肝移植术后感染病原菌分布及耐药性分析
DOI: 10.3969/j.issn.1001-5256.2023.06.017
Distribution and drug resistance of pathogenic bacteria for infection after liver transplantation
-
摘要:
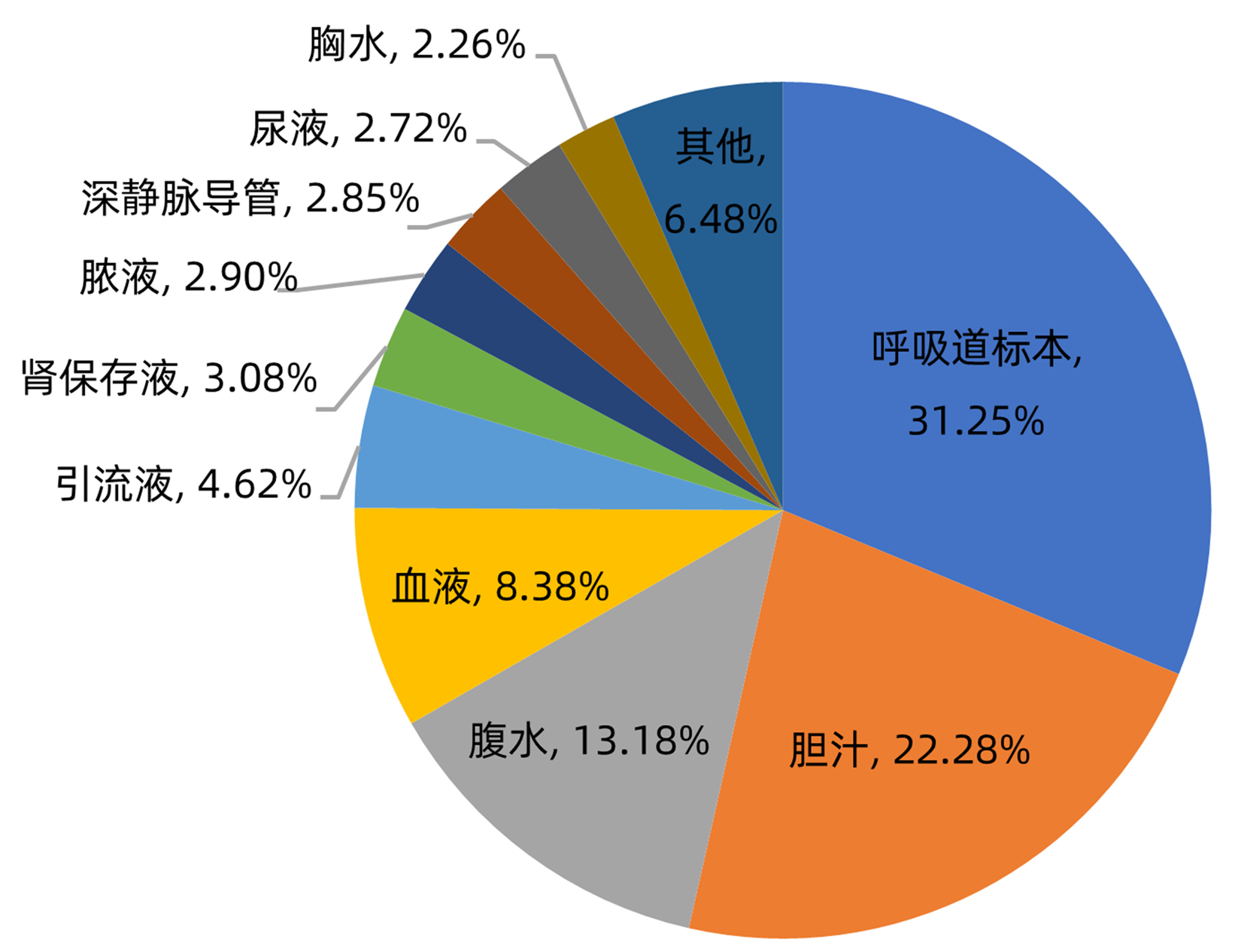
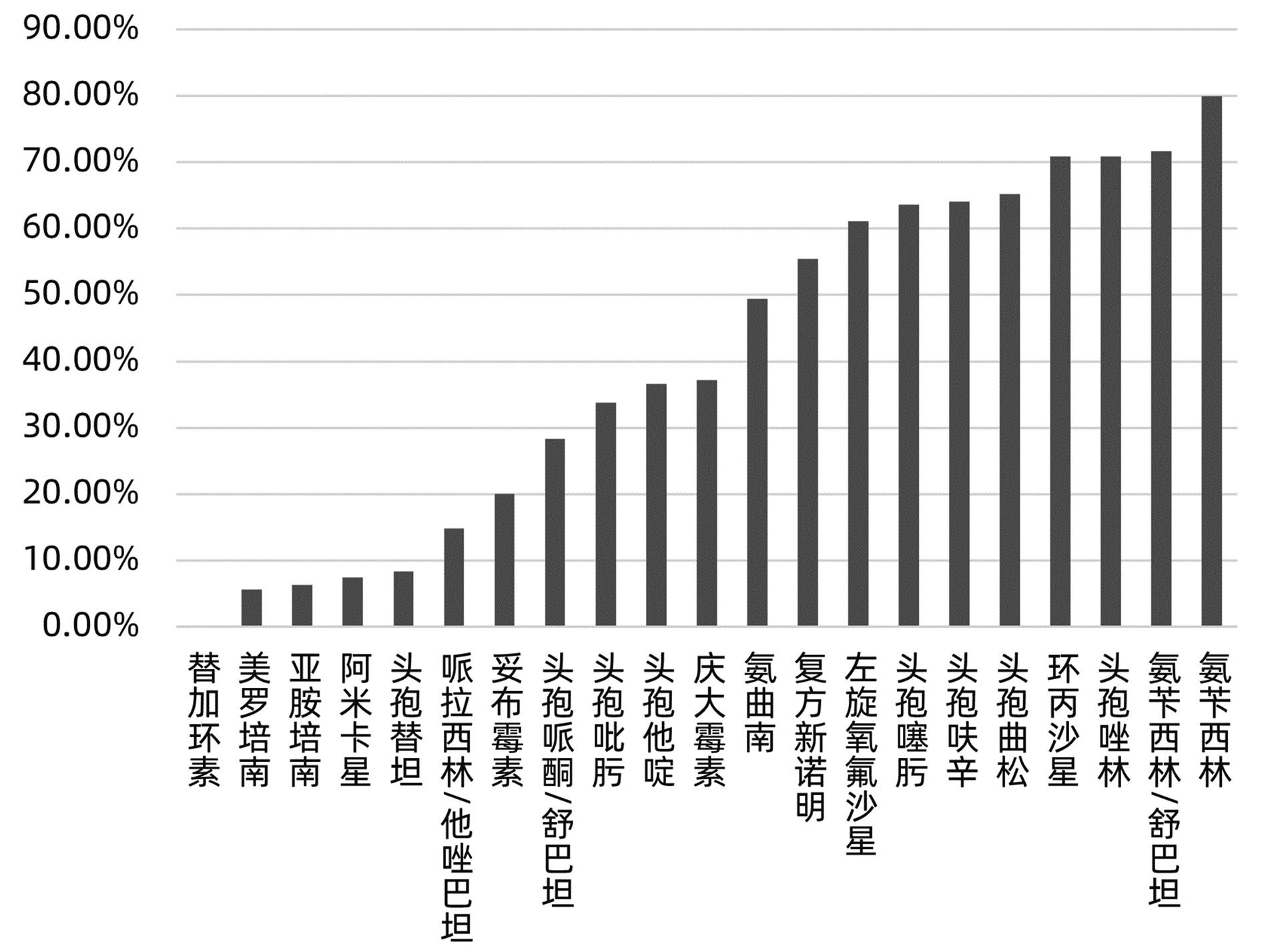
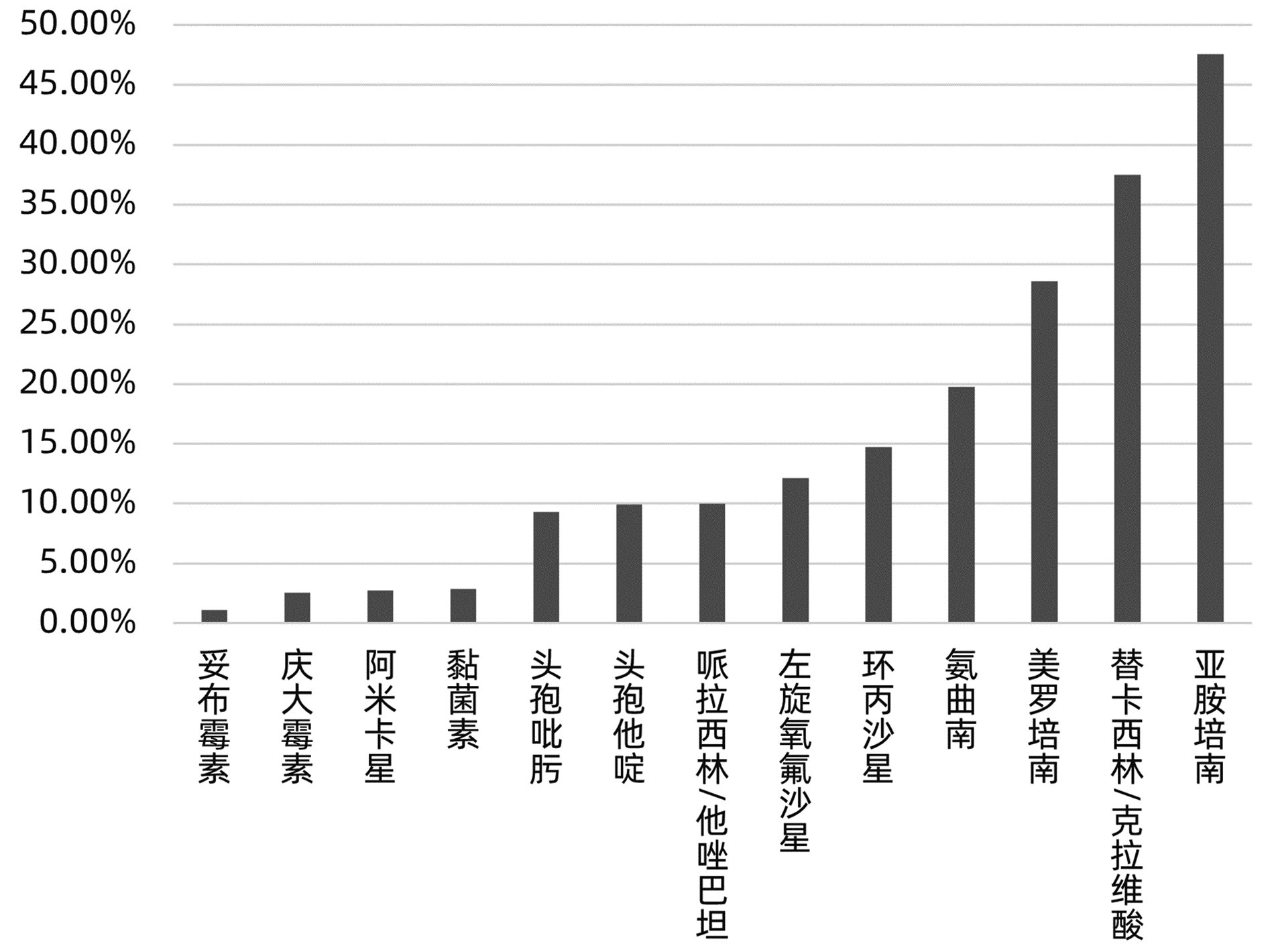
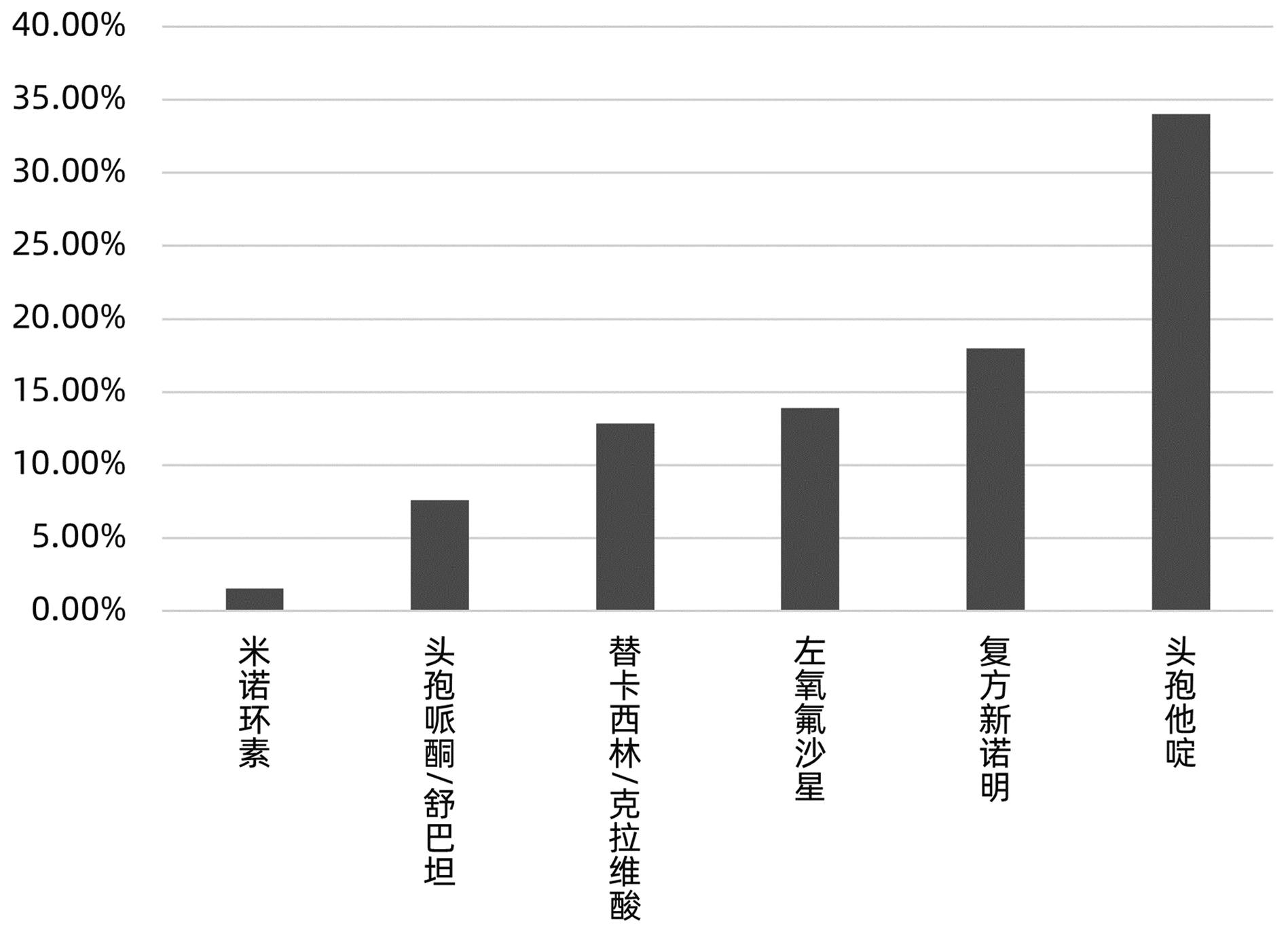
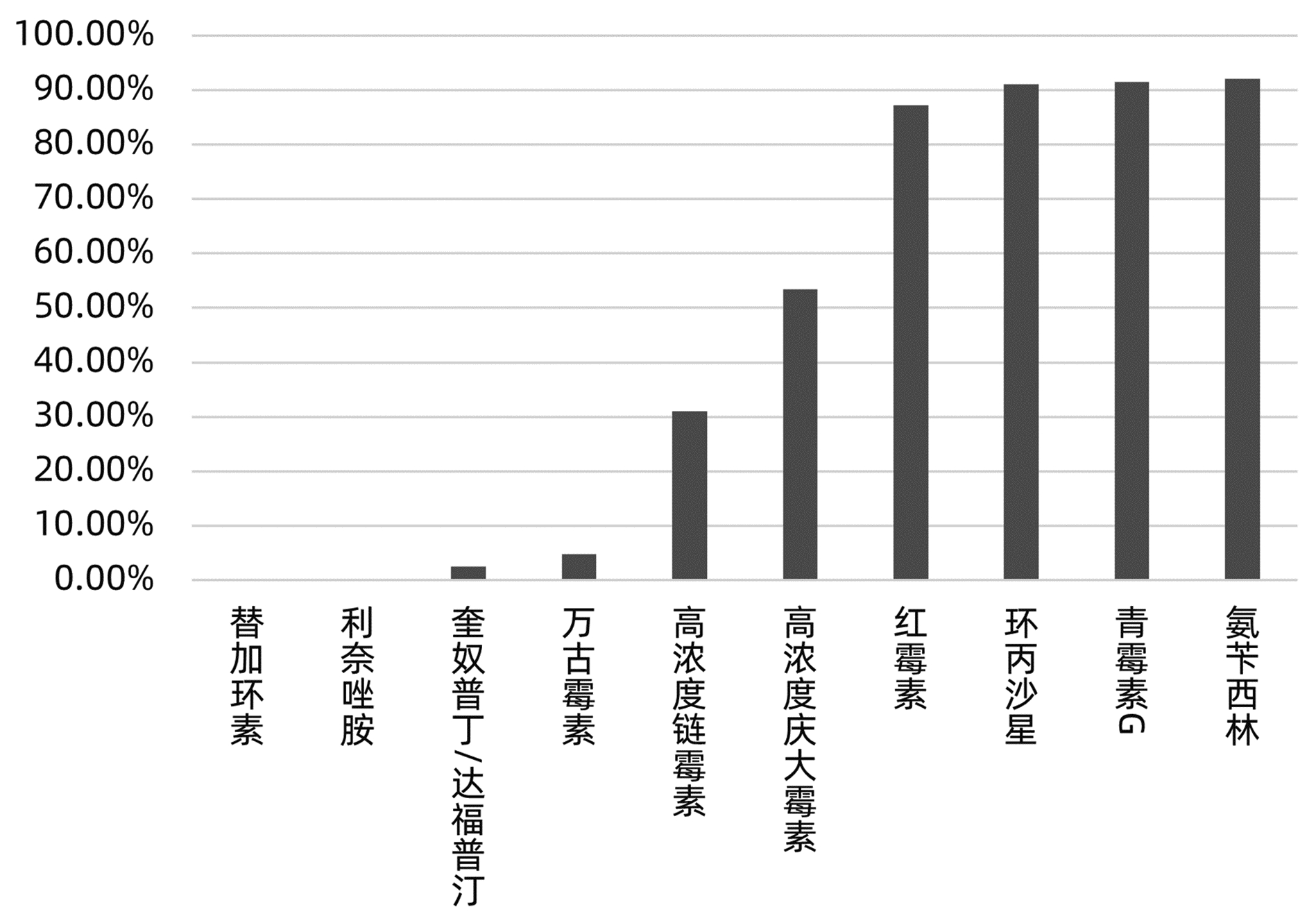
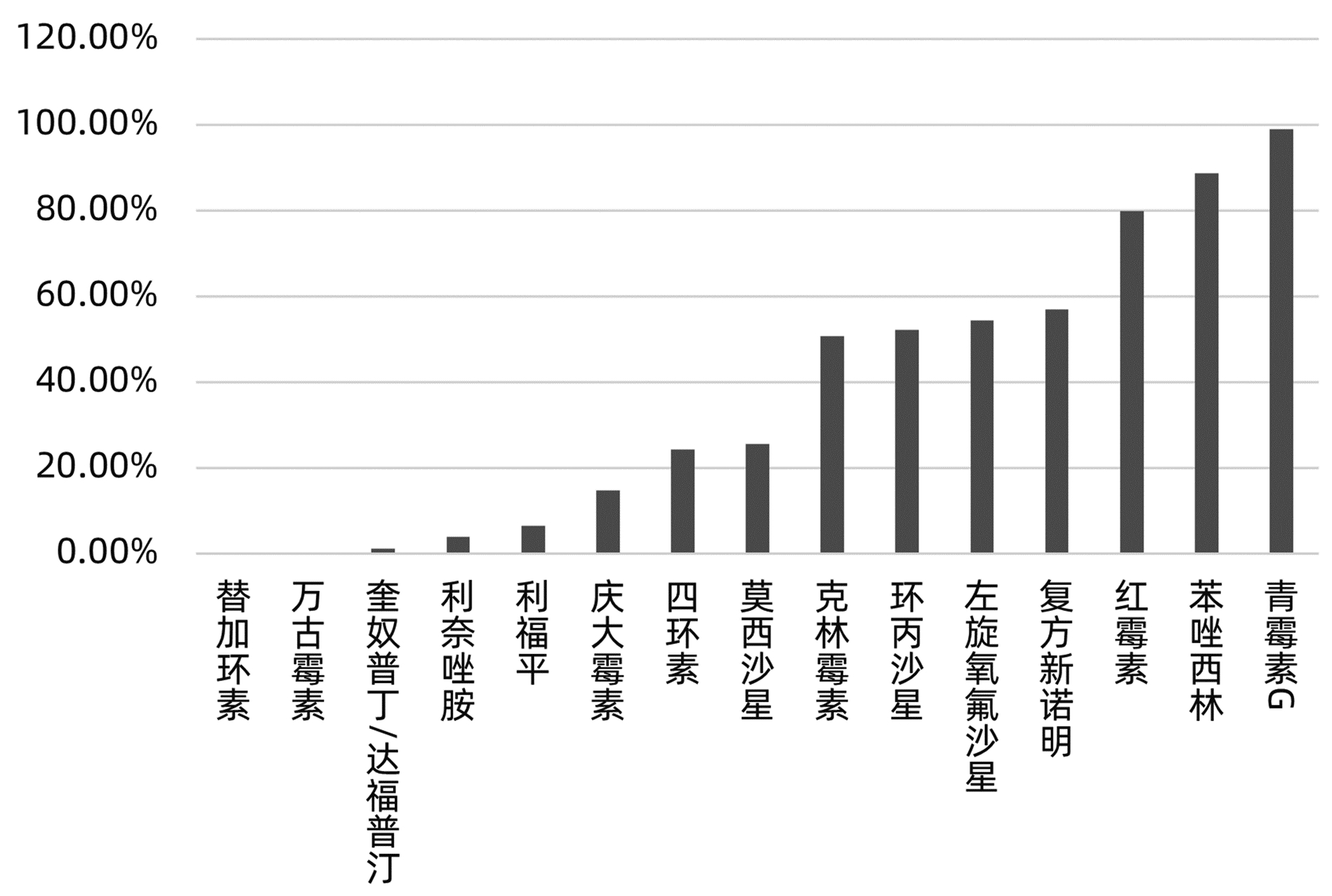
目的 分析肝移植术后感染病原菌分布及耐药性,为临床合理应用抗菌药物提供科学依据。 方法 对2014年3月—2021年12月青岛大学附属医院904例肝移植术后感染患者送检的各类标本分离出的病原菌分布及耐药性进行分析。采用WHONET 5.6软件对菌株列表及细菌耐药率进行统计;采用Excel对标本来源、构成比及病原菌分布情况等进行统计分析。 结果 共分离出非重复性病原菌2 208株,主要分离自呼吸道(31.25%)、胆汁(22.28%)、腹水(13.18%)、血液(8.38%)和引流液(4.62%),前10位病原菌为肺炎克雷伯菌肺炎亚种(10.69%)、屎肠球菌(10.42%)、大肠埃希菌(8.24%)、铜绿假单胞菌(8.24%)、表皮葡萄球菌(8.06%)、鲍曼不动杆菌(7.93%)、嗜麦芽窄食单胞菌(6.61%)、阴沟肠杆菌(3.22%)、溶血葡萄球菌(3.08%)和金黄色葡萄球菌(2.94%),共占比69.43%。呼吸道标本的主要致病菌为铜绿假单胞菌、嗜麦芽窄食单胞菌、肺炎克雷伯菌肺炎亚种和鲍曼不动杆菌,胆汁、腹水和引流液标本的主要致病菌为屎肠球菌,血液标本的主要致病菌为大肠埃希菌、表皮葡萄球菌和肺炎克雷伯菌肺炎亚种。药敏数据显示:肠杆菌目细菌对头孢菌素类和氟喹诺酮类均有较高的耐药率,对氨基糖苷类中阿米卡星的耐药率<10%,未检出对替加环素耐药菌株,其中肺炎克雷伯菌肺炎亚种对美罗培南、亚胺培南的耐药率分别为14.71%、11.35%,均高于大肠埃希菌(5.66%、6.29%);非发酵菌对碳青霉烯类药物均有较高的耐药率,但对替加环素和黏菌素耐药率均<10%。革兰阳性球菌中,屎肠球菌对万古霉素和奎奴普丁/达福普汀的耐药率分别为6.17%和2.44%,未发现对替加环素和利奈唑胺耐药的屎肠球菌;表皮葡萄球菌对大环内酯类、氟喹诺酮类、磺胺类和林可霉素类抗菌药物的耐药率均>50%,少部分菌株对利奈唑胺和奎奴普丁/达福普汀耐药(<3%),未检出对替加环素和万古霉素耐药的表皮葡萄球菌。重点监测耐药菌287株,占比13%,分别为耐碳青霉烯类鲍曼不动杆菌128株、耐碳青霉烯类铜绿假单胞菌88株、耐碳青霉烯类肺炎克雷伯菌肺炎亚种26株、耐碳青霉烯类大肠埃希菌11株、耐甲氧西林金黄色葡萄球菌23株和耐万古霉素肠球菌11株,其中,耐碳青霉烯类肺炎克雷伯菌肺炎亚种主要产丝氨酸碳青霉烯酶,耐碳青霉烯类大肠埃希菌主要产金属β-内酰胺酶。 结论 肝移植术后感染的病原菌以革兰阴性菌为主,不同标本类型病原菌的种类分布不同,部分菌株耐药率呈增长趋势,需要加强医院感染和抗菌药物的管理。 Abstract:Objective To investigate the distribution and drug resistance of pathogenic bacteria for infection after liver transplantation, and to provide a scientific basis for the rational clinical application of antibiotics. Methods The pathogenic bacteria isolated from the specimens of 904 patients with infection after liver transplantation in The Affiliated Hospital of Qingdao University from March 2014 to December 2021 were analyzed in terms of distribution and drug resistance. WHONET 5.6 software was used to perform a statistical analysis of strains and bacterial resistance rate, and Excel was used to analyze the sources of specimens, composition ratios, and distribution of pathogenic bacteria. Results A total of 2 208 non-repetitive pathogenic bacteria were isolated, mainly from the specimens of respiratory tract (31.25%), bile (22.28%), ascites (13.18%), blood (8.38%), and drainage fluid (4.62%). The top 10 pathogenic bacteria were Klebsiella pneumoniae subspecies (10.69%), Enterococcus faecium (10.42%), Escherichia coli (8.24%), Pseudomonas aeruginosa (8.24%), Staphylococcus epidermidis (8.06%), Acinetobacter baumannii (7.93%), Stenotrophomonas maltophilia (6.61%), Enterobacter cloacae (3.22%), Staphylococcus haemolyticus (3.08%), and Staphylococcus aureus (2.94%), accounting for 69.43% of the total pathogenic bacteria. Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Klebsiella pneumoniae subspecies, and Acinetobacter baumannii were the main pathogenic bacteria isolated from respiratory tract specimens; Enterococcus faecium was the main pathogenic bacterium isolated from bile, ascites, and drainage fluid specimens; Escherichia coli, Staphylococcus epidermidis, and Klebsiella pneumoniae subspecies were the main pathogenic bacteria isolated from blood specimens. Drug sensitivity data showed that Enterobacterales bacteria had a relatively high resistance rate to cephalosporins and fluoroquinolones and a resistance rate of < 10% to amikacin among aminoglycosides, with no strains resistant to tigecycline; compared with Escherichia coli, Klebsiella pneumoniae subspecies had a higher resistance rate to meropenem (14.71% vs 5.66%) and imipenem (11.35% vs 6.29%); non-fermentative bacteria had a relatively high resistance rate to carbapenems, with a resistance rate of < 10% to tigecycline and colistin. Among Gram-positive cocci, Enterococcus faecium had a resistance rate of 6.17% to vancomycin and 2.44% to quinupristin/dalfopristin, with no strains resistant to tigecycline and linezolid; Staphylococcus epidermidis had a resistance rate of > 50% to macrolides, fluoroquinolones, sulfonamides, and lincomycin, and a small part of these strains were resistant to linezolid and quinupristin/dalfopristin (< 3%), with no Staphylococcus epidermidis strains resistant to tigecycline and vancomycin. A total of 287 drug-resistant strains were monitored, accounting for 13%, among which there were 128 carbapenem-resistant Acinetobacter baumannii strains, 88 carbapenem-resistant Pseudomonas aeruginosa strains, 26 carbapenem-resistant Klebsiella pneumoniae subspecies strains, 11 carbapenem-resistant Escherichia coli strains, 23 methicillin-resistant Staphylococcus aureus strains, and 11 vancomycin-resistant Enterococcus strains. The carbapenem-resistant Klebsiella pneumoniae subspecies strains mainly produced serine carbapenemase, and the carbapenem-resistant Escherichia coli strains mainly produced metal β-lactamase. Conclusion Gram-negative bacteria are the main pathogenic bacteria for infection after liver transplantation, and there are differences in the distribution of pathogenic bacteria between different types of specimens. The resistance rate of some strains tend to increase, and therefore, it is necessary to strengthen the management of nosocomial infection and antibiotics. -
Key words:
- Liver Transplantation /
- Bacterial Infections /
- Drug Resistance, Bacterial
-
表 1 肝移植术后感染的不同标本病原菌构成比
Table 1. Pathogen composition ratio of different specimens infected after liver transplantation
标本种类 病原菌种类 菌株数 构成比 呼吸道 铜绿假单胞菌 127 5.75% 嗜麦芽窄食单胞菌 105 4.76% 肺炎克雷伯菌肺炎亚种 103 4.66% 鲍曼不动杆菌 101 4.57% 金黄色葡萄球菌 41 1.86% 其他 213 9.65% 胆汁 屎肠球菌 94 4.26% 大肠埃希菌 54 2.45% 肺炎克雷伯菌肺炎亚种 41 1.86% 其他 303 13.72% 腹水 屎肠球菌 53 2.40% 表皮葡萄球菌 34 1.54% 肺炎克雷伯菌肺炎亚种 19 0.86% 其他 185 8.38% 血液 大肠埃希菌 28 1.27% 表皮葡萄球菌 25 1.13% 肺炎克雷伯菌肺炎亚种 23 1.04% 其他 109 4.94% 引流液 屎肠球菌 18 0.82% 大肠埃希菌 15 0.68% 表皮葡萄球菌 13 0.59% 其他 56 2.54% 其他 448 20.29% -
[1] ZHAO Y, ZHAO LJ. Research advances in the risk factors for infection after liver transplantation[J]. J Clin Hepatol, 2021, 37(8): 1957-1962. DOI: 10.3969/j.issn.1001-5256.2021.08.046.赵云, 赵礼金. 肝移植术后感染相关危险因素的研究进展[J]. 临床肝胆病杂志, 2021, 37(8): 1957-1962. DOI: 10.3969/j.issn.1001-5256.2021.08.046. [2] WANG XC, GE J, CHEN YW, et al. Research progress in detection of pathogen infection after organ transplantation[J]. Ogran Transplant, 2018, 9(6): 474-477. DOI: 10.3969/j.issn.1674-7445.2018.06.015.王啸晨, 葛军, 陈雅文, 等. 器官移植术后病原体感染检测研究进展[J]. 器官移植, 2018, 9(6): 474-477. DOI: 10.3969/j.issn.1674-7445.2018.06.015. [3] KIM SI. Bacterial infection after liver transplantation[J]. World J Gastroenterol, 2014, 20(20): 6211-6220. DOI: 10.3748/wjg.v20.i20.6211. [4] LEE JS, LEE SH, KIM KS, et al. Bacterial infection monitoring in the early period after liver transplantation[J]. Ann Surg Treat Res, 2018, 94(3): 154-158. DOI: 10.4174/astr.2018.94.3.154. [5] KAWECKI D, PACHOLCZYK M, LAGIEWSKA B, et al. Bacterial and fungal infections in the early post- transplantation period after liver transplantation: etiologic agents and their susceptibility[J]. Transplant Proc, 2014, 46(8): 2777-2781. DOI: 10.1016/j.transproceed.2014.08.031. [6] DORSCHNER P, MCELROY LM, ISON MG. Nosocomial infections within the first month of solid organ transplantation[J]. Transpl Infect Dis, 2014, 16(2): 171-187. DOI: 10.1111/tid.12203. [7] SHANG H, WANG YS, SHEN ZY. National Clinical Laboratory Operating Procedures (4th Edition)[M]. Beijing: People's Health Publishing House, 2015: 580-831.尚红, 王毓三, 申子瑜. 全国临床检验操作规程(第四版)[M]. 北京: 人民卫生出版社, 2015: 580-831. [8] Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 29th. CLSI M100[S]. USA: Clinical and Laboratory Standards Institute, 2019. [9] Society of Clinical Microbiology and Infection of China International Exchange and Promotion Association for Medical and Healthcare, Clinical Microbiology Group of the Laboratory Medicine Society of the Chinese Medical Association, Clinical Microbiology Group of the Microbiology and Immunology Society of the Chinese Medical Association. Expert consensus on polymyxins, tigecycline and ceftazidime/avibactam susceptibility testing[J]. Chin J Lab Med, 2020, 43(10): 964-972. DOI: 10.3760/cma.j.cn114452-20200719-00619.中国医疗保健国际交流促进会临床微生物与感染分会, 中华医学会检验医学分会临床微生物学组, 中华医学会微生物学与免疫学分会临床微生物学组. 多黏菌素类与替加环素及头孢他啶/阿维巴坦药敏方法和报告专家共识[J]. 中华检验医学杂志, 2020, 43(10): 964-972. DOI: 10.3760/cma.j.cn114452-20200719-00619. [10] Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 32nd. CLSI M100[S]. USA: Clinical and Laboratory Standards Institute, 2022. [11] XING F, YANG CS, JIN Y, et al. Distribution and drug resistance of microbial pathogens in 120 patients with infectious diseases in a hospital[J]. Clin J Med Offic, 2021, 49(9): 1023-1025. DOI: 10.16680/j.1671-3826.2021.09.21.邢飞, 杨春生, 金跃, 等. 某院120例感染性患者微生物病原菌分布及耐药性分析[J]. 临床军医杂志, 2021, 49(9): 1023-1025. DOI: 10.16680/j.1671-3826.2021.09.21. [12] CHENG YJ. Analysis of distribution and drug resistance of 145 pathogenic bacteria causing postoperative infections in liver transplantation patients[J]. Chin J Health Lab Technol, 2018, 28(4): 470-472. https://www.cnki.com.cn/Article/CJFDTOTAL-ZWJZ201804027.htm程燕洁. 145例肝移植患者术后医院感染病原菌的分布及耐药性分析[J]. 中国卫生检验杂志, 2018, 28(4): 470-472. https://www.cnki.com.cn/Article/CJFDTOTAL-ZWJZ201804027.htm [13] WU JD, MU XX, HAN GY, et al. Analysis on distribution and drug resistance of 1380 strains of pathogens infected after liver transplantation from 2012 to 2015[J]. Drugs & Clinic, 2015, 30(12): 1546-1549. DOI: 10.7501/j.issn.1674-5515.2015.12.027.吴金道, 母小新, 韩国勇, 等. 2012—2015年1380株肝移植术后感染病原菌的分布及耐药性分析[J]. 现代药物与临床, 2015, 30(12): 1546-1549. DOI: 10.7501/j.issn.1674-5515.2015.12.027. [14] JIANG AD, YANG JY, XU T. Analysis on distribution and drug resistance of pathogens infected in adult deceased donor liver transplant recipients[J]. J Chin Antibiotics, 2020, 45(9): 924-928. DOI: 10.3969/j.issn.1001-8689.2020.09.014.蒋艾豆, 杨家印, 徐珽. 成人尸体肝移植受者感染病原菌分布及耐药性分析[J]. 中国抗生素杂志, 2020, 45(9): 924-928. DOI: 10.3969/j.issn.1001-8689.2020.09.014. [15] YAO S, YAGI S, UOZUMI R, et al. A high portal venous pressure gradient increases gut-related bacteremia and consequent early mortality after living donor liver transplantation[J]. Transplantation, 2018, 102(4): 623-631. DOI: 10.1097/TP.0000000000002047. [16] ANESI JA, BLUMBERG EA, ABBO LM. Perioperative antibiotic prophylaxis to prevent surgical site infections in solid organ transplantation[J]. Transplantation, 2018, 102(1): 21-34. DOI: 10.1097/TP.0000000000001848. [17] Chinese Society of Organ Transplantation, Chinese Medical Association. Technical specifications for the diagnosis and treatment of drug resistant bacteria infection after organ transplantation (2019 edition)[J]. Ogran Transplant, 2019, 10(4): 352-358. DOI: 10.3969/j.issn.1674-7445.2019.04.002.中华医学会器官移植学分会. 器官移植术后耐药菌感染诊疗技术规范(2019版)[J]. 器官移植, 2019, 10(4): 352-358. DOI: 10.3969/j.issn.1674-7445.2019.04.002. [18] HU FP, GUO Y, ZHU DM, et al. CHINET surveillance of bacterial resistance: results of 2020[J]. Chin J Infect Chemother, 2021, 21(4): 377-387. DOI: 10.16718/j.1009-7708.2021.04.001.胡付品, 郭燕, 朱德妹, 等. 2020年CHINET中国细菌耐药监测[J]. 中国感染与化疗杂志, 2021, 21(4): 377-387. DOI: 10.16718/j.1009-7708.2021.04.001. [19] KIM YJ, YOON JH, KIM SI, et al. High mortality associated with Acinetobacter species infection in liver transplant patients[J]. Transplant Proc, 2011, 43(6): 2397-2399. DOI: 10.1016/j.transproceed.2011.06.011. [20] SHEN CY, WANG J, LIN T, et al. Distribution and risk factors of multi-drug resistant bacteria infection after liver transplantation[J]. J Hepatopancreatobiliary Surg, 2020, 32(6): 355-360. DOI: 10.11952/j.issn.1007-1954.2020.06.008.申存毅, 王婧, 林婷, 等. 肝移植术后多重耐药菌感染分布特征及危险因素[J]. 肝胆胰外科杂志, 2020, 32(6): 355-360. DOI: 10.11952/j.issn.1007-1954.2020.06.008. [21] YU H, XU XS, LI M, et al. Consensus statement on laboratory detection and clinical report of carbapenemases among Enterobacterales[J]. Chin J Infect Chemother, 2020, 20(6): 671-680. DOI: 10.16718/j.1009-7708.2020.06.015.喻华, 徐雪松, 李敏, 等. 肠杆菌目细菌碳青霉烯酶的实验室检测和临床报告规范专家共识[J]. 中国感染与化疗杂志, 2020, 20(6): 671-680. DOI: 10.16718/j.1009-7708.2020.06.015. 期刊类型引用(3)
1. 张艳培,梁健,邓鑫,梁明坤,张冰冰,徐粤娟. 浅析肝纤维化发生机制. 大众科技. 2021(02): 41-44+12 .  百度学术
百度学术2. 曾静,熊庆,张丽丽,袁娟,何莹,邱黎菲. SPACE肽修饰成纤维细胞生长因子21的制备及对小鼠皮肤瘢痕形成的抑制作用. 中国免疫学杂志. 2021(11): 1308-1312 .  百度学术
百度学术3. 唐青蓝,李红梅,张礼林,王玉丰,颜礼完,周君. 小鼠FGF21基因克隆及his-mFGF21融合蛋白的诱导表达. 贵州医科大学学报. 2019(05): 552-556 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 3720 KB)
PDF下载 ( 3720 KB)

 下载:
下载:






 百度学术
百度学术

