肝硬化合并脓毒症患者短期住院死亡的Cox比例风险预测模型建立及评价
DOI: 10.3969/j.issn.1001-5256.2023.05.014
Establishment and evaluation of a multivariate Cox proportional-hazards prediction model for mortality during short-term hospitalization in patients with liver cirrhosis and sepsis
-
摘要:
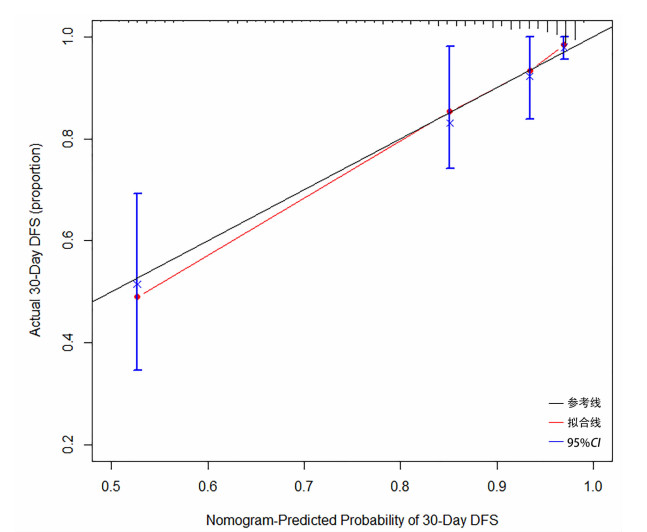
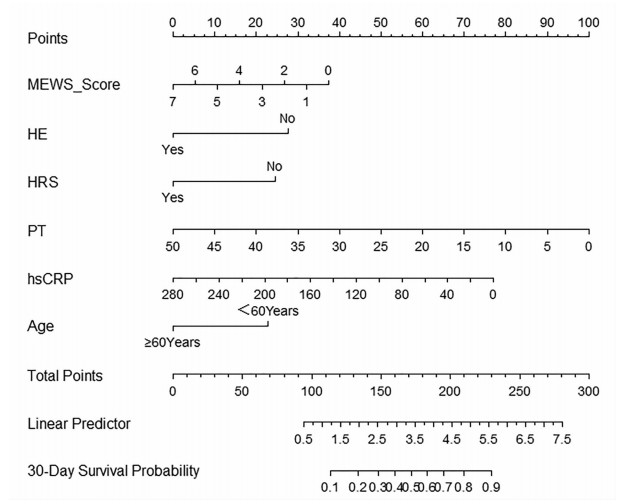
目的 构建肝硬化合并脓毒症患者短期住院死亡的Cox比例风险预测模型。 方法 回顾性收集2012年1月—2022年8月昆明市第三人民医院收治的336例肝硬化合并脓毒症患者的临床资料。依据患者短期住院期间是否死亡,分为死亡组(n=40)和生存组(n=296)。收集人口学资料、合并症及临床生化指标,比较各观察指标在两组中的差异。计量资料呈正态分布两组间比较采用成组t检验;计量资料呈偏态分布两组间比较采用Mann-Whitney U检验;计数资料两组间比较采用χ2检验。采用Cox多因素分析筛选变量,据此构建Cox比例风险预测模型并计算风险比(HR)及95%CI。计算C指数来检验其预测精度。绘制列线图可视化展示Cox比例风险预测模型,绘制校准曲线以观察模型预测结果与实际情况的符合程度。 结果 336例患者中男261例(77.7%),女75例(22.3%),平均年龄(50.0±10.6)岁。其中死亡40例,住院时间8.2~23.0天,平均住院时间(16.8±11.3)天。与生存组相比较,死亡组的年龄(≥60岁)、近2周有创操作史、消化道出血、HE、HRS、改良MEWS评分、PT、APTT、INR、D-D、CD4/CD8、Lac、WBC、NE、TBil、IL-6、PCT、hsCRP、BUN、Cr水平均较高,差异具有统计学意义(P值均<0.05);RBC、Hb、TP、白蛋白(Alb)、TC、LDL、HDL水平均较低,差异均有统计学意义(P值均<0.05)。多因素Cox回归分析,年龄(HR=2.602;95%CI:1.277~5.303,P=0.008)、HE(HR=2.516;95%CI:1.258~5.033,P=0.009)、HRS(HR=2.324;95%CI:1.010~5.349,P=0.047)、hsCRP(HR=1.008;95%CI:1.003~1.013,P=0.004)、改良早期预警评分(MEWS)(HR=1.205;95%CI:1.022~1.422,P=0.027)、PT(HR=1.076,95%CI:1.030~1.124,P=0.027)是肝硬化合并脓毒症患者死亡的独立影响因素。C-指数为0.857(95%CI:0.815~0.920),提示模型的预测准确性较高。绘制校准图提示模型预测风险与实际发生风险的一致性较好。 结论 构建的肝硬化合并脓毒症患者短期住院死亡Cox比例风险预测模型可用于预测新诊断肝硬化合并脓毒症患者在短期住院期间发生死亡的风险,从而指导临床医护人员采取针对性的干预措施,最终避免或降低患者发生死亡的可能性。 Abstract:Objective To establish a Cox proportional-hazards prediction model for mortality during short-term hospitalization in patients with liver cirrhosis and sepsis. Methods A retrospective analysis was performed for the clinical data of 336 patients with liver cirrhosis and sepsis who were admitted to The Third People's Hospital of Kunming from January 2012 to August 2022, and according to whether the patient died during short-term hospitalization, they were divided into death group with 40 patients and survival group with 296 patients. Demographic data, comorbidities, and clinical biochemical parameters were collected and compared between the two groups. The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. The multivariate Cox analysis was used for screening of variables, then a Cox proportional-hazards prediction model was established, and hazard ratio (HR) and its 95% confidence interval [CI] were calculated; C-index index was used to evaluate the prediction accuracy of the model. The Cox proportional-hazards prediction model was visualized by a nomogram, and calibration curve was plotted to evaluate the consistency between the prediction results of the model and the actual condition. Results Among the 336 patients, there were 261 male patients (77.7%) and 75 female patients (22.3%), with a mean age of 50.0±10.6 years, and 40 patients died, with a mean hospital stay of 16.8±11.3 days (range 8.2-23.0 days). Compared with the survival group, the death group had a significantly higher proportion of patients with an age of ≥60 years, a history of invasive operation within the past two weeks, gastrointestinal bleeding, hepatic encephalopathy (HE) or hepatorenal syndrome (HRS), a significantly higher Modified Early Warning Score (MEWS) score, and significantly higher levels of prothrombin time (PT), activated partial thromboplastin time, international normalized ratio, D-dimer, CD4/CD8 ratio, lactate, white blood cell count, norepinephrine, total bilirubin, interleukin-6, procalcitonin, high-sensitivity C-reactive protein (hsCRP), blood urea nitrogen, and creatinine (all P < 0.05), as well as significantly lower levels of red blood cell count, hemoglobin, albumin, total cholesterol, low-density lipoprotein, and high-density lipoprotein (all P < 0.05). The multivariate Cox regression analysis showed that age (HR=2.602, 95%CI: 1.277-5.303, P=0.008), HE (HR=2.516, 95%CI: 1.258-5.033, P=0.009), HRS (HR=2.324, 95%CI: 1.010-5.349, P=0.047), hsCRP (HR=1.008, 95%CI: 1.003-1.013, P=0.004), MEWS score (HR=1.205, 95%CI: 1.022-1.422, P=0.027), and PT (HR=1.076, 95%CI: 1.030-1.124, P=0.027) were independent influencing factors for death in patients with liver cirrhosis and sepsis. The model showed a C-index of 0.857 (95%CI: 0.815-0.920), suggesting that the model had relatively high prediction accuracy, and the calibration curve showed good consistency between the predicted risk and the actual risk. Conclusion The Cox proportional-hazards prediction model established for death during short-term hospitalization in patients with liver cirrhosis and sepsis can be used to predict the risk of death during short-term hospitalization in patients with liver cirrhosis and sepsis, thereby guiding clinical medical staff to take targeted intervention measures to avoid or reduce the possibility of death in patients. -
Key words:
- Liver Cirrhosis /
- Sepsis /
- Risk Factors /
- Proportional Hazards Models
-
表 1 两组基线特征的单因素分析
Table 1. Univariate analysis of baseline characteristics of general data
项目 生存组(n=296) 死亡组(n=40) 统计值 P值 性别[例(%)] χ2=0.19 0.665 男 231(78.0) 30(75.0) 女 65(22.0) 10(25.0) 年龄[例(%)] χ2=10.03 0.002 ≥60岁 49(16.6) 15(37.5) <60岁 247(83.4) 25(62.5) 糖尿病[例(%)] 38(12.8) 9(22.5) χ2=2.73 0.098 高血压[例(%)] 48(16.2) 5(12.5) χ2=0.37 0.545 输注人血白蛋白[例(%)] 143(48.3) 19(47.5) χ2=0.01 0.923 质子泵抑制剂[例(%)] 133(44.9) 15(37.5) χ2=0.79 0.374 近2周有创操作史[例(%)] 132(44.6) 26(65.0) χ2=5.10 0.024 腹水[例(%)] 131(44.3) 19(47.5) χ2=0.15 0.699 消化道出血[例(%)] 64(21.6) 18 (45.0) χ2=9.21 0.002 HE[例(%)] 40(13.5) 18(45.0) χ2=24.46 <0.001 HRS[例(%)] 14(4.7) 9(22.5) χ2=17.45 <0.001 改良MEWS评分 1.0(0.0 ~ 2.0) 3.0(0.0~5.0) Z=-3.36 0.001 PT(s) 17.0(14.6~20.5) 24.3(17.9~30.6) Z=-4.91 <0.001 TT(s) 19.6(17.7~21.1) 20.8(18.4~22.9) Z=-1.79 0.073 APTT(s) 41.2(35.9~46.2) 47.6(39.7~56.3) Z=-3.78 <0.001 INR 1.5(1.2~1.7) 2.1(1.5~2.5) Z=-4.60 <0.001 D-D(ug/mL) 9.7(5.3~9.7) 17.5(7.7~18.3) Z=-4.31 <0.001 CD4/CD8 1.9(1.6~1.9) 2.3(2.3~2.3) Z=-4.87 <0.001 PLT(×109/L) 83.0(55.0~132.5) 83.0(59.0~140.3) Z=-0.47 0.637 Lac (mmol/L) 3.3(2.3~3.3) 9.0(3.8~9.0) Z=-6.56 <0.001 WBC(×109/L) 5.3(3.7~7.6) 7.5(4.7~13.5) Z=-2.91 0.004 NE(×109/L) 3.4(2.2~5.9) 6.3(3.2~11.8) Z=-3.77 <0.001 LYM(×109/L) 1.0(0.5~1.5) 0.9(0.5~1.3) Z=-1.09 0.276 RBC(×109/L) 3.5(2.8~4.5) 3.2(2.5~3.9) Z=-2.36 0.018 Hb(g/L) 119.5(87.3~145.0) 107.5(73.3~127.8) Z=-2.50 0.013 TBil(μmol /L) 33.4(17.4~79.5) 74.8(34.0~206.0) Z=-3.04 0.002 ALT(U/L) 38.0(22.0~69.0) 43.0(24.8~76.0) Z=-0.58 0.559 AST(U/L) 53.0(34.0~99.7) 77.0(40.3~121.3) Z=-1.72 0.085 TP(g/L) 62.9(54.8~70.6) 56.2(48.1~65.5) Z=-3.29 0.001 Alb(g/L) 29.1(23.8~36.0) 23.8(20.2~29.4) Z=-3.87 <0.001 PAB(mg/L) 104.8(58.7~136.5) 90.2(60.9~112.4) Z=-1.59 0.111 IL-6(pg/mL) 52.7(17.6~952.8) 1 951.5(197.8~5 414.3) Z=-5.86 <0.001 PCT(ng/mL) 1.1(0.1~4.8) 2.8(0.4~14.0) Z=-3.07 0.002 hsCRP(mg/L) 14.6(2.5~29.8) 60.7(15.4~106.3) Z=-4.91 <0.001 C1q(mg/L) 176.1(138.9~194.8) 170.7(127.0~200.0) Z=-0.92 0.359 BUN(mmol/L) 5.8(4.1~9.1) 15.1(6.3~24.6) Z=-5.07 <0.001 Cr(mmol/L) 73.0(57.0~103.8) 130.5(73.5~235.0) Z=-4.36 <0.001 TG(mmol/L) 1.1(0.7~3.1) 2.2(0.7~2.4) Z=-0.87 0.385 TC(mmol/L) 2.9(2.0~3.3) 2.3(1.4~2.9) Z=-2.73 0.006 LDL(mmol/L) 1.6(1.0~1.9) 1.3(0.7~1.6) Z=-2.06 0.039 HDL(mmol/L) 0.7(0.4~1.0) 0.4(0.2~0.4) Z=-4.62 <0.001 表 2 各变量的多因素Cox比例风险回归分析
Table 2. Multivariate Cox proportional risk regression analysis of each variable
变量 β值 SE Wald HR 95%CI P值 年龄(≥60岁) 0.956 0.363 6.931 2.602 1.277~5.303 0.008 HE 0.923 0.354 6.808 2.516 1.258~5.033 0.009 HRS 0.843 0.425 3.030 2.324 1.010~5.349 0.047 hsCRP 0.008 0.003 8.466 1.008 1.003~1.013 0.004 改良MEWS评分 0.187 0.084 4.905 1.205 1.022~1.422 0.027 PT 0.073 0.022 10.662 1.076 1.030~1.124 0.001 -
[1] DELLINGER RP, LEVY MM, SCHORR CA, et al. 50 years of sepsis investigation/enlightenment among adults-the long and winding road[J]. Crit Care Med, 2021, 49(10): 1606-1625. DOI: 10.1097/CCM.0000000000005203. [2] HUANG HK, CHEN HY, HSU YC. Comparing the prognosis of patient with alcohol and nonalcohol-associated cirrhosis with bacteremia[J]. Alcohol Alcohol, 2020, 55(5): 512-517. DOI: 10.1093/alcalc/agaa057. [3] CHEN HY, HSU YC. Afebrile bacteremia in adult emergency department patients with liver cirrhosis: clinical characteristics and outcomes[J]. Sci Rep, 2020, 10(1): 7617. DOI: 10.1038/s41598-020-64644-7. [4] CHATTERJEE S, KAWAHARA R, TJONDRO HC, et al. Serum N-glycomics stratifies bacteremic patients infected with different pathogens[J]. J Clin Med, 2021, 10(3): 516. DOI: 10.3390/jcm10030516. [5] JOHNSON AL, RATNASEKERA IU, IRVINE KM, et al. Bacteraemia, sepsis and antibiotic resistance in Australian patients with cirrhosis: a population-based study[J]. BMJ Open Gastroenterol, 2021, 8(1): e000695. DOI: 10.1136/bmjgast-2021-000695. [6] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2019 version)[J]. J Clin Hepatol, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008.中华医学会肝病学分会, 中华医学会感染病学分会. 丙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008. [7] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B(2010 version)[J]. J Clin Hepatol, 2011, 27(1): 113-128. http://lcgdbzz.org/article/id/LCGD201101035中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2010年版)[J]. 临床肝胆病杂志, 2011, 27(1): 113-128. http://lcgdbzz.org/article/id/LCGD201101035 [8] Fatty Liver Expert Committee, Chinese Medical Doctor Association, National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association. Guidelines of prevention and treatment for alcoholic liver disease: a 2018 update[J]. J Clin Hepatol, 2018, 34(5): 939-946. DOI: 10.3969/j.issn.1001-5256.2018.05.006.中国医师协会脂肪性肝病专家委员会, 中华医学会肝病学分会脂肪肝和酒精性肝病学组. 酒精性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 939-946. DOI: 10.3969/j.issn.1001-5256.2018.05.006. [9] National Workshop on Fatty Liver and AlcoholicLiver Disease, Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the diagnosis and management ofprimary biliary cholangitis (2021)[J]. J Clin Hepatol, 2022, 38(1): 35-41. DOI: 10.3969/j.issn.1001-5256.2022.01.007中华医学会肝病学分会. 原发性胆汁性胆管炎的诊断和治疗指南(2021)[J]﹒临床肝胆病杂志, 2022, 38(1): 35-41. DOI: 10.3969/j.issn.1001-5256.2022.01.007 [10] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and management of autoimmune hepatitis(2015)[J]. J Clin Hepatol, 2016, 32(1): 9-22. DOI: 10.3969/j.issn.1001-5256.2016.01.002.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 自身免疫性肝炎诊断和治疗共识(2015)[J]. 临床肝胆病杂志, 2016, 32(1): 9-22. DOI: 10.3969/j.issn.1001-5256.2016.01.002. [11] SINGER M, DEUTSCHMAN CS, SEYMOUR CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. DOI: 10.1001/jama.2016.0287. [12] ZHENG RQ, ZHANG YF, RONG ZQ, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021, interpretation and expectation[J]. Chin Critical Care Med, 2021, 33(10): 1159- 1164. DOI: 10.3760/cma.j.cn121430-20211009-01442.郑瑞强, 张艺芬, 荣子琪, 等. 《拯救脓毒症运动: 脓毒症与感染性休克治疗国际指南2021版》解读与展望[J]. 中华危重病急救医学, 2021, 33(10): 1159-1164. DOI: 10.3760/cma.j.cn121430-20211009-01442. [13] FLEISCHMANN-STRUZEK C, MIKOLAJETZ A, SCHWARZKOPF D, et al. Challenges in assessing the burden of sepsis and understanding the inequalities of sepsis outcomes between National Health Systems: secular trends in sepsis and infection incidence and mortality in Germany[J]. Intensive Care Med, 2018, 44(11): 1826-1835. DOI: 10.1007/s00134-018-5377-4. [14] HASSAN EA, ABDEL REHIM AS, AHMED AO, et al. Clinical value of presepsin in comparison to hsCRP as a monitoring and early prognostic marker for sepsis in critically ill patients[J]. Medicina (Kaunas), 2019, 55(2): 36. DOI: 10.3390/medicina55020036. [15] MILBRANDT EB, ELDADAH B, NAYFIELD S, et al. Toward an integrated research agenda for critical illness in aging[J]. Am J Respir Crit Care Med, 2010, 182(8): 995-1003. DOI: 10.1164/rccm.200904-0630CP. [16] SCHMITT F, MANOLOV V, MORGENSTERN J, et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study[J]. Ann Intensive Care, 2019, 9(1): 19. DOI: 10.1186/s13613-019-0499-6. [17] VAN VUGHT LA, UHEL F, DING C, et al. Consumptive coagulopathy is associated with a disturbed host response in patients with sepsis[J]. J Thromb Haemost, 2021, 19(4): 1049-1063. DOI: 10.1111/jth.15246. [18] BATES SM. D-dimer assays in diagnosis and management of thrombotic and bleeding disorders[J]. Semin Thromb Hemost, 2012, 38(7): 673-682. DOI: 10.1055/s-0032-1326782. [19] INNOCENTI F, GORI AM, GIUSTI B, et al. Prognostic value of sepsis-induced coagulation abnormalities: an early assessment in the emergency department[J]. Intern Emerg Med, 2019, 14(3): 459-466. DOI: 10.1007/s11739-018-1990-z. [20] BAKKER J. Lost in translation: on lactate, hypotension, sepsis- induced tissue hypoperfusion, quantitative resuscitation and surviving sepsis campaign bundles[J]. Crit Care Med, 2015, 43(3): 705-706. DOI: 10.1097/CCM.0000000000000870. [21] BOHRA A, WORLAND T, HUI S, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis treated with current standards of care[J]. World J Gastroenterol, 2020, 26(18): 2221-2231. DOI: 10.3748/wjg.v26.i18.2221. [22] KROUPINA K, BÉMEUR C, ROSE CF. Amino acids, ammonia, and hepatic encephalopathy[J]. Anal Biochem, 2022, 649: 114696. DOI: 10.1016/j.ab.2022.114696. [23] TRANAH TH, BALLESTER MP, CARBONELL-ASINS JA, et al. Plasma ammonia levels predict hospitalisation with liver-related complications and mortality in clinically stable outpatients with cirrhosis[J]. J Hepatol, 2022, 77(6): 1554-1563. DOI: 10.1016/j.jhep.2022.07.014. [24] ARORA V, MAIWALL R, RAJAN V, et al. Terlipressin is superior to noradrenaline in the management of acute kidney injury in acute on chronic liver failure[J]. Hepatology, 2020, 71(2): 600-610. DOI: 10.1002/hep.30208. [25] FEBRES ALDANA C, POPPITI RJ. Cholangitis lenta causing bile cast nephropathy: A unique model of hepatorenal failure in sepsis[J]. Fetal Pediatr Pathol, 2018, 37(6): 424-432. DOI: 10.1080/15513815.2018.1520945. [26] GINÈS P, FERNÁNDEZ J, DURAND F, et al. Management of critically-ill cirrhotic patients[J]. J Hepatol, 2012, 56 (Suppl 1): S13-24. DOI: 10.1093/ckj/sfac025. [27] BARLOW B, PONNALURI S, BARLOW A, et al. Targeting the gut microbiome in the management of sepsis-associated encephalopathy[J]. Front Neurol, 2022, 13: 999035. DOI: 10.3389/fneur.2022.999035. [28] ALI WA, BAZAN NS, ELBERRY AA, et al. A randomized trial to compare procalcitonin and C-reactive protein in assessing severity of sepsis and in guiding antibacterial therapy in Egyptian critically ill patients[J]. Ir J Med Sci, 2021, 190(4): 1487-1495. DOI: 10.1007/s11845-020-02494-y. [29] GREALISH M, CHIEW AL, VARNDELL W, Depczynski B. The relationship between admission glucose and lactate with critical illness amongst adult patients presenting to the emergency department[J]. Acta Diabetol, 2021, 58(10): 1343-1349. DOI: 10.1007/s00592-021-01725-7. [30] GÖK R, GÖK A, BULUT M. Assessing prognosis with modified early warning score, rapid emergency medicine score and worthing physiological scoring system in patients admitted to intensive care unit from emergency department[J]. Int Emerg Nurs, 2019, 43: 9-14. DOI: 10.1016/j.ienj.2018.06.002. [31] TANG R, SHI LP, WEI YY, et al. Correlation analysis of MEWS, length of stay in lCU and death of patients who were unplanned transferred to lCU before transfer[J]. Nursing Prac Res, 2022, 19(2): 179-183. DOI: 10.3969/j.issn.1672-9676.2022.02.005.唐蓉, 石兰萍, 魏莹莹, 等. 非计划性转入ICU患者转入前MEWS与ICU住院时长及死亡情况的相关性分析[J]. 护理实践与研究, 2022, 19(2): 179-183. DOI: 10.3969/j.issn.1672-9676.2022.02.005. [32] KHAN A, SARMA D, GOWDA C, et al. The Role of Modified Early Warning Score (MEWS) in the prognosis of acute pancreatitis[J]. Oman Med J, 2021, 36(3): e272. DOI: 10.5001/omj.2021.72. -



 PDF下载 ( 2199 KB)
PDF下载 ( 2199 KB)

 下载:
下载: