Toll样受体4在对乙酰氨基酚致小鼠肝损伤过程中对肝脏再生的影响
DOI: 10.3969/j.issn.1001-5256.2023.05.017
Effect of Toll-like receptor 4 on liver regeneration during acetaminophen-induced liver injury in mice
-
摘要:
目的 观察抑制Toll样受体4(TLR4)是否影响对乙酰氨基酚(APAP)致小鼠肝损伤过程中的肝脏再生,初步探讨TLR4参与肝脏再生的机制。 方法 将78只雄性CD-1小鼠采用随机数字表法分为9组,其中对照组(正常对照组、溶剂对照组、抑制剂对照组)每组6只,实验组(APAP 24 h组、TAK-242+APAP 24 h组、APAP 48 h组、TAK-242+APAP 48 h组、APAP 72 h组、TAK-242+APAP 72 h组)每组10只。实验组小鼠给予单剂量腹腔注射APAP(300 mg/kg),TAK-242在APAP注射前3 h以3 mg/kg剂量腹腔注射。在不同时间点收集各组小鼠血清和肝脏组织。采用生化方法检测小鼠血清ALT水平,HE染色检测肝脏组织病理改变。RT-PCR、Western blot、免疫组化方法检测Cyclin D1、PCNA、Ki-67、STAT3、p-STAT3的表达。正态分布的计量资料两组间比较采用成组t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。非正态分布的计量资料两组间比较采用Mann-Whitney U检验;多组间比较及进一步两两比较均采用Kruskal-Wallis H检验。 结果 与正常对照组相比,APAP 24 h组和APAP 48 h组的血清ALT水平均明显较高(P值均<0.05);TAK-242+APAP 24 h和48 h组的血清ALT水平均明显高于同时间点APAP组(P值均<0.05)。HE染色结果显示,APAP处理的小鼠肝脏可见典型的小叶中心性坏死,TAK-242+APAP 24 h和48 h组小鼠肝脏的坏死面积均显著大于同时间点APAP组(P值均<0.05)。RT-PCR、Western blot、免疫组化结果显示,TAK-242+APAP 24 h、48 h和72 h组Cyclin D1 mRNA和蛋白表达水平均明显低于同时间点的APAP组(P值均<0.05);TAK-242+APAP 24 h、48 h和72 h组PCNA mRNA表达水平均明显低于同时间点的APAP组(P值均<0.05),TAK-242+APAP 24 h和48 h组PCNA蛋白表达水平均明显低于同时间点的APAP组(P值均<0.05);TAK-242+APAP 24 h和72 h组Ki-67 mRNA表达水平均明显低于同时间点的APAP组(P值均<0.05),TAK-242+APAP 24 h、48 h和72 h组Ki-67蛋白表达水平均明显低于同时间点的APAP组(P值均<0.05)。此外,TAK-242+APAP 24 h和48 h组STAT3磷酸化水平均显著低于同时点的APAP组(P值均<0.05)。 结论 TLR4可能通过提高STAT3磷酸化水平促进APAP诱导的小鼠肝损伤过程中的肝脏再生。 -
关键词:
- 化学性与药物性肝损伤 /
- 醋氨酚 /
- Toll样受体4 /
- 肝再生 /
- 小鼠, 近交ICR
Abstract:Objective To investigate whether Toll-like receptor 4 (TLR4) inhibition affects liver regeneration during acetaminophen (APAP)-induced liver injury in mice, as well as the mechanism of TLR4 involved in liver regeneration. Methods A total of 78 male CD-1 mice were divided into nine groups using a random number table, i.e., three control groups (normal control group, solvent control group, inhibitor control group) with 6 mice in each group and six experimental groups (APAP 24-hour group, TAK-242+APAP 24-hour group, APAP 48-hour group, TAK-242+APAP 48-hour group, APAP 72-hour group, TAK-242+APAP 72-hour group) with 10 mice in each group. The mice in the experimental groups were given a single dose of intraperitoneally injected APAP (300 mg/kg), and TAK-242 was intraperitoneally injected at a dose of 3 mg/kg at 3 hours before APAP administration. Serum and liver tissue samples were collected at different time points. The biochemical method was used to measure the serum level of alanine aminotransferase (ALT); HE staining was used to observe liver pathological changes; RT-PCR, Western blot, and immunohistochemistry were used to measure the expression levels of Cyclin D1, PCNA, Ki-67, STAT3, and p-STAT3. The t-test was used for comparison of normally distributed continuous data between two groups; a one-way analysis of variance was used for comparison between multiple groups, and the least significant difference t-test was used for further comparison between two groups. The Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups, and the Kruskal-Wallis H test was used for comparison between multiple groups and further comparison between two groups. Results Compared with the normal control group, the APAP 24-hour and 48-hour groups had a significantly higher serum level of ALT (both P < 0.05), and the TAK-242+APAP 24-hour and 48-hour groups had a significantly higher serum level of ALT than the APAP group at the same time point (both P < 0.05). HE staining showed typical central lobular necrosis in the liver of APAP-treated mice, and the TAK-242+APAP 24-hour and 48-hour groups had a significantly larger necrotic area than the APAP group at the same time point (both P < 0.05). RT-PCR, Western blot, and immunohistochemistry showed that the TAK-242+APAP 24-hour, 48-hour, and 72-hour groups had significantly lower mRNA and protein expression levels of Cyclin D1 than the APAP group at the same time point (all P < 0.05); the TAK-242+APAP 24-hour, 48-hour, and 72-hour groups had a significantly lower mRNA expression level of PCNA than the APAP group at the same time point (all P < 0.05), and the TAK-242+APAP 24-hour and 48-hour groups had a significantly lower protein expression level of PCNA than the APAP group at the same time point (all P < 0.05); the TAK-242+APAP 24-hour and 72-hour groups had a significantly lower mRNA expression level of Ki-67 than the APAP group at the same time point (all P < 0.05), and the TAK-242+APAP 24-hour, 48-hour, and 72-hour groups had a significantly lower protein expression level of Ki-67 than the APAP group at the same time point (all P < 0.05). In addition, the TAK-242+APAP 24-hour and 48-hour groups had a significantly lower phosphorylation level of STAT3 than the APAP group at the same time point (both P < 0.05). Conclusion TLR4 may promote liver regeneration by increasing the phosphorylation level of STAT3 during APAP-induced liver injury in mice. -
近年来药物性肝损伤发病率逐渐上升,日益受到人们的关注。引起药物性肝损伤的原因较多,例如中草药、抗菌药、非甾体类抗炎药等,且可能在正常剂量使用下引起药物性肝损伤[1],严重的可导致急性肝衰竭甚至需要肝移植[2],因此药物性肝损伤也逐渐引起全球公共卫生系统关注并成为加重肝病医疗系统经济负担的因素之一[3]。对乙酰氨基酚(Acetaminophen, APAP)是引起药物性肝损伤的主要病因之一,也是导致急性肝衰竭的重要原因[4-5]。肝脏再生对肝损伤修复和预后极为关键,药物性肝损伤后肝再生失败可能会导致严重的后果[6-7]。Toll样受体4(TLR4)是模式识别受体的一种,主要参与机体免疫和炎症反应的调节[8],但Marlini等[9]研究发现,TLR4可能对肝脏修复和再生过程有影响。与野生型小鼠相比,C3H/HeJ小鼠(TLR4基因发生错义突变而导致TLR4表达缺陷型小鼠[10])行部分肝脏切除术后肝脏重量恢复和肝细胞增殖所需时间明显延长。TLR4是否在APAP引起的肝损伤过程肝脏再生中发挥作用,目前研究甚少。TAK-242是目前较常用的小分子TLR4抑制剂,可以特异性阻断TLR4信号传导[11]。因此,本研究利用TAK-242来初步探索TLR4在APAP肝损伤过程肝脏再生中的作用,以期为研究肝再生机制以及药物性肝损伤治疗提供新的见解。
1. 材料与方法
1.1 主要试剂
APAP(HY-66005)、TAK-242(HY-11109)购买于中国MedChemExpress公司;DMSO(D8371)购买于北京索莱宝科技有限公司;ALT检测试剂盒(C009-2-1)购买于南京建成生物工程研究所;RT-PCR逆转录试剂盒(R-202)、SYBR Green染料(Q204)购买于新贝生物科技有限公司;Trizol(15596026)购买于美国Thermo Fisher Scientific公司;GAPDH抗体(TA-08)、免疫组化试剂盒(SP-9001)、DAB显色试剂盒(ZLI-9018)购买于北京中杉金桥生物技术有限公司;4%多聚甲醛组织固定液(BL539A)购买于中国白鲨生物科技有限公司;PCNA抗体(2586)、Ki-67抗体(12202)、STAT3抗体(9139)和p-STAT3抗体(9145)购买于美国Cell Signaling Technology公司;Cyclin D1抗体(26939-1-AP)购买于武汉三鹰生物技术有限公司;WB超敏显影液(K-12043-D10)购买于美国Advansta公司;引物由上海生工生物工程股份有限公司合成。
1.2 动物分组及造模
雄性CD-1(ICR)小鼠78只,6~8周龄,购买于维通利华实验动物技术有限公司[生产许可证编号:SCXK(浙)2019-0001;使用许可证编号:SYXK(皖)2017-006]。采用随机数字表法将小鼠分为9组:对照组(正常对照组、溶剂对照组、抑制剂对照组)每组6只,实验组(APAP 24 h组、TAK-242+APAP 24 h组、APAP 48 h组、TAK-242+APAP 48 h组、APAP 72 h组、TAK-242+APAP 72 h组)每组10只。APAP溶液用生理盐水配置,TAK-242溶液用0.5% DMSO配置。所有小鼠适应性喂养1周后再进行造模。给药前所有小鼠禁食12 h。实验组小鼠给予单剂量腹腔注射APAP(300 mg/kg),TAK-242在APAP注射前3 h以3 mg/kg剂量腹腔注射于相应组小鼠(剂量参照文献[12-13])。正常对照组小鼠腹腔注射与APAP组等体积生理盐水,抑制剂对照组小鼠注射与实验组小鼠等体积TAK-242溶液,溶剂对照组小鼠注射与抑制剂对照组等体积的0.5% DMSO溶液。在注射APAP后24 h、48 h、72 h麻醉后处理小鼠,收集小鼠血液和肝脏并及时冷冻保存,以便进行后续实验。
1.3 实验方法
1.3.1 血清ALT检测
将血液样本在室温下静置2 h,3 000 r/min离心20 min,将血清转移至另一EP管保存。按照ALT检测试剂盒说明测定ALT水平。
1.3.2 肝脏组织病理观察
使用4%多聚甲醛组织固定液对肝左叶进行固定,按照常规步骤进行脱水、浸蜡、包埋和切片。将肝组织切片进行HE染色,然后在显微镜下观察组织损伤情况并进行拍照。
1.3.3 RT-PCR检测mRNA水平
取40 mg肝组织放入玻璃研磨器中,同时加入1 mL Trizol,在冰上充分研磨,经过氯仿、异丙醇、75%乙醇萃取、沉淀和洗涤等步骤,提取总RNA。检测RNA浓度后,按照逆转录试剂盒说明书进行逆转录得到cDNA。以GAPDH为内参,使用RT-PCR方法检测并以2-△△Ct法计算PCNA、Ki-67、Cyclin D1的mRNA水平。引物序列见表 1。
表 1 RT-PCR引物序列Table 1. RT-PCR primer sequences基因 上游(5′-3′) 下游(5′-3′) Cyclin D1 AGGCGGATGAGAACAAGCAG CCTTGTTTAGCCAGAGGCCG Ki-67 CCATCATTGACCGCTCCTT CTGCCAGTGTGCTGTTCTAC PCNA GGGTTGGTAGTTGTCGCTGT CCAAGGAGACGTGAGACGAG GAPDH GACATGCCGCCTGGAGAAAC AGCCCAGGATGCCCTTTAGT 1.3.4 Western blot检测蛋白水平
在玻璃研磨器中加入70 mg肝脏组织及1 mL RIPA裂解液,充分研磨并经过离心后,得到总蛋白。利用BCA法测定蛋白浓度,加入上样缓冲液后将蛋白放在加热器上100 ℃加热10 min使其变性。将蛋白样品加入到SDS-PAGE凝胶样品孔中进行电泳,结束后在冰上进行转膜,将蛋白转移至PVDF膜上。室温下使用脱脂牛奶封闭2~3 h,TBST溶液清洗3次,一抗4 ℃孵育过夜(GAPDH,1∶ 1 000;STAT3,1∶ 1 000;p-STAT3,1∶ 2 000;PCNA,1∶ 1 000;Cyclin D1,1∶ 5 000)。第2天PVDF洗涤干净后,二抗室温孵育1 h。最后显影,使用Image J进行灰度值分析。
1.3.5 免疫组化检测
肝脏组织切片进行常规的烘烤、脱蜡、水化,使用柠檬酸钠进行抗原修复,然后阻断内源性过氧化物酶,经过封闭后,一抗(Ki-67,1∶ 300)4 ℃孵育过夜。第2天室温下复温30 min,洗涤后二抗孵育45 min。DAB显色,最后使用中性树胶封片并拍照。
1.4 统计学方法
采用SPSS 23.0统计软件进行数据分析,Graphpad Prism 8.0软件进行绘图。正态分布的计量资料以x±s表示,两组间比较采用成组t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验;多组间比较及进一步两两比较均采用Kruskal-Wallis H检验。P<0.05为差异有统计学意义。
2. 结果
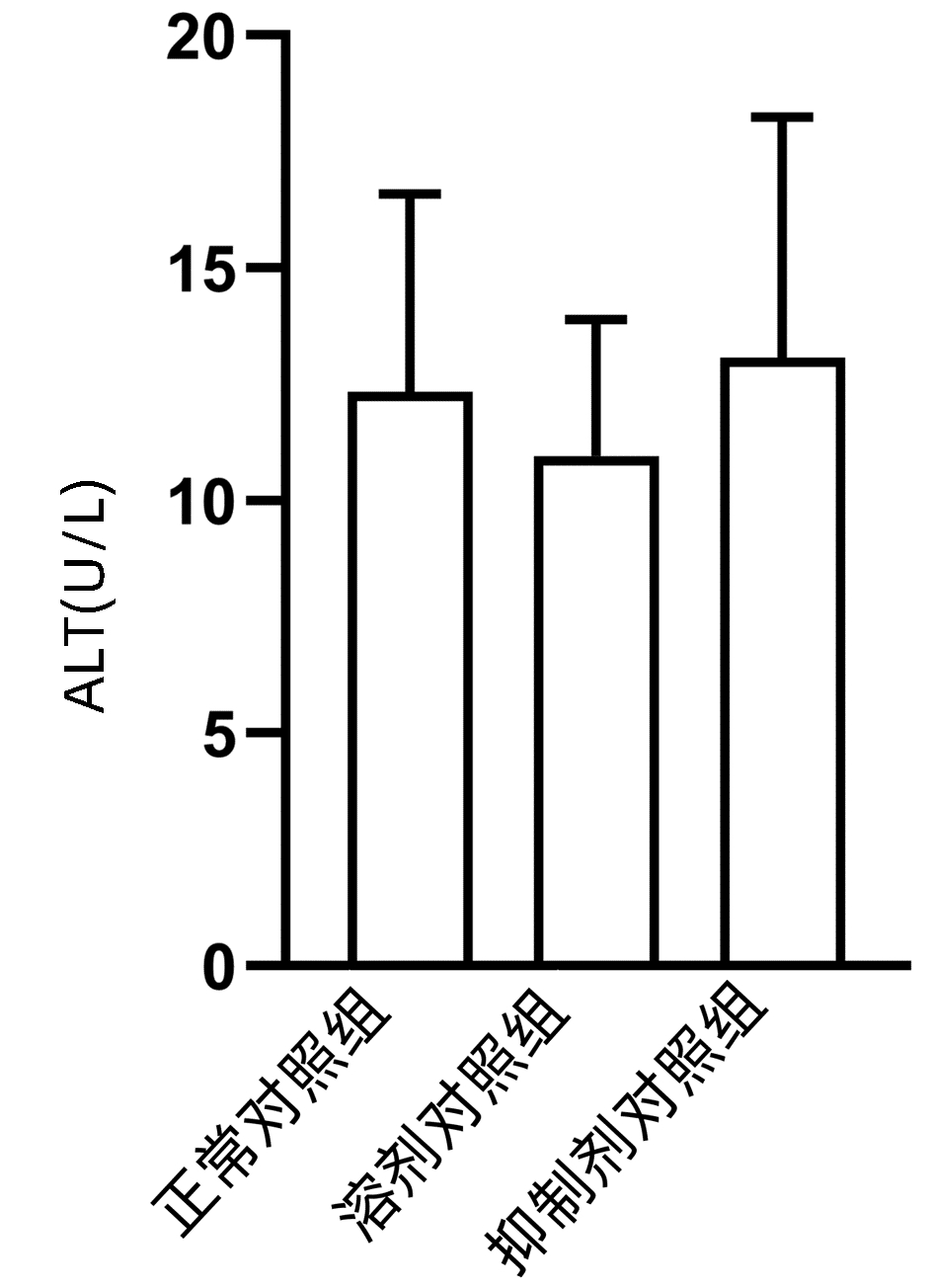
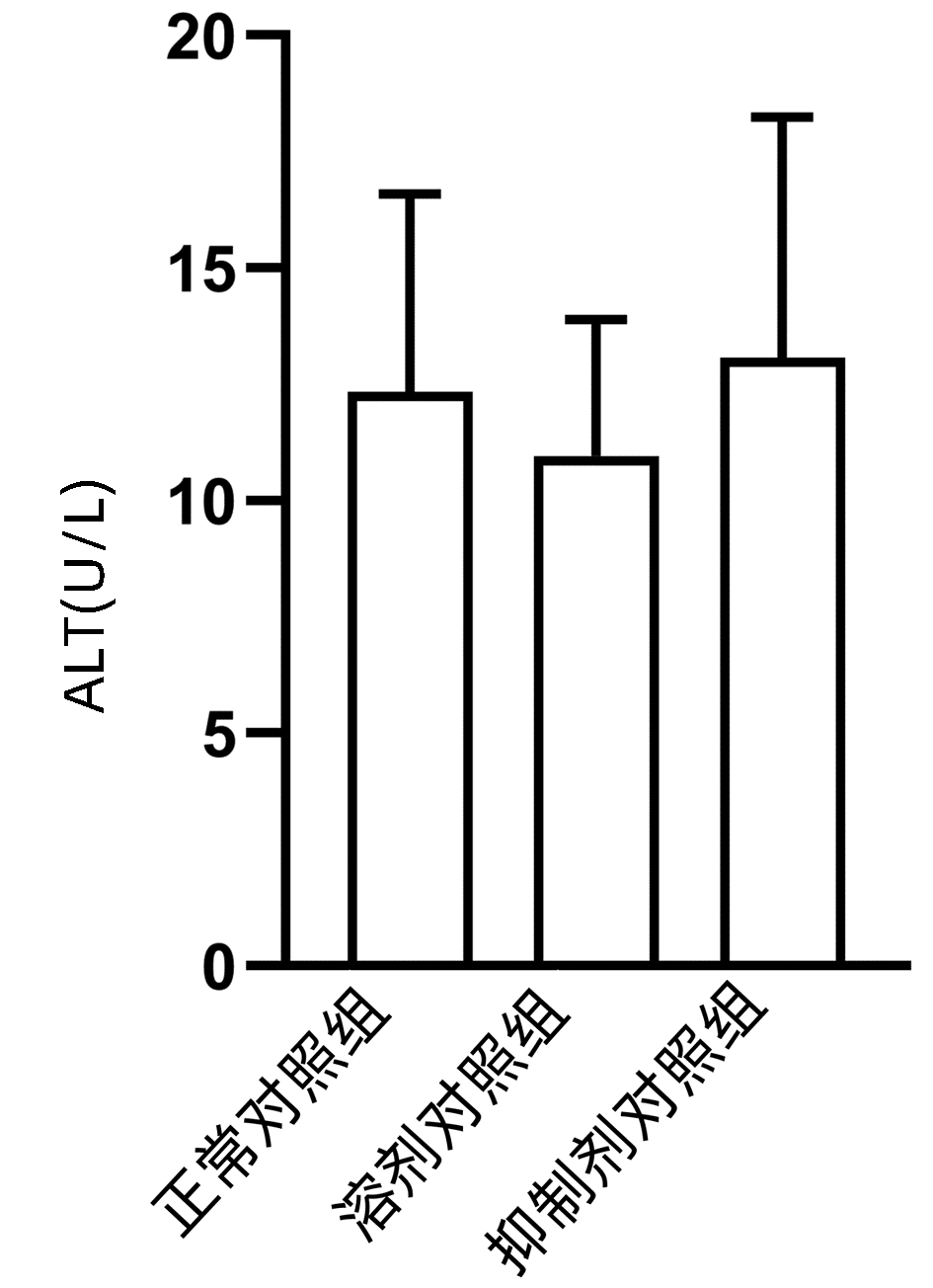
2.1 观察抑制剂和溶剂对正常小鼠肝脏的影响
结果显示,正常对照组、溶剂对照组和抑制剂对照组小鼠血清ALT水平差异无统计学意义(P>0.05)(图 1),且与正常对照组相比,溶剂对照组和抑制剂对照组小鼠的肝脏HE染色未发现明显的病理改变(图 2)。
2.2 抑制TLR4可能延迟APAP诱导的肝损伤过程中肝脏的恢复
APAP 24 h组和APAP 48 h组血清ALT水平均明显高于正常对照组(P值均<0.05),而TAK-242+APAP 24 h组及TAK-242+APAP 48 h组的ALT水平明显高于同时间点APAP组(P值均<0.05);小鼠肝组织HE染色结果显示,正常对照组小鼠肝小叶结构清晰,肝细胞排列整齐,APAP处理的小鼠肝脏可见典型的小叶中心性坏死。与相同时间点APAP组相比,TAK-242+APAP 24 h组和TAK-242+APAP 48 h组小鼠肝脏坏死面积明显较大(P值均<0.05)(表 2、3,图 3)。
表 2 APAP对小鼠血清ALT及肝脏组织的影响Table 2. Effects of APAP on serum ALT and liver tissue in mice组别 动物数(只) ALT(U/L) 肝脏坏死面积(%) 正常对照组 6 12.339±4.245 0 APAP 24 h组 10 1 449.848±209.4911) 41.600(38.617~46.502)1) APAP 48 h组 10 281.702±140.5921) 36.050(25.127~45.718)1) APAP 72 h组 10 45.251±4.298 0 (0~1.903) H值 17.857 16.035 P值 <0.001 0.001 注:与正常对照组比较,1)P<0.05。 表 3 抑制TLR4在各时间点对APAP肝损伤小鼠血清ALT及肝脏组织的影响Table 3. Effects of TLR4 inhibition on serum ALT and liver tissue of mice with APAP-induced liver injury at different time point组别 动物数(只) 24 h 48 h 72 h ALT(U/L) APAP组 10 1 449.848±209.491 281.702±140.592 45.251±4.298 TAK-242+APAP组 10 3 484.212±960.336 1 151.505±656.722 56.995±24.763 t值 -4.628 -2.896 -1.045 P值 0.002 0.040 0.352 肝脏坏死面积(%) APAP组 10 41.600(38.617~46.502) 36.050(25.127~45.718) 0(0~1.903) TAK-242+APAP组 10 58.554(55.889~73.409) 59.558(54.856~60.591) 2.713(0~8.289) Z值 -2.610 -2.611 -1.294 P值 0.008 0.008 0.310 2.3 抑制TLR4下调肝脏Cyclin D1、PCNA、Ki-67的mRNA水平和蛋白表达
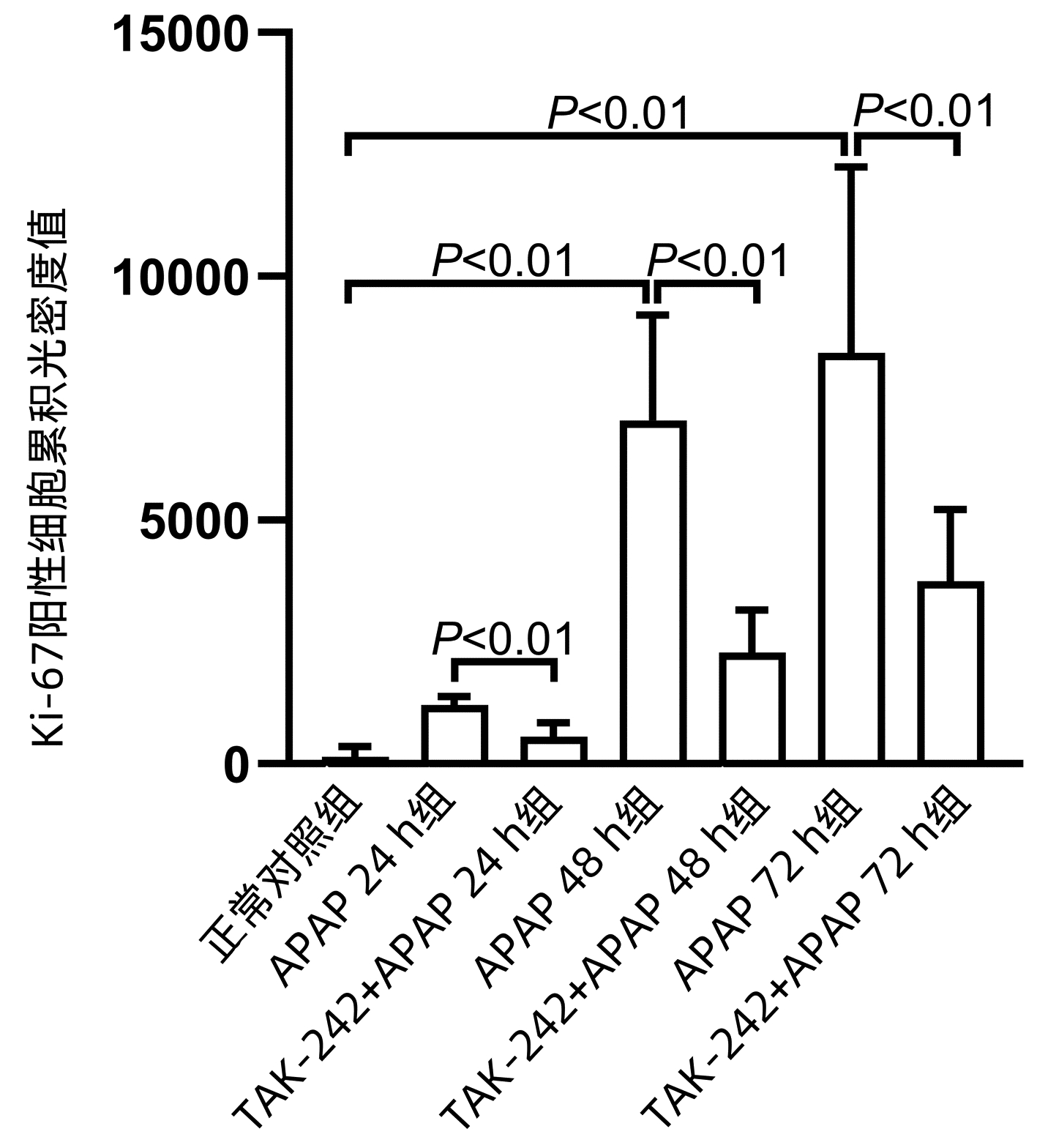
APAP 48 h组和APAP72 h组Cyclin D1、PCNA、Ki-67 mRNA水平均明显高于正常对照组(P值均<0.05),APAP 24 h组PCNA mRNA水平显著高于正常对照组(P<0.05);TAK-242+ APAP 24 h组、TAK-242+APAP 48 h组和TAK-242+APAP 72 h组的Cyclin D1、PCNA mRNA水平均低于同时间点APAP组,TAK-242+APAP 24 h组和TAK-242+APAP 72 h组的Ki-67 mRNA水平均低于同时间点APAP组,差异均有统计学意义(P值均<0.05) (表 4、5)。Western blot检测Cyclin D1、PCNA蛋白表达结果显示,TAK-242+APAP 24 h组和TAK-242+APAP 48 h组的Cyclin D1、PCNA蛋白表达明显低于同时间点APAP组(P值均<0.05)(图 4)。免疫组化染色及Ki-67阳性细胞累积光密度值比较结果显示,APAP 48 h组和APAP 72 h组Ki-67蛋白表达显著高于正常对照组(P值均<0.05),而TAK-242+APAP 24 h组、TAK-242+APAP 48 h组及TAK-242+APAP 72 h组的Ki-67蛋白表达明显低于同时间点APAP组(P值均<0.05)(图 5、6)。
表 4 APAP对小鼠肝组织Ki-67 mRNA、Cyclin D1 mRNA、PCNA mRNA表达的影响Table 4. Effects of APAP on the expression of Ki-67 mRNA, Cyclin D1 mRNA and PCNA mRNA in mouse liver组别 动物数(只) Ki-67 mRNA相对表达量 Cyclin D1 mRNA相对表达量 PCNA mRNA相对表达量 正常对照组 6 1.002±0.072 1.034±0.325 1.009±0.162 APAP 24 h组 10 1.027±0.184 1.061±0.102 1.347±0.1151) APAP 48 h组 10 83.566±23.3621) 3.707±0.2551) 5.327±0.1621) APAP 72 h组 10 39.316±10.9161) 2.417±0.3361) 1.739±0.0861) F值 27.853 88.659 657.231 P值 <0.001 <0.001 <0.001 注:与正常对照组比较,1)P<0.05。 表 5 抑制TLR4对小鼠肝组织Ki-67 mRNA、Cyclin D1 mRNA、PCNA mRNA表达的影响Table 5. Effects of TLR4 inhibition on the expression of Ki-67 mRNA, Cyclin D1 mRNA and PCNA mRNA in mouse liver组别 动物数(只) 24 h 48 h 72 h Ki-67 mRNA相对表达量 APAP组 10 1.027±0.184 83.566±23.362 39.316±10.916 TAK-242+APAP组 10 0.366±0.129 70.145±31.141 5.950±0.678 t值 5.107 0.597 5.284 P值 0.007 0.583 0.006 Cyclin D1 mRNA相对表达量 APAP组 10 1.061±0.102 3.707±0.255 2.417±0.336 TAK-242+APAP组 10 0.678±0.073 2.918±0.487 1.837±0.202 t值 6.116 2.871 2.957 P值 0.001 0.028 0.025 PCNA mRNA相对表达量 APAP组 10 1.347±0.115 5.327±0.162 1.739±0.086 TAK-242+APAP组 10 0.830±0.075 2.937±0.144 0.620±0.035 t值 6.529 19.074 20.909 P值 0.003 <0.001 <0.001 2.4 抑制TLR4下调p-STAT3水平
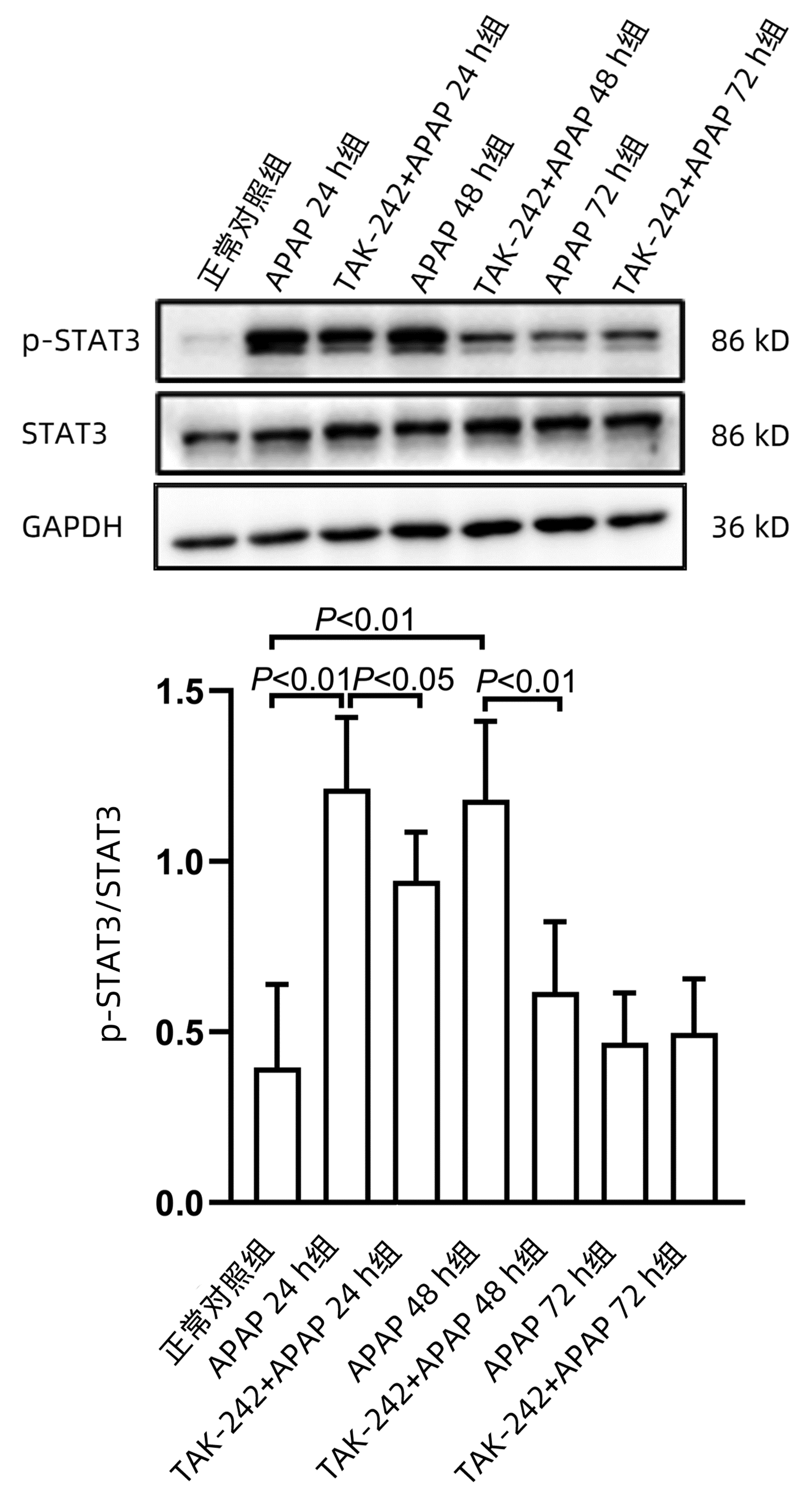
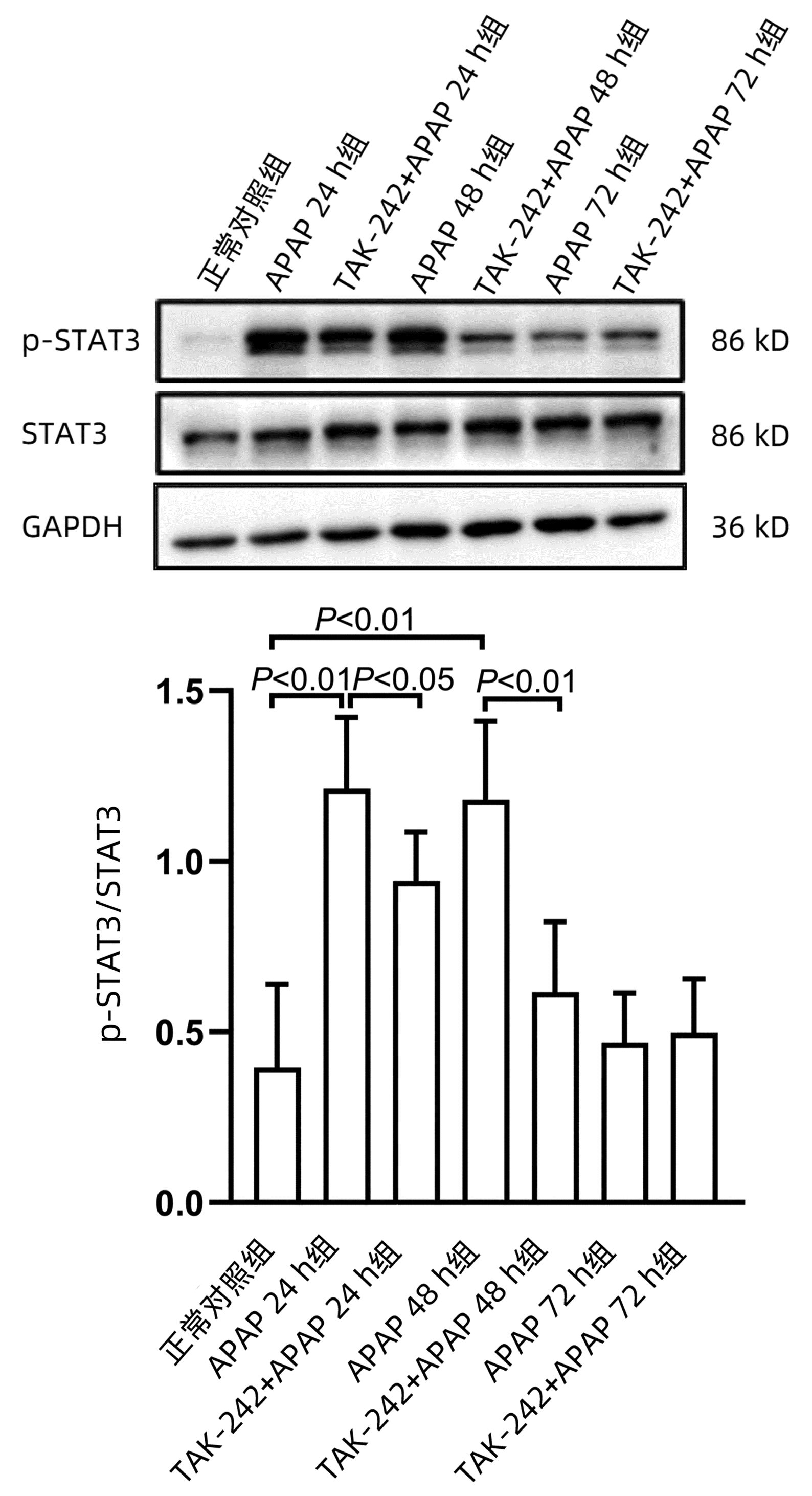
课题组前期体外研究[14]发现,STAT3在APAP肝损伤后肝细胞再生过程中发挥重要作用,为探索TLR4参与肝脏再生过程是否与STAT3有关,应用Western blot方法检测STAT3蛋白磷酸化(p-STAT3)水平。结果显示,APAP 24 h组和APAP 48 h组p-STAT3水平均显著高于正常对照组(P值均<0.01),而TAK-242+APAP 24 h组及TAK-242+APAP 48 h组p-STAT3水平均明显低于同时间点APAP组(P值均<0.05)(图 7)。
3. 讨论
肝脏是具有极强再生能力的器官,也是唯一能够在被部分/大部分切除后恢复原有重量的内脏器官[15]。据报道[16],存在肝脏炎症和肝细胞坏死的肝脏疾病都可能发生肝再生,包括药物性肝损伤。N-乙酰半胱氨酸(NAC)是最常用于治疗药物性肝损伤的药物,特别是对于APAP肝损伤,治疗效果较好。但是NAC的治疗时间窗较短,对晚期发现的肝损伤治疗效果较差,甚至长时间高剂量使用NAC会不利于APAP肝损伤恢复[17]。在毒性化学物引起的肝损伤中,肝损伤和再生是此消彼长的关系。一项急性肝损伤的动态研究[18]发现,在轻度急性肝损伤小鼠体内,肝损伤进展时,肝再生也在增强,当肝再生强于肝损伤,即开始进入恢复阶段;而在重度肝损伤小鼠模型中,肝再生一开始即受到抑制,因肝再生速度不及损伤的速度,损伤加速进展,造成小鼠死亡。据报道[19-20],在APAP肝损伤中,明显的肝再生发现在24 h以后,因此本研究选择分别于24 h、48 h和72 h处死小鼠。
TLR4是一种跨膜多功能分子,可以与来自细胞内外的配体结合,在肝、肾、心、肺、皮肤等多个器官和组织中表达,参与炎症反应、免疫调控和组织修复等过程[21]。有研究[22]报道,早期阻断TLR4可以预防肾小管坏死和急性肾损伤,但是在愈合期阻断TLR4会导致IL-22生成减少,从而损害肾再生。与野生型小鼠相比,TLR4敲除的小鼠对博来霉素引起的肺损伤更敏感,肺泡上皮损伤也更严重,Ⅱ型肺泡上皮细胞增殖数较少[23]。最近一项研究[24]发现,在行部分肝切除术小鼠体内,TLR4与脂多糖结合可以激活肝细胞转化,促进肝再生。TLR4敲除小鼠行部分肝切除术后,细胞增殖指标Ki-67和PCNA的表达明显受到抑制。本文利用TLR4特异性抑制剂TAK-242进行研究发现,与24 h和48 h的APAP组相比,使用抑制剂组处理的小鼠ALT水平和肝脏坏死面积恢复均较慢,这表明抑制TLR4可能会阻碍APAP肝损伤过程肝脏的修复。进一步检测再生相关指标发现,在APAP诱导小鼠肝损伤中,抑制TLR4后小鼠肝脏的Cyclin D1、PCNA和Ki-67 mRNA及蛋白水平均下降,提示TLR4可能参与APAP肝损伤过程中肝再生,并对肝再生起到一定的促进作用。
STAT3是细胞增殖和组织再生过程的重要分子,在肝脏再生过程中发挥重要作用。在行部分肝切除术的小鼠体内,STAT3信号通路激活增加,肝细胞STAT3特异性敲除的小鼠肝脏再生会受到抑制[25-26]。此外,在四氯化碳诱导的肝损伤小鼠模型中发现,STAT3表达显著增加,抑制STAT3后,肝脏增殖指标表达明显降低[27]。在本研究过程中,笔者发现与正常对照组相比,APAP 24 h组和APAP 48 h组STAT3磷酸化水平明显升高,但同时间点TAK-242+APAP组STAT3磷酸化水平降低,这提示p-STAT3可能参与了TLR4在APAP肝损伤中促进肝脏再生的过程。
肝脏再生的机制比较复杂,涉及的分子及通路众多。本研究仅对TLR4在APAP肝损伤过程肝再生中的作用进行了初步探讨,更深层的机制有待课题组后期进一步研究。
综上所述,TLR4可能通过STAT3信号通路对APAP诱导的小鼠肝损伤过程中的肝脏再生起到一定的促进作用。
-
表 1 RT-PCR引物序列
Table 1. RT-PCR primer sequences
基因 上游(5′-3′) 下游(5′-3′) Cyclin D1 AGGCGGATGAGAACAAGCAG CCTTGTTTAGCCAGAGGCCG Ki-67 CCATCATTGACCGCTCCTT CTGCCAGTGTGCTGTTCTAC PCNA GGGTTGGTAGTTGTCGCTGT CCAAGGAGACGTGAGACGAG GAPDH GACATGCCGCCTGGAGAAAC AGCCCAGGATGCCCTTTAGT 表 2 APAP对小鼠血清ALT及肝脏组织的影响
Table 2. Effects of APAP on serum ALT and liver tissue in mice
组别 动物数(只) ALT(U/L) 肝脏坏死面积(%) 正常对照组 6 12.339±4.245 0 APAP 24 h组 10 1 449.848±209.4911) 41.600(38.617~46.502)1) APAP 48 h组 10 281.702±140.5921) 36.050(25.127~45.718)1) APAP 72 h组 10 45.251±4.298 0 (0~1.903) H值 17.857 16.035 P值 <0.001 0.001 注:与正常对照组比较,1)P<0.05。 表 3 抑制TLR4在各时间点对APAP肝损伤小鼠血清ALT及肝脏组织的影响
Table 3. Effects of TLR4 inhibition on serum ALT and liver tissue of mice with APAP-induced liver injury at different time point
组别 动物数(只) 24 h 48 h 72 h ALT(U/L) APAP组 10 1 449.848±209.491 281.702±140.592 45.251±4.298 TAK-242+APAP组 10 3 484.212±960.336 1 151.505±656.722 56.995±24.763 t值 -4.628 -2.896 -1.045 P值 0.002 0.040 0.352 肝脏坏死面积(%) APAP组 10 41.600(38.617~46.502) 36.050(25.127~45.718) 0(0~1.903) TAK-242+APAP组 10 58.554(55.889~73.409) 59.558(54.856~60.591) 2.713(0~8.289) Z值 -2.610 -2.611 -1.294 P值 0.008 0.008 0.310 表 4 APAP对小鼠肝组织Ki-67 mRNA、Cyclin D1 mRNA、PCNA mRNA表达的影响
Table 4. Effects of APAP on the expression of Ki-67 mRNA, Cyclin D1 mRNA and PCNA mRNA in mouse liver
组别 动物数(只) Ki-67 mRNA相对表达量 Cyclin D1 mRNA相对表达量 PCNA mRNA相对表达量 正常对照组 6 1.002±0.072 1.034±0.325 1.009±0.162 APAP 24 h组 10 1.027±0.184 1.061±0.102 1.347±0.1151) APAP 48 h组 10 83.566±23.3621) 3.707±0.2551) 5.327±0.1621) APAP 72 h组 10 39.316±10.9161) 2.417±0.3361) 1.739±0.0861) F值 27.853 88.659 657.231 P值 <0.001 <0.001 <0.001 注:与正常对照组比较,1)P<0.05。 表 5 抑制TLR4对小鼠肝组织Ki-67 mRNA、Cyclin D1 mRNA、PCNA mRNA表达的影响
Table 5. Effects of TLR4 inhibition on the expression of Ki-67 mRNA, Cyclin D1 mRNA and PCNA mRNA in mouse liver
组别 动物数(只) 24 h 48 h 72 h Ki-67 mRNA相对表达量 APAP组 10 1.027±0.184 83.566±23.362 39.316±10.916 TAK-242+APAP组 10 0.366±0.129 70.145±31.141 5.950±0.678 t值 5.107 0.597 5.284 P值 0.007 0.583 0.006 Cyclin D1 mRNA相对表达量 APAP组 10 1.061±0.102 3.707±0.255 2.417±0.336 TAK-242+APAP组 10 0.678±0.073 2.918±0.487 1.837±0.202 t值 6.116 2.871 2.957 P值 0.001 0.028 0.025 PCNA mRNA相对表达量 APAP组 10 1.347±0.115 5.327±0.162 1.739±0.086 TAK-242+APAP组 10 0.830±0.075 2.937±0.144 0.620±0.035 t值 6.529 19.074 20.909 P值 0.003 <0.001 <0.001 -
[1] LOUVET A, NTANDJA WANDJI LC, LEMAÎTRE E, et al. Acute liver injury with therapeutic doses of acetaminophen: A prospective study[J]. Hepatology, 2021, 73(5): 1945-1955. DOI: 10.1002/hep.31678. [2] WANG YW, LIANG YR. Research progress on liver transplantation for drug-induced liver injury[J]. Ogran Transplant, 2022, 13(3): 338-343. DOI: 10.3969/j.issn.1674-7445.2022.03.009.王砚伟, 梁雨荣. 药物性肝损伤肝移植治疗进展[J]. 器官移植, 2022, 13(3): 338-343. DOI: 10.3969/j.issn.1674-7445.2022.03.009. [3] ASRANI SK, DEVARBHAVI H, EATON J, et al. Burden of liver diseases in the world[J]. J Hepatol, 2019, 70(1): 151-171. DOI: 10.1016/j.jhep.2018.09.014. [4] NASH E, SABIH AH, CHETWOOD J, et al. Drug-induced liver injury in Australia, 2009-2020: the increasing proportion of non-paracetamol cases linked with herbal and dietary supplements[J]. Med J Aust, 2021, 215(6): 261-268. DOI: 10.5694/mja2.51173. [5] STRAVITZ RT, LEE WM. Acute liver failure[J]. Lancet, 2019, 394(10201): 869-881. DOI: 10.1016/S0140-6736(19)31894-X. [6] BHUSHAN B, APTE U. Liver regeneration after acetaminophen hepatotoxicity: Mechanisms and therapeutic opportunities[J]. Am J Pathol, 2019, 189(4): 719-729. DOI: 10.1016/j.ajpath.2018.12.006. [7] BHUSHAN B, GUNEWARDENA S, EDWARDS G, et al. Comparison of liver regeneration after partial hepatectomy and acetaminophen-induced acute liver failure: A global picture based on transcriptome analysis[J]. Food Chem Toxicol, 2020, 139: 111186. DOI: 10.1016/j.fct.2020.111186. [8] ROCHA DM, CALDAS AP, OLIVEIRA LL, et al. Saturated fatty acids trigger TLR4-mediated inflammatory response[J]. Atherosclerosis, 2016, 244: 211-215. DOI: 10.1016/j.atherosclerosis.2015.11.015. [9] MARLINI M, MABUCHI A, MALLARD BL, et al. Delayed liver regeneration in C3H/HeJ mice: possible involvement of haemodynamic and structural changes in the hepatic microcirculation[J]. Exp Physiol, 2016, 101(12): 1492-1505. DOI: 10.1113/EP085727. [10] HOSHINO K, TAKEUCHI O, KAWAI T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product[J]. J Immunol, 1999, 162(7): 3749-3752. [11] MATSUNAGA N, TSUCHIMORI N, MATSUMOTO T, et al. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules[J]. Mol Pharmacol, 2011, 79(1): 34-41. DOI: 10.1124/mol.110.068064. [12] ONO Y, MAEJIMA Y, SAITO M, et al. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice[J]. Sci Rep, 2020, 10(1): 694. DOI: 10.1038/s41598-020-57714-3. [13] WANG H, LI X, DONG G, et al. Toll-like receptor 4 inhibitor TAK-242 improves fulminant hepatitis by regulating accumulation of myeloid-derived suppressor cell[J]. Inflammation, 2021, 44(2): 671-681. DOI: 10.1007/s10753-020-01366-y. [14] YU W, ZHANG LJ, LU Y, et al. Role of STAT3 in hepatocyte regeneration after acetaminophen-induced hepatocellular injury in mice[J]. J Clin Hepatol, 2021, 37(4): 857-862. DOI: 10.3969/j.issn.1001-5256.2021.04.026.余旺, 章礼久, 路燕, 等. STAT3在对乙酰氨基酚所致小鼠肝损伤后肝细胞再生中的作用[J]. 临床肝胆病杂志, 2021, 37(4): 857-862. DOI: 10.3969/j.issn.1001-5256.2021.04.026. [15] YAGI S, HIRATA M, MIYACHI Y, et al. Liver regeneration after hepatectomy and partial liver transplantation[J]. Int J Mol Sci, 2020, 21(21): 8414. DOI: 10.3390/ijms21218414. [16] FAUSTO N, CAMPBELL JS, RIEHLE KJ. Liver regeneration[J]. Hepatology, 2006, 43(2 Suppl 1): S45-S53. DOI: 10.1002/hep.20969. [17] YANG R, MIKI K, HE X, et al. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity[J]. Crit Care, 2009, 13(2): R55. DOI: 10.1186/cc7782. [18] SHAO S, ZHANG Y, LI G, et al. The dynamics of cell death patterns and regeneration during acute liver injury in mice[J]. FEBS Open Bio, 2022, 12(5): 1061-1074. DOI: 10.1002/2211-5463.13383. [19] BHUSHAN B, EDWARDS G, DESAI A, et al. Liver-specific deletion of integrin-linked kinase in mice attenuates hepatotoxicity and improves liver regeneration after acetaminophen overdose[J]. Gene Expr, 2016, 17(1): 35-45. DOI: 10.3727/105221616X691578. [20] FAN X, CHEN P, TAN H, et al. Dynamic and coordinated regulation of KEAP1-NRF2-ARE and p53/p21 signaling pathways is associated with acetaminophen injury responsive liver regeneration[J]. Drug Metab Dispos, 2014, 42(9): 1532-1539. DOI: 10.1124/dmd.114.059394. [21] YU L, WANG L, CHEN S. Endogenous toll-like receptor ligands and their biological significance[J]. J Cell Mol Med, 2010, 14(11): 2592-2603. DOI: 10.1111/j.1582-4934.2010.01127.x. [22] KULKARNI OP, HARTTER I, MULAY SR, et al. Toll-like receptor 4-induced IL-22 accelerates kidney regeneration[J]. J Am Soc Nephrol, 2014, 25(5): 978-989. DOI: 10.1681/ASN.2013050528. [23] LIANG J, ZHANG Y, XIE T, et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice[J]. Nat Med, 2016, 22(11): 1285-1293. DOI: 10.1038/nm.4192. [24] SHAO C, JING Y, ZHAO S, et al. LPS/Bcl3/YAP1 signaling promotes Sox9+HNF4α+ hepatocyte-mediated liver regeneration after hepatectomy[J]. Cell Death Dis, 2022, 13(3): 277. DOI: 10.1038/s41419-022-04715-x. [25] YIN S, WANG H, PARK O, et al. Enhanced liver regeneration in IL-10-deficient mice after partial hepatectomy via stimulating inflammatory response and activating hepatocyte STAT3[J]. Am J Pathol, 2011, 178(4): 1614-1621. DOI: 10.1016/j.ajpath.2011.01.001. [26] FU H, DONG R, ZHANG Y, et al. Tmub1 negatively regulates liver regeneration via inhibiting STAT3 phosphorylation[J]. Cell Signal, 2019, 55: 65-72. DOI: 10.1016/j.cellsig.2018.12.013. [27] HU K, XU J, FAN K, et al. Nuclear accumulation of pyruvate kinase M2 promotes liver regeneration via activation of signal transducer and activator of transcription 3[J]. Life Sci, 2020, 250: 117561. DOI: 10.1016/j.lfs.2020.117561. 期刊类型引用(1)
1. 努尔孜叶·阿布里克木,热娜古丽·努尔,李静,陆晨. Toll样受体9水平与免疫球蛋白A肾病患者临床病理特征及预后关系. 临床军医杂志. 2025(01): 64-68 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 4566 KB)
PDF下载 ( 4566 KB)

 下载:
下载:







 下载:
下载:




 百度学术
百度学术