Sonic Hedgehog信号通路在重症急性胰腺炎大鼠模型肠黏膜屏障损伤中的作用探讨
DOI: 10.3969/j.issn.1001-5256.2023.05.020
Role of the Sonic Hedgehog signaling pathway in intestinal mucosal barrier injury in rats with severe acute pancreatitis
-
摘要:
目的 探讨Sonic Hedgehog(Shh)信号通路在重症急性胰腺炎(SAP)大鼠肠道黏膜屏障损伤中的表达及作用。 方法 48只SD大鼠按随机数字表法分为:假手术组(Sham组)、SAP模型组(SAP组)、SAP模型组+Shh信号通路特异性激动剂嘌呤胺组(PUR+SAP组)、SAP模型组+Shh信号通路特异性抑制剂环巴胺组(CYC+SAP组),各组又分为12 h、24 h两个亚组,每个亚组6只大鼠。SAP造模采用5%牛磺胆酸钠胰胆管逆行注射,干预组分别在造模前腹腔注射0.69 mg/kg嘌呤胺及0.69 mg/kg环巴胺。在造模后12 h和24 h取材。HE染色观察大鼠胰腺及回肠病理学变化;ELISA法检测大鼠血清淀粉酶、脂肪酶、DAO和EndoCAb的表达水平;TUNEL法检测肠上皮细胞凋亡情况;Western Blot检测回肠组织Shh、Ptch1和Gli1的表达。计量资料符合正态分布时多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验法;计量资料不符合正态分布时多组间比较及进一步两两比较均采用Kruskal-Wallis H检验法。 结果 与Sham组相比,SAP组胰腺和回肠组织病理学评分均明显增加,血清脂肪酶、淀粉酶、DAO和EndoCAb水平明显升高,肠上皮细胞凋亡增加,回肠组织Shh、Ptch1和Gli1蛋白表达升高(P值均<0.05)。与SAP组对比,PUR+SAP组胰腺和肠道病理损伤及功能障碍减轻,肠道上皮细胞凋亡显著减少,回肠组织中的Shh、Ptch1和Gli1蛋白表达明显升高(P值均<0.05)。与SAP组对比,CYC+SAP组胰腺和肠道病理损伤及功能障碍加重,肠道上皮细胞凋亡显著增加,回肠组织中的Shh、Ptch1和Gli1蛋白表达明显降低(P值均<0.05)。 结论 Shh信号通路可能参与了SAP肠黏膜屏障损伤并且发挥保护作用。 Abstract:Objective To investigate the expression and role of the Sonic Hedgehog (Shh) signaling pathway in intestinal mucosal barrier injury in rats with severe acute pancreatitis (SAP). Methods A total of 48 Sprague-Dawley rats were divided into sham-operation group (Sham group), SAP model group (SAP group), SAP+Shh signaling pathway-specific agonist purmorphamine group (PUR+SAP group), and SAP+Shh signaling pathway-specific antagonist cyclopamine group (CYC+SAP group) using a random number table, with 12 rats in each group, and each group was further divided into 12-hour and 24-hour subgroups, with 6 rats in each subgroup. Rats were given retrograde injection of 5% sodium taurocholate into the pancreatic and bile ducts to establish a model of SAP, and rats in the intervention groups were given intraperitoneal injection of 0.69 mg/kg purmorphamine and 0.69 mg/kg cyclopamine before modeling. Related samples were collected at 12 and 24 hours after modeling. HE staining was used to observe the pathological changes of the pancreas and the ileum; ELISA was used to measure the serum levels of amylase, lipase, diamine oxidase (DAO), and endotoxin-core antibody (EndoCAb); the TUNEL method was used to observe the apoptosis of intestinal epithelial cells; Western blot was used to measure the expression levels of Shh, Ptch1, and Gli1 in ileal tissue. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups; the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups and further comparison between two groups. Results Compared with the Sham group, the SAP group had significant increases in the pathological scores of pancreatic and ileum tissue, the serum levels of lipase, amylase, DAO, and EndoCAb, the apoptosis of intestinal epithelial cells, and the protein expression levels of Shh, Ptch1, and Gli1 in ileal tissue (all P < 0.05). Compared with the SAP group, the PUR+SAP group had significantly alleviated pathological injury and dysfunction of the pancreas and intestine, a significant reduction in the apoptosis of intestinal epithelial cells, and significant increases in the protein expression levels of Shh, Ptch1, and Gli1 in ileal tissue (all P < 0.05). Compared with the SAP group, the CYC+SAP group had significant aggravation of the pathological injury and dysfunction of the pancreas and intestine, a significant increase in the apoptosis of intestinal epithelial cells, and significant reductions in the protein expression levels of Shh, Ptch1, and Gli1 in ileal tissue (all P < 0.05). Conclusion The Shh signaling pathway may be involved in intestinal mucosal barrier injury in SAP and exerts a protective effect. -
Key words:
- Pancreatitis /
- Intestinal Mucosal Barrier /
- Signal Transduction /
- Apoptosis
-
肝细胞癌(HCC)是我国最常见的恶性肿瘤之一。国家癌症中心最新数据显示,我国每年新发肝癌约37万人,约32.6万人死于肝癌,其中94.7%与慢性HBV感染有关[1-2]。早期发现、早期诊断和早期治疗是改善原发性肝癌预后的关键。然而目前尚缺乏原发性肝癌高敏感度和高特异度的诊断指标。自噬是细胞自我吞噬胞内长寿蛋白或受损细胞器并通过溶酶体途径进行的分解代谢过程,从而维持细胞稳态[3]。近年研究[4-7]显示自噬在HBV感染和HCC的发生发展起重要作用。但目前关于自噬相关蛋白在HBV相关HCC(HBV-HCC)患者血清中的表达及意义鲜有报道。本研究通过分析HBV-HCC、非HBV相关HCC(nonHBV-HCC)、慢性乙型肝炎(CHB)和健康对照者血清自噬相关蛋白7(ATG7)的表达水平,探讨ATG7对HBV-HCC的诊断价值。
1. 资料与方法
1.1 研究对象
选取2018年6月—2020年12月于本院住院的HCC患者89例。HCC的诊断标准符合《原发性肝癌诊疗规范(2017年版)》[8]并经病理确认。排除标准如下:病理类型为肝内胆管癌或HCC-肝内胆管癌混合型者;已接受过肝移植术、局部消融、肝动脉化疗栓塞术、放化疗等抗肿瘤治疗者;继发性HCC患者;同时伴有其他肿瘤患者。CHB诊断符合《慢性乙型肝炎防治指南(2019年版)》[9]。根据血清是否检出HBsAg和/或HBV DNA分为HBV-HCC组及nonHBV-HCC组。选取同期50例CHB患者(CHB组)和20例健康体检者作为对照组(HC组)。
1.2 资料收集
收集研究对象性别和年龄,总蛋白(TP)、白蛋白(Alb)、TBil、DBil、ALT、AST、GGT和ALP等实验室指标。另收集HBV-HCC组患者的BCLC分期和肿瘤直径等病理资料。
1.3 血清ATG7检测
所有研究对象清晨空腹肘静脉采血,采血后1 h内送实验室,3000 r/min离心10 min分离血清,-80 ℃冰箱保存。ATG7检测前4 h室温下复溶。使用上海江莱生物技术有限公司人ATG7酶联免疫吸附测定试剂盒(批号:Aug 2020)进行检测,严格按试剂盒说明书操作,在加入终止液15 min内用PHOMO酶标仪(安图生物,合肥)在450 nm波长测定各孔光密度(OD值),用WPS表格以标准品的浓度为纵坐标,OD值为横坐标绘制标准曲线并取得多项式公式,根据多项式公式计算每个样本的ATG7浓度。
1.4 伦理学审查
本研究方案经由福建医科大学孟超肝胆医院伦理委员会审批,批号:科审2020-029-01。
1.5 统计学方法
采用SPSS 22.0进行统计分析。非正态分布的计量资料以M(P25~P75)表示,多组间比较采用Kruskal-Wallis H检验,2组间比较采用Mann-Whitney U检验;计数资料组间比较采用χ2检验;采用Spearman进行相关性分析。运用GraphPad Prism7.0绘制散点图,采用Medcalc18.11.3绘制受试者工作特征曲线(ROC曲线)并比较不同指标曲线下面积(AUC)。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
HCC、CHB及HC 3组比较,男女比例差异无统计学意义,其余指标差异均有统计学意义(P值均<0.01)(表 1)。89例HCC患者中,67例(75.28%)血清HBsAg和/或HBV DNA阳性,纳入HBV-HCC组;剩余22例(24.72%)纳入nonHBV-HCC组,包括5例酒精性肝硬化,8例非酒精性脂肪肝,1例慢性丙型肝炎,2例肝硬化(病因未明),6例慢性肝炎(病因未明)。HBV-HCC组血清TBil水平及具有肝硬化背景病例数高于nonHBV-HCC组,而nonHBV-HCC组的血清AST水平和最大肿瘤直径高于HBV-HCC组,差异均有统计学意义(P值均<0.05),其余参数差异均无统计学意义(P值均>0.05)(表 2)。
表 1 3组人口学和实验室特征比较参数 HCC组(n=89) CHB组(n=50) HC组(n=20) χ2值 P值 年龄(岁) 56.0(48.0~66.5) 42.0(32.8~52.5) 37.5(30.0~48.8) 38.807 <0.001 男性[例(%)] 77(86.52) 38(76.00) 13(65.00) 5.480 0.076 TBil(μmol/L) 19.30(12.65~26.10) 19.55(12.78~29.28) 12.15(9.48~18.63) 10.478 0.005 DBil(μmol/L) 7.60(4.70~10.85) 4.45(2.20~7.05) 5.30(4.15~6.35) 17.168 <0.001 TP(g/L) 61.0(54.5~67.5) 66.5(60.8~73.3) 72.0(70.0~77.0) 34.168 <0.001 Alb(g/L) 34.0(31.0~38.5) 37.5(34.0~41.0) 44.0(41.0~47.0) 42.516 <0.001 ALT(U/L) 115.0(62.0~218.5) 87.5(26.5~206.8) 19.0(12.3~26.5) 37.212 <0.001 AST(U/L) 149.0(54.0~286.0) 57.0(31.5~110.5) 17.0(14.3~20.0) 54.345 <0.001 GGT(U/L) 52.0(34.0~110.5) 68.5(29.3~112.0) 17.0(14.3~24.5) 31.527 <0.001 ALP(U/L) 81.0(60.5~103.0) 102.0(82.5~117.0) 66.5(52.8~88.3) 21.160 <0.001 AFP(ng/mL) 37.90(5.00~712.32) 7.20(3.05~241.27) 2.85(2.18~4.48) 33.048 <0.001 ATG7(ng/mL) 21.11(17.76~22.73) 19.21(16.65~20.82) 13.82(8.70~17.82) 33.134 <0.001 表 2 HBV-HCC组与nonHBV-HCC组生化和病理特征比较参数 HBV-HCC组(n=67) nonHBV-HCC组(n=22) 统计值 P值 年龄(岁) 55.0(46.0~ 65.0) 63.5(50.8~71.8) U=535.000 0.055 男/女(例) 58/9 19/3 χ2=0.001 0.981 TBil(μmol/L) 20.60(13.30~27.40) 16.00(11.15~21.48) U=524.500 0.043 DBil(μmol/L) 7.70(4.80~10.90) 7.15(3.80~9.65) U=602.500 0.201 TP(g/L) 61.0(50.0~67.0) 58.5(51.3~68.3) U=638.500 0.348 Alb(g/L) 34.0(31.0~38.0) 33.0(30.3~39.0) U=709.500 0.793 ALT(U/L) 95.0(54.0~198.0) 152.0(89.8~268.0) U=557.000 0.087 AST(U/L) 136.0(37.0~240.0) 236.5(93.8~348.8) U=523.000 0.042 GGT(U/L) 51.0(30.0~111.0) 58.0(35.0~113.3) U=658.000 0.452 ALP(U/L) 76.0(60.0~99.0) 92.0(62.8~127.8) U=575.500 0.124 AFP(ng/mL) 58.00(7.10~945.80) 18.28(2.48~405.33) U=536.500 0.057 BCLC(A/B/C/D,例) 51/4/12/0 12/5/5/0 χ2=5.229 0.073 最大肿瘤直径(cm) 3.95(2.50~7.70) 8.00(3.70~11.00) U=520.000 0.047 肝硬化[例(%)] 55(82.09) 9(40.91) χ2=13.904 <0.001 2.2 HBV-HCC血清ATG7表达水平升高
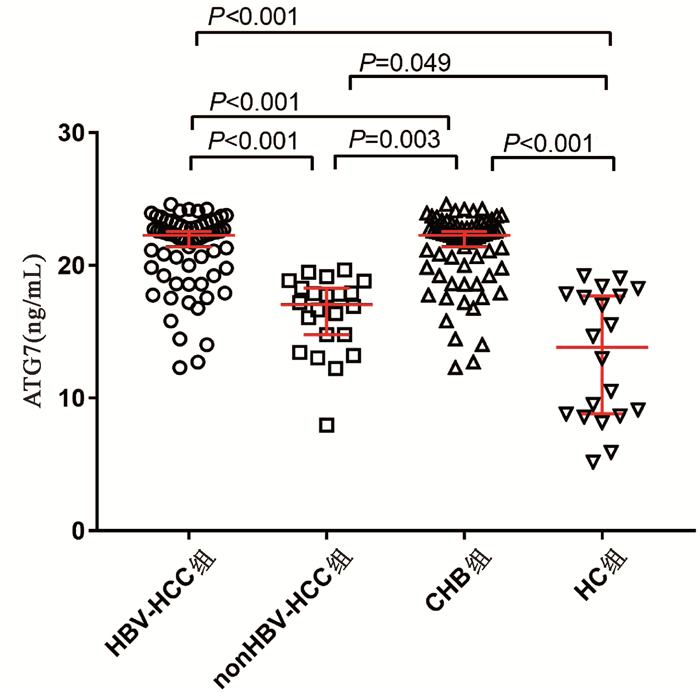
HBV- HCC组、nonHBV-HCC组、CHB组和HC组血清ATG7水平分别为22.88(19.79~23.04)ng/mL、17.06(14.45~19.40)ng/mL、19.21(16.65~20.82)ng/mL和13.82(8.70~17.82)ng/mL,差异有统计学意义(χ2=65.144,P<0.001)。两两比较显示HBV-HCC组血清ATG7不仅高于CHB组(U=758.5,P<0.001)和HC组(U=94.0,P<0.001),也高于nonHBV-HCC组(U=142.0,P<0.001);而nonHBV-HCC组血清ATG7水平不仅低于HBV-HCC组,也低于CHB组(U=311.0,P=0.003),仅稍高于HC组(U=142.0,P=0.049)(图 1)。此外,在67例HBV-HCC患者中,有肝硬化背景和无肝硬化背景患者的血清ATG7水平分别为22.32 (19.79~22.99)ng/mL和20.89(19.40~23.37)ng/mL,差异无统计学意义(P>0.05)。
2.3 血清ATG7表达水平和血清AFP水平的相关性
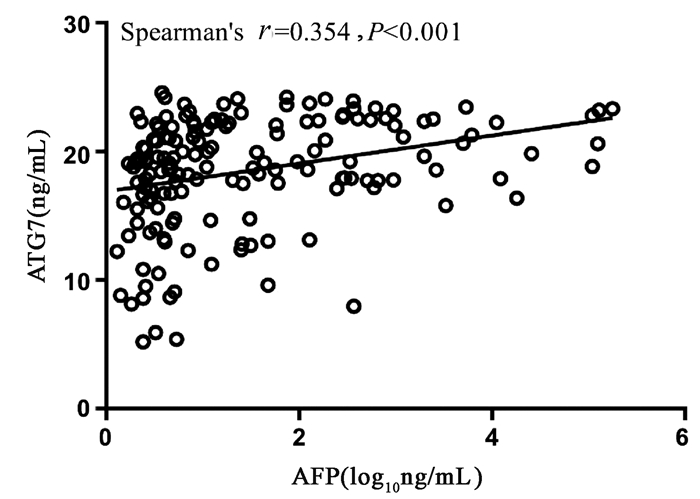
Spearman相关性分析显示,血清ATG7表达水平和血清AFP水平呈正相关,但相关性较弱(r=0.354,95%CI:0.205~0.486,P<0.001)(图 2)。
2.4 ATG7在HBV-HCC中的诊断性能
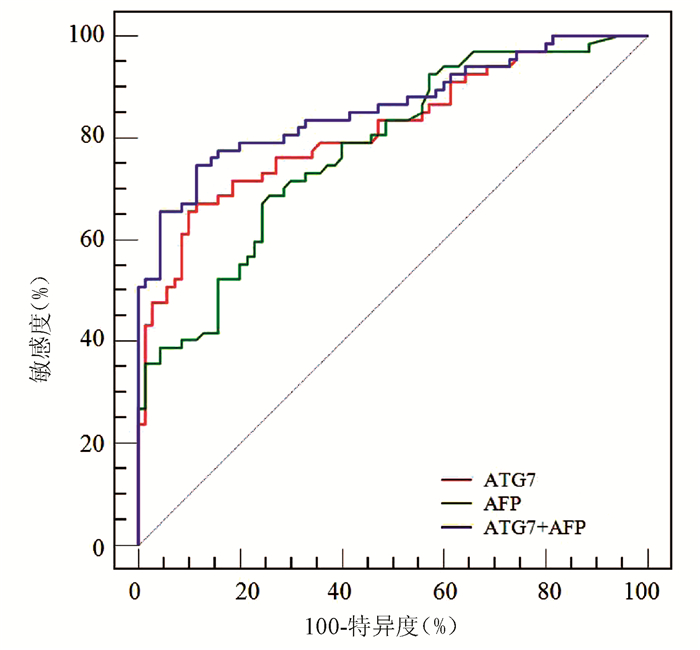
ROC曲线分析显示,ATG7诊断HBV-HCC的AUC为0.818(95% CI:0.743~0.879),稍高于AFP(AUC=0.777,95%CI:0.698~0.843),但二者差异无统计学意义(Z=0.852,P=0.394)(图 3);ATG7诊断HBV-HCC的cut-off值为20.08 ng/mL,敏感度和特异度分别为71.64%和77.14%,均略高于AFP(68.66%和74.29%)。ATG7联合AFP的二元logistic回归预测概率的AUC为0.859 (95%CI:0.790~0.913),显著高于ATG7(Z=2.192,P=0.028)和AFP(Z=2.076,P=0.038)(图 3, 表 3)。
表 3 ATG7、AFP及其联合检测诊断HBV-HCC的性能项目 AUC cut-off值 敏感度(%) 特异度(%) 阳性预测值(%) 阴性预测值(%) AFP 0.777 12.20 ng/mL 68.66 74.29 71.9 71.2 ATG7 0.818 20.08 ng/mL 71.64 77.14 75.0 74.0 ATG7+AFP 0.859 0.56 74.63 88.57 86.2 78.5 3. 讨论
本研究通过ELISA技术检测HCC患者血清ATG7表达水平,结果显示HBV-HCC患者血清ATG7显著升高,血清ATG7是诊断HBV-HCC的良好标志物,ATG7与AFP联合诊断可显著提高HBV-HCC的诊断效率。
ATG7作为一种泛素样修饰物激活酶,通过激活ATG8和ATG12调节自噬体的延伸,是自噬体形成过程的关键蛋白之一[10]。近年研究[11-12]提示ATG7及自噬在HBV-HCC发生发展中发挥重要作用。首先,HBV感染或HBV病毒蛋白的表达可诱导ATG7表达及自噬反应。本研究中CHB组血清ATG7显著高于健康对照组,印证了自噬与HBV感染有关。其次,多项研究[13-14]显示包括ATG7在内的多种自噬相关蛋白在HCC组织表达显著提高。近年的分子细胞学试验进一步揭示多种分子可通过ATG7调节自噬促进HCC发生发展[15-17]。研究[15]显示长链非编码RNA HOX转录反义RNA(HOTAIR)可通过上调ATG7表达激活自噬促进HCC细胞系增殖。癌性锚蛋白重复序列可直接与胞质中的ATG7蛋白相互作用诱导自噬从而促进HCC细胞进展[16]。肿瘤坏死因子α诱导蛋白8(TNFAIP8)则通过与ATG3-ATG7复合物相互作用调节自噬促进HCC细胞增殖[17]。相反,靶向敲减ATG7可抑制自噬,从而促进细胞凋亡、延缓细胞周期及抑制HCC细胞增殖[13]。上述研究结果表明ATG7与HBV-HCC密切相关。本研究数据显示HBV-HCC患者血清ATG7显著高于CHB和健康对照组,进一步证实了自噬与HBV-HCC的相关性,提示血清ATG7是一种潜在HBV-HCC标志物。而且HBV-HCC患者血清ATG7也显著高于nonHBV-HCC,提示不同病因的HCC发病机制不同,其血清标志物也可不同。
虽然AFP已在临床应用数十年,但诊断敏感度不高。最近的一项系统综述[18]显示,AFP诊断HCC的合并敏感度仅为63.9%。与此相符,本研究中AFP诊断HBV-HCC的敏感度、特异度和AUC分别为68.66%、74.29%和0.777。虽然ATG7的敏感度与AFP相仿,但其特异度较高,总体诊断性能较好。此外,ATG7和AFP相关性较弱,提示二者联合检测可有效提高诊断性能。早前有学者[19]提出应用logistic回归分析有助于提高多指标联合诊断效率。本研究结果显示ATG7联合AFP的二元logistic回归预测模型对HBV-HCC的整体诊断性能较好,AUC及诊断敏感度和特异度均显著高于ATG7及AFP。
总之,本研究显示ATG7是HBV-HCC的良好标志物,与AFP有较好的互补性,二者联合检测可显著提高HBV-HCC的诊断效率。然而本研究为单中心横断面研究,纳入研究病例有限,因此其诊断性能仍有待进一步扩大样本的多中心临床验证。
-
表 1 胰腺和回肠组织的病理学评分
Table 1. The pancreas and ileum tissue pathology score
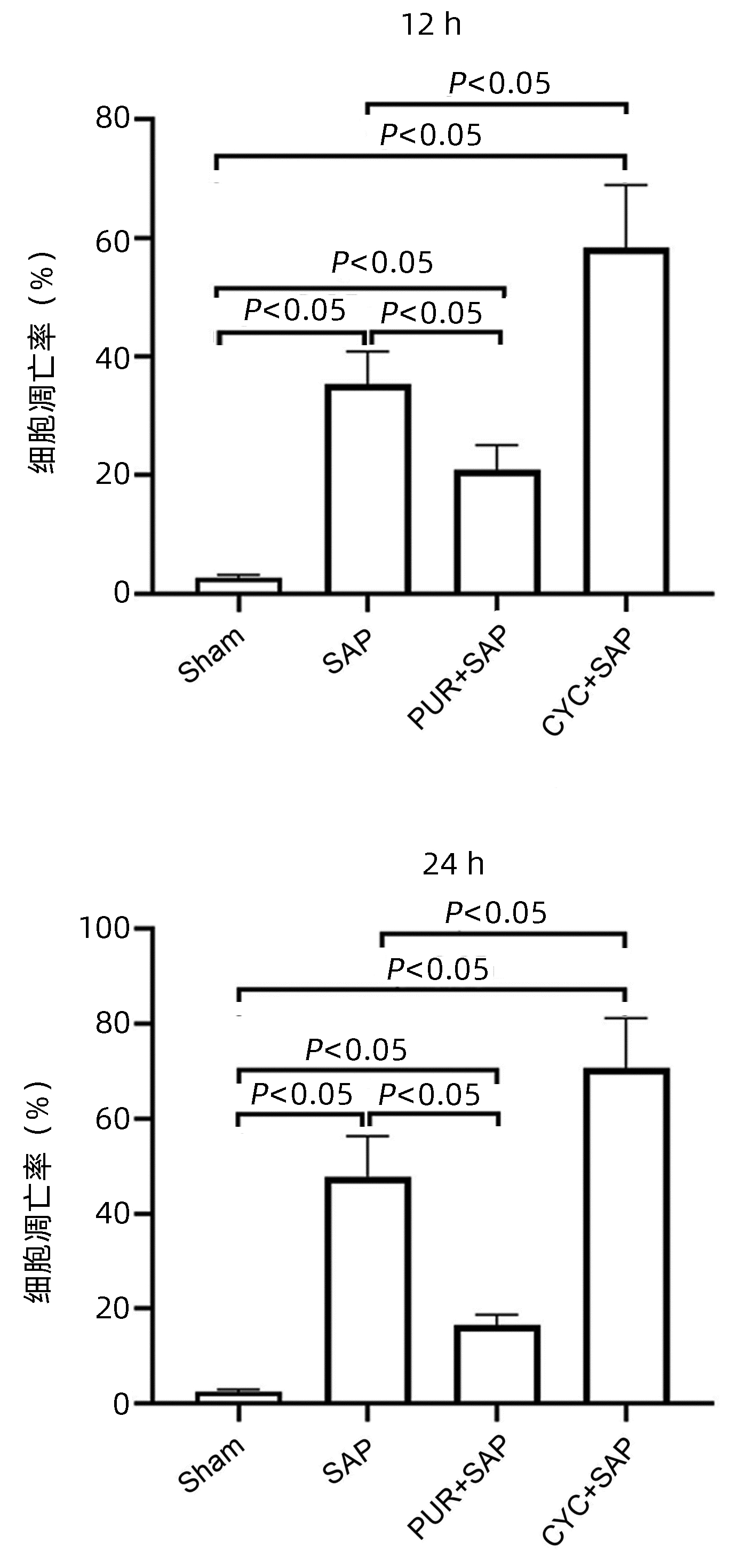
组别 动物数(只) 胰腺组织12 h 胰腺组织24 h 回肠组织12 h 回肠组织24 h Sham组 6 0.50(0.50~0.63) 0.50(0.50~0.63) 0.50(0.38~0.50) 0.75(0.50~1.00) SAP组 6 7.76(7.36~8.50)1) 8.75(8.38~9.50)1) 3.62(3.53~3.73)1) 4.25(4.07~4.73)1) PUR+SAP组 6 2.25(1.88~2.50)1)2) 2.75(2.38~3.13)1)2) 2.16(2.08~2.29)1)2) 1.20(1.08~1.60)1)2) CYC+SAP组 6 13.25(12.38~13.63)1)2) 15.50(15.00~15.63)1)2) 4.75(4.50~5.00)1)2) 5.00(5.00~5.00)1)2) H值 21.84 21.88 21.78 21.73 P值 <0.05 <0.05 <0.05 <0.05 注: 与Sham组比较,1)P<0.05;与SAP组比较,2)P<0.05。 -
[1] Chinese Society for Emergency Medicine; Beijing-Tianjin-Hebei Alliance of Emergency Treatment and First Aid; Emergency Medicine Branch, Beijing Medical Association, et al. Expert consensus on emergency diagnosis and treatment of acute pancreatitis[J]. J Clin Hepatol, 2021, 37(5): 1034-1041. DOI: 10.3969/j.issn.1001-5256.2021.05.012.中华医学会急诊分会, 京津冀急诊急救联盟, 北京医学会急诊分会, 等. 急性胰腺炎急诊诊断及治疗专家共识[J]. 临床肝胆病杂志, 2021, 37(5): 1034-1041. DOI: 10.3969/j.issn.1001-5256.2021.05.012. [2] TERAO K, WAKE H, ADACHI N, et al. Histidine-rich glycoprotein suppresses hyperinflammatory responses of lung in a severe acute pancreatitis mouse model[J]. Pancreas, 2018, 47(9): 1156-1164. DOI: 10.1097/MPA.0000000000001153. [3] CHEN X, ZHAO HX, FU XS, et al. Glucagonlike peptide 2 protects intestinal barrier in severe acute pancreatitis through regulating intestinal epithelial cell proliferation and apoptosis[J]. Pancreas, 2012, 41(7): 1080-1085. DOI: 10.1097/MPA.0b013e31824966b0. [4] ZHAO HX, FU XS, ZHOU XY, et al. Endoplasmic reticulum stress may not be involved in intestinal epithelial cell apoptosis in experimental acute pancreatitis[J]. Dig Dis Sci, 2015, 60(6): 1690-1698. DOI: 10.1007/s10620-015-3542-y. [5] INGHAM PW, MCMAHON AP. Hedgehog signaling in animal development: paradigms and principles[J]. Genes Dev, 2001, 15(23): 3059-3087. DOI: 10.1101/gad.938601. [6] PERIDES G, van ACKER GJ, LAUKKARINEN JM, et al. Experimental acute biliary pancreatitis induced by retrograde infusion of bile acids into the mouse pancreatic duct[J]. Nat Protoc, 2010, 5(2): 335-341. DOI: 10.1038/nprot.2009.243. [7] KUGLER MC, JOYNER AL, LOOMIS CA, et al. Sonic hedgehog signaling in the lung. From development to disease[J]. Am J Respir Cell Mol Biol, 2015, 52(1): 1-13. DOI: 10.1165/rcmb.2014-0132TR. [8] ZHOU X, LIU Z, JANG F, et al. Autocrine Sonic hedgehog attenuates inflammation in cerulein-induced acute pancreatitis in mice via upregulation of IL-10[J]. PLoS One, 2012, 7(8): e44121. DOI: 10.1371/journal.pone.0044121. [9] HUANG J, ZHENG YQ, ZHOU XY. The role of Hedgehog signal in mice with acute pancreatitis[J]. J Luzhou Med Coll, 2013, 36(2): 121-123. https://www.cnki.com.cn/Article/CJFDTOTAL-LXYB201302010.htm黄娟, 郑英强, 周翔宇. 小鼠急性胰腺炎时Hedgehog信号通路mRNA的表达及意义[J]. 泸州医学院学报, 2013, 36(2): 121-123. https://www.cnki.com.cn/Article/CJFDTOTAL-LXYB201302010.htm [10] LAI K, LUO L, WANG F, et al. Expression of Shh in different tissues in acute pancreatitis[J]. J Southwest Med Univ, 2017, 40(1): 26-30. DOI: 10.3969/j.issn.1000-2669.2017.01.008.赖坤, 罗澜, 王芳, 等. 小鼠急性胰腺炎中Shh在胰腺局部和远隔器官的表达变化[J]. 西南医科大学学报, 2017, 40(1): 26-30. DOI: 10.3969/j.issn.1000-2669.2017.01.008. [11] SINHA S, CHEN JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened[J]. Nat Chem Biol, 2006, 2(1): 29-30. DOI: 10.1038/nchembio753. [12] van den HEUVEL M, INGHAM PW. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling[J]. Nature, 1996, 382(6591): 547-551. DOI: 10.1038/382547a0. [13] LIAO X, SIU MK, AU CW, et al. Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation[J]. Carcinogenesis, 2009, 30(1): 131-140. DOI: 10.1093/carcin/bgn230. [14] MAHESHWARI R, SUBRAMANIAN RM. Severe acute pancreatitis and necrotizing pancreatitis[J]. Crit Care Clin, 2016, 32(2): 279-290. DOI: 10.1016/j.ccc.2015.12.006. [15] DENG W, ABLIZ A, XU S, et al. Severity of pancreatitis-associated intestinal mucosal barrier injury is reduced following treatment with the NADPH oxidase inhibitor apocynin[J]. Mol Med Rep, 2016, 14(4): 3525-3534. DOI: 10.3892/mmr.2016.5678. [16] TIAN R, TAN JT, WANG RL, et al. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis[J]. Eur Rev Med Pharmacol Sci, 2013, 17(3): 349-355. [17] CAPURSO G, ZERBONI G, SIGNORETTI M, et al. Role of the gut barrier in acute pancreatitis[J]. J Clin Gastroenterol, 2012, 46 (Suppl): S46-S51. DOI: 10.1097/MCG.0b013e3182652096. [18] Pancreas Study Group, Chinese Society of Gastroenterology, Chinese Medical Association, Editorial Board of Chinese Journal of Pancreatology, Editorial Board of Chinese Journal of Digestion. Chinese guidelines for the management of acute pancreatitis (Shanghai, 2013)[J]. J Clin Hepatol, 2013, 29(9): 656-660. DOI: 10.3969/j.issn.1001-5256.2013.09.006.中华医学会消化病学分会胰腺疾病学组, 《中华胰腺病杂志》编辑委员会, 《中华消化杂志》编辑委员会. 中国急性胰腺炎诊治指南(2013年, 上海)[J]. 临床肝胆病杂志, 2013, 29(9): 656-660. DOI: 10.3969/j.issn.1001-5256.2013.09.006. [19] WISCHMEYER PE. Nutrition therapy in sepsis[J]. Crit Care Clin, 2018, 34(1): 107-125. DOI: 10.1016/j.ccc.2017.08.008. [20] LUAN ZG, ZHANG H, MA XC, et al. Role of high-mobility group box 1 protein in the pathogenesis of intestinal barrier injury in rats with severe acute pancreatitis[J]. Pancreas, 2010, 39(2): 216-223. DOI: 10.1097/MPA.0b013e3181bab5c5. [21] PAN LY, CHEN YF, LI HC, et al. Dachengqi Decoction attenuates intestinal vascular endothelial injury in severe acute pancreatitis in vitro and in vivo[J]. Cell Physiol Biochem, 2017, 44(6): 2395-2406. DOI: 10.1159/000486155. [22] LEE JH, CHUNG YC, BOK E, et al. Injury-stimulated Sonic hedgehog expression in microglia contributes to neuroinflammatory response in the MPTP model of Parkinson's disease[J]. Biochem Biophys Res Commun, 2017, 482(4): 980-986. DOI: 10.1016/j.bbrc.2016.11.144. [23] XIA YP, HE QW, LI YN, et al. Recombinant human sonic hedgehog protein regulates the expression of ZO-1 and occludin by activating angiopoietin-1 in stroke damage[J]. PLoS One, 2013, 8(7): e68891. DOI: 10.1371/journal.pone.0068891. [24] LV T, SHEN L, YANG L, et al. Polydatin ameliorates dextran sulfate sodium-induced colitis by decreasing oxidative stress and apoptosis partially via Sonic hedgehog signaling pathway[J]. Int Immunopharmacol, 2018, 64: 256-263. DOI: 10.1016/j.intimp.2018.09.009. [25] IKEDA H, SUZUKI Y, SUZUKI M, et al. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium[J]. Gut, 1998, 42(4): 530-537. DOI: 10.1136/gut.42.4.530. [26] YAMAMOTO S, TANABE M, WAKABAYASHI G, et al. The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine[J]. J Surg Res, 2001, 99(1): 134-141. DOI: 10.1006/jsre.2001.6106. [27] MENG QH, LIU HB, WANG JB. Polydatin ameliorates renal ischemia/reperfusion injury by decreasing apoptosis and oxidative stress through activating sonic hedgehog signaling pathway[J]. Food Chem Toxicol, 2016, 96: 215-225. DOI: 10.1016/j.fct.2016.07.032. [28] LIANG R, MORRIS P, CHO SS, et al. Hedgehog signaling displays a biphasic expression pattern during intestinal injury and repair[J]. J Pediatr Surg, 2012, 47(12): 2251-2263. DOI: 10.1016/j.jpedsurg.2012.09.016. [29] TIAN A, SHI Q, JIANG A, et al. Injury-stimulated Hedgehog signaling promotes regenerative proliferation of Drosophila intestinal stem cells[J]. J Cell Biol, 2015, 208(6): 807-819. DOI: 10.1083/jcb.201409025. 期刊类型引用(2)
1. 郭佳佳,张文果,王文秀,张晓丽,马徜徉. 血清Nox2、ATG7水平对新生儿窒息心肌损伤的评估价值. 安徽医药. 2024(09): 1791-1795 .  百度学术
百度学术2. 蔡卓玮,胡刚峰,章波. miR-203在乙型肝炎病毒相关肝细胞癌诊断及预后中的意义. 广西医科大学学报. 2022(11): 1773-1780 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 5731 KB)
PDF下载 ( 5731 KB)

 下载:
下载:




 下载:
下载:




 百度学术
百度学术