骨形态发生蛋白4在非酒精性脂肪性肝病发病机制中的作用
DOI: 10.3969/j.issn.1001-5256.2023.05.027
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:王宇威负责收集文献,撰写论文;柴茹负责收集、筛选文献;刘近春负责拟定写作思路,指导撰写文章并最后定稿。
Role of bone morphogenetic protein-4 in the pathogenesis of nonalcoholic fatty liver disease
-
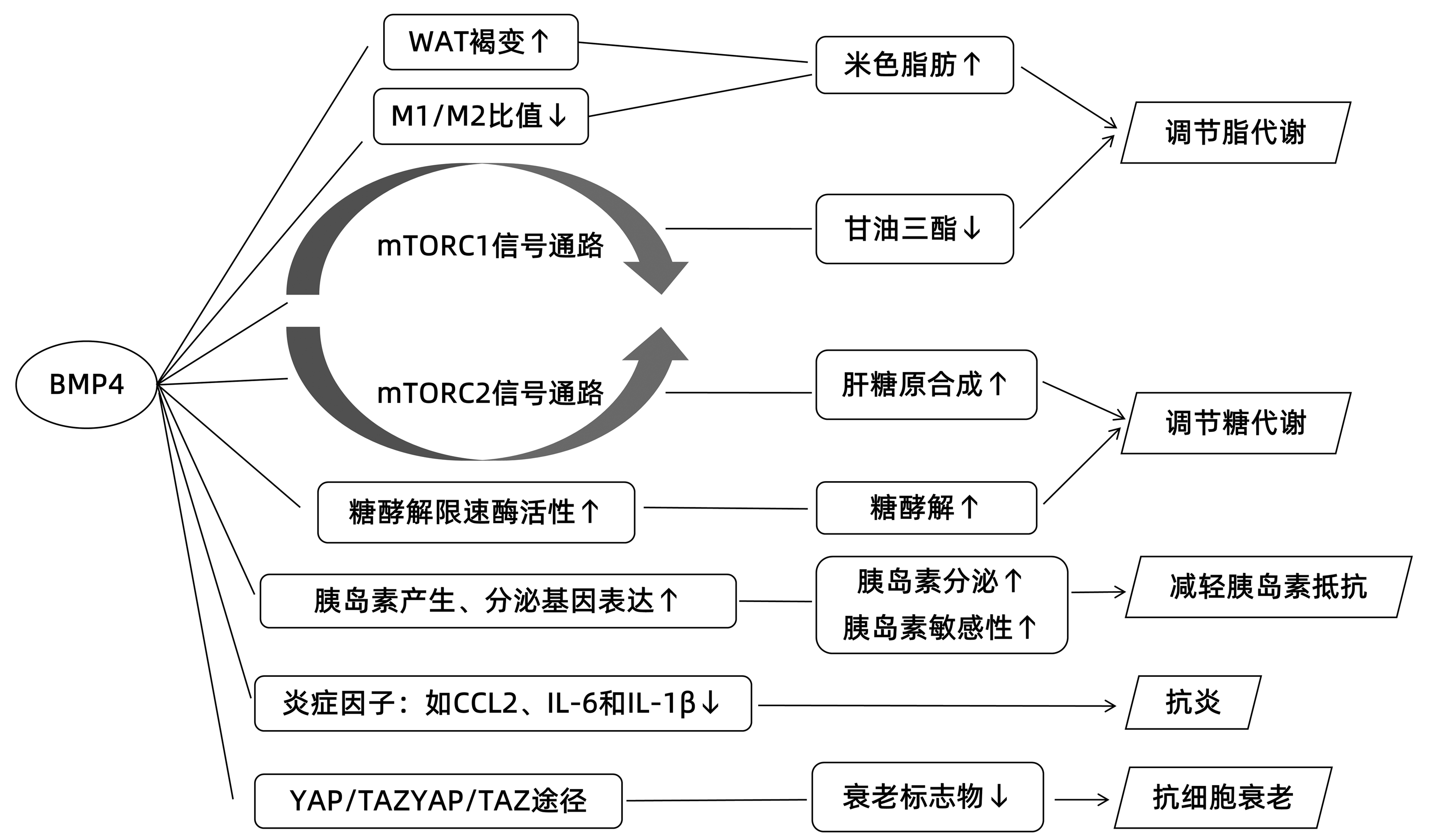
摘要: 非酒精性脂肪性肝病(NAFLD)目前已成为全球最常见的慢性肝病之一,严重危害人类健康。近年来研究发现骨形态发生蛋白4(BMP4)与NAFLD之间可能存在一定相关性。本综述旨在依据国内外关于BMP4与NAFLD相关研究的最新进展,探究BMP4作用于NAFLD的潜在机制,以期为NAFLD的预防及治疗提供新的思路。Abstract: Nonalcoholic fatty liver disease (NAFLD) has become one of the most common chronic liver diseases in the world, and it seriously harms human health. Recent studies have found that bone morphogenetic protein 4 (BMP4) might be associated with NAFLD. This article reviews the latest advances in the research on the association between BMP4 and NAFLD in China and globally and explores the potential mechanism of action of BMP4 on NAFLD, in order to provide new ideas for the prevention and treatment of NAFLD.
-
[1] LI J, ZOU B, YEO YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2019, 4(5): 389-398. DOI: 10.1016/S2468-1253(19)30039-1. [2] ESLAM M, SARIN SK, WONG VW, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease[J]. Hepatol Int, 2020, 14(6): 889-919. DOI: 10.1007/s12072-020-10094-2. [3] YOUNOSSI ZM, COREY KE, LIM JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review[J]. Gastroenterology, 2021, 160(3): 912-918. DOI: 10.1053/j.gastro.2020.11.051. [4] BABOOTA RK, BLVHER M, SMITH U. Emerging role of bone morphogenetic protein 4 in metabolic disorders[J]. Diabetes, 2021, 70(2): 303-312. DOI: 10.2337/db20-0884. [5] ZOU ML, CHEN ZH, TENG YY, et al. The Smad dependent TGF-β and BMP signaling pathway in bone remodeling and therapies[J]. Front Mol Biosci, 2021, 8: 593310. DOI: 10.3389/fmolb.2021.593310. [6] WU J, JIANG H, WANG L. Relationship between serum lipocalin 2, bone morphogenetic protein 4 levels and osteoporosis in elderly patients with type 2 diabetes mellitus[J]. China Med Herald, 2022, 19(12): 66-69, 74. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202212015.htm吴静, 姜惠, 王丽. 老年2型糖尿病患者血清脂质运载蛋白2、骨形态发生蛋白4与骨质疏松的关系[J]. 中国医药导报, 2022, 19(12): 66-69, 74. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202212015.htm [7] MOSTAFA S, PAKVASA M, COALSON E, et al. The wonders of BMP9: From mesenchymal stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism to regenerative medicine[J]. Genes Dis, 2019, 6(3): 201-223. DOI: 10.1016/j.gendis.2019.07.003. [8] ZHANG L, LUO Q, SHU Y, et al. Transcriptomic landscape regulated by the 14 types of bone morphogenetic proteins (BMPs) in lineage commitment and differentiation of mesenchymal stem cells (MSCs)[J]. Genes Dis, 2019, 6(3): 258-275. DOI: 10.1016/j.gendis.2019.03.008. [9] EHATA S, MIYAZONO K. Bone morphogenetic protein signaling in cancer; some topics in the recent 10 years[J]. Front Cell Dev Biol, 2022, 10: 883523. DOI: 10.3389/fcell.2022.883523. [10] BACH DH, PARK HJ, LEE SK. The dual role of bone morphogenetic proteins in cancer[J]. Mol Ther Oncolytics, 2018, 8: 1-13. DOI: 10.1016/j.omto.2017.10.002. [11] VACCA M, LESLIE J, VIRTUE S, et al. Bone morphogenetic protein 8B promotes the progression of non-alcoholic steatohepatitis[J]. Nat Metab, 2020, 2(6): 514-531. DOI: 10.1038/s42255-020-0214-9. [12] SUN QJ, CAI LY, JIAN J, et al. The role of bone morphogenetic protein 9 in nonalcoholic fatty liver disease in mice[J]. Front Pharmacol, 2020, 11: 605967. DOI: 10.3389/fphar.2020.605967. [13] YANG Z, LI P, SHANG Q, et al. CRISPR-mediated BMP9 ablation promotes liver steatosis via the down-regulation of PPARα expression[J]. Sci Adv, 2020, 6(48): eabc5022. DOI: 10.1126/sciadv.abc5022. [14] CHUNG YH, HUANG YH, CHU TH, et al. BMP-2 restoration aids in recovery from liver fibrosis by attenuating TGF-β1 signaling[J]. Lab Invest, 2018, 98(8): 999-1013. DOI: 10.1038/s41374-018-0069-9. [15] BABOOTA RK, RAWSHANI A, BONNET L, et al. BMP4 and Gremlin 1 regulate hepatic cell senescence during clinical progression of NAFLD/NASH[J]. Nat Metab, 2022, 4(8): 1007-1021. DOI: 10.1038/s42255-022-00620-x. [16] YANG J, UEHARU H, MISHINA Y. Energy metabolism: A newly emerging target of BMP signaling in bone homeostasis[J]. Bone, 2020, 138: 115467. DOI: 10.1016/j.bone.2020.115467. [17] GANNON M. BuMP-ing up insulin secretion by pancreatic beta cells[J]. Cell Metab, 2007, 5(3): 157-159. DOI: 10.1016/j.cmet.2007.02.003. [18] ROH HC, TSAI L, SHAO M, et al. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity[J]. Cell Metab, 2018, 27(5): 1121-1137. DOI: 10.1016/j.cmet.2018.03.005. [19] WU Y, KINNEBREW MA, KUTYAVIN VI, et al. Distinct signaling and transcriptional pathways regulate peri-weaning development and cold-induced recruitment of beige adipocytes[J]. Proc Natl Acad Sci U S A, 2020, 117(12): 6883-6889. DOI: 10.1073/pnas.1920419117. [20] HOFFMANN JM, GRVNBERG JR, CHURCH C, et al. BMP4 gene therapy in mature mice reduces BAT activation but protects from obesity by browning subcutaneous adipose tissue[J]. Cell Rep, 2017, 20(5): 1038-1049. DOI: 10.1016/j.celrep.2017.07.020. [21] QIAN SW, TANG Y, LI X, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis[J]. Proc Natl Acad Sci U S A, 2013, 110(9): E798-E807. DOI: 10.1073/pnas.1215236110. [22] QIAN SW, WU MY, WANG YN, et al. BMP4 facilitates beige fat biogenesis via regulating adipose tissue macrophages[J]. J Mol Cell Biol, 2019, 11(1): 14-25. DOI: 10.1093/jmcb/mjy011. [23] HOFFMANN JM, GRVNBERG JR, HAMMARSTEDT A, et al. BMP4 gene therapy enhances insulin sensitivity but not adipose tissue browning in obese mice[J]. Mol Metab, 2020, 32: 15-26. DOI: 10.1016/j.molmet.2019.11.016. [24] PENG Q, CHEN B, WANG H, et al. Bone morphogenetic protein 4 (BMP4) alleviates hepatic steatosis by increasing hepatic lipid turnover and inhibiting the mTORC1 signaling axis in hepatocytes[J]. Aging (Albany NY), 2019, 11(23): 11520-11540. DOI: 10.18632/aging.102552. [25] SAXTON RA, SABATINI DM. mTOR signaling in growth, metabolism, and disease[J]. Cell, 2017, 169(2): 361-371. DOI: 10.1016/j.cell.2017.03.035. [26] LAMMING DW, SABATINI DM. A central role for mTOR in lipid homeostasis[J]. Cell Metab, 2013, 18(4): 465-469. DOI: 10.1016/j.cmet.2013.08.002. [27] YOUNOSSI ZM, MARCHESINI G, PINTO-CORTEZ H, et al. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation[J]. Transplantation, 2019, 103(1): 22-27. DOI: 10.1097/TP.0000000000002484. [28] ZHONG J, KANG Q, CAO Y, et al. BMP4 augments the survival of hepatocellular carcinoma (HCC) cells under hypoxia and hypoglycemia conditions by promoting the glycolysis pathway[J]. Am J Cancer Res, 2021, 11(3): 793-811. [29] AN L, SHI Q, ZHU Y, et al. Bone morphogenetic protein 4 (BMP4) promotes hepatic glycogen accumulation and reduces glucose level in hepatocytes through mTORC2 signaling pathway[J]. Genes Dis, 2021, 8(4): 531-544. DOI: 10.1016/j.gendis.2020.11.004. [30] SAKHNENY L, MUELLER L, SCHONBLUM A, et al. The postnatal pancreatic microenvironment guides β cell maturation through BMP4 production[J]. Dev Cell, 2021, 56(19): 2703-2711. DOI: 10.1016/j.devcel.2021.08.014. [31] SHEN H, HUANG GJ, GONG YW. Effect of transforming growth factor beta and bone morphogenetic proteins on rat hepatic stellate cell proliferation and trans-differentiation[J]. World J Gastroenterol, 2003, 9(4): 784-787. DOI: 10.3748/wjg.v9.i4.784. [32] PEGORIER S, CAMPBELL GA, KAY AB, et al. Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-beta1 in normal human lung fibroblasts (NHLF)[J]. Respir Res, 2010, 11(1): 85. DOI: 10.1186/1465-9921-11-85. [33] OMAR R, YANG J, ALRUSHAID S, et al. Inhibition of BMP4 and alpha smooth muscle actin expression in LX-2 hepatic stellate cells by BMP4-siRNA lipid based nanoparticle[J]. J Pharm Pharm Sci, 2018, 21(1): 119-134. DOI: 10.18433/jpps29584. [34] ZENG XY, ZHANG YQ, HE XM, et al. Suppression of hepatic stellate cell activation through downregulation of gremlin1 expression by the miR-23b/27b cluster[J]. Oncotarget, 2016, 7(52): 86198-86210. DOI: 10.18632/oncotarget.13365. [35] ZHANG YQ, WAN LY, HE XM, et al. Gremlin1 accelerates hepatic stellate cell activation through upregulation of TGF-beta expression[J]. DNA Cell Biol, 2017, 36(7): 603-610. DOI: 10.1089/dna.2017.3707. [36] BARABAN E, CHAVAKIS T, HAMILTON BS, et al. Anti-inflammatory properties of bone morphogenetic protein 4 in human adipocytes[J]. Int J Obes (Lond), 2016, 40(2): 319-327. DOI: 10.1038/ijo.2015.141. [37] JIN R, WANG XX, LIU F, et al. Research advances in pharmacotherapy for nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2022, 38(7): 1634-1640. DOI: 10.3969/j.issn.1001-5256.2022.07.033.靳睿, 王晓晓, 刘峰, 等. 非酒精性脂肪性肝病的药物治疗进展[J]. 临床肝胆病杂志, 2022, 38(7): 1634-1640. DOI: 10.3969/j.issn.1001-5256.2022.07.033. [38] OGRODNIK M, MIWA S, TCHKONIA T, et al. Cellular senescence drives age-dependent hepatic steatosis[J]. Nat Commun, 2017, 8: 15691. DOI: 10.1038/ncomms15691. [39] ARAVINTHAN A, SCARPINI C, TACHTATZIS P, et al. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease[J]. J Hepatol, 2013, 58(3): 549-556. DOI: 10.1016/j.jhep.2012.10.031. [40] MIYAJIMA C, KAWARADA Y, INOUE Y, et al. Transcriptional coactivator TAZ negatively regulates tumor suppressor p53 activity and cellular senescence[J]. Cells, 2020, 9(1): 171. DOI: 10.3390/cells9010171. [41] HAYASHI Y, HSIAO EC, SAMI S, et al. BMP-SMAD-ID promotes reprogramming to pluripotency by inhibiting p16/INK4A-dependent senescence[J]. Proc Natl Acad Sci U S A, 2016, 113(46): 13057-13062. DOI: 10.1073/pnas.1603668113. [42] LU L, FINEGOLD MJ, JOHNSON RL. Hippo pathway coactivators Yap and Taz are required to coordinate mammalian liver regeneration[J]. Exp Mol Med, 2018, 50(1): e423. DOI: 10.1038/emm.2017.205. [43] KONISHI T, SCHUSTER RM, LENTSCH AB. Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury[J]. Am J Physiol Gastrointest Liver Physiol, 2018, 314(4): G471-G482. DOI: 10.1152/ajpgi.00153.2017. [44] MOORING M, FOWL BH, LUM S, et al. Hepatocyte stress increases expression of Yes-associated protein and transcriptional coactivator with PDZ-binding motif in hepatocytes to promote parenchymal inflammation and fibrosis[J]. Hepatology, 2020, 71(5): 1813-1830. DOI: 10.1002/hep.30928. [45] HAAK AJ, KOSTALLARI E, SICARD D, et al. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis[J]. Sci Transl Med, 2019, 11(516): eaau6296. DOI: 10.1126/scitranslmed.aau6296. -



 PDF下载 ( 2189 KB)
PDF下载 ( 2189 KB)

 下载:
下载:


