir Immune Defic Syndr, 2020, 85(2): 227-232. DOI: 10.1097/QAI.0000000000002429 [15] LUO X, QU Y, CAI XB, et al. Effects of antiviral therapy on the reversal of liver fibrosis[J]. J Clin Hepatol, 2022, 38(11): 2596-2598. DOI: 10.3969/j.issn.1001-5256.2022.11.032.罗昕, 曲颖, 蔡晓波, 等. 抗病毒治疗对肝纤维化逆转的影响[J]. 临床肝胆病杂志, 2022, 38(11): 2596-2598. DOI: 10.3969/j.issn.1001-5256.2022.11.032. [16] CHEN P, WEI W, JIN L, et al. Efficacy and safety of tenofovir alafenamide fumarate in nucleoside analogue treatment-naïve patients with chronic hepatitis B[J]. Exp Ther Med, 2021, 22(5): 1325. DOI: 10.3892/etm.2021.10760. [17] LI C, MA Y, YANG C, et al. Association of cystatin C kidney function measures with long-term deficit-accumulation frailty trajectories and physical function decline[J]. JAMA Netw Open, 2022, 5(9): e2234208. DOI: 10.1001/jamanetworkopen.2022.34208. -



 PDF下载 ( 2730 KB)
PDF下载 ( 2730 KB)


 下载:
下载: