重症急性胰腺炎继发持续性炎症-免疫抑制-分解代谢综合征的影响因素及预测模型构建
DOI: 10.3969/j.issn.1001-5256.2023.06.019
Influencing factors for persistent inflammation, immunosuppression, and catabolism syndrome in patients with severe acute pancreatitis and establishment of a predictive model
-
摘要:
目的 探讨重症急性胰腺炎(SAP)继发持续性炎症-免疫抑制-分解代谢综合征(PICS)的影响因素,并构建预测模型。 方法 回顾性分析2012年5月—2022年5月广西医科大学第一附属医院163例因SAP收入重症医学科、急诊重症监护室患者的临床资料。依据PICS诊断标准分成2组:PICS组(65例,SAP发生PICS患者)和非PICS组(98例,SAP未发生PICS患者)。符合正态分布的计量资料2组间比较采用成组t检验;不符合正态分布的计量资料2组间比较采用Mann-Whitney U秩和检验。计数资料2组间比较采用χ2检验或Fisher确切概率法。计算方差膨胀因子、相关系数矩阵热图评估变量间多重共线性,采用Lasso回归及多因素Logistic回归筛选出独立危险因素,构建列线图预测模型。采用受试者工作特征曲线、校准曲线及Hosmer-Lemeshow拟合优度检验对模型进行内部验证;采用临床决策曲线评估模型的临床实用性。 结果 单因素分析结果显示,平均动脉压、血红蛋白、红细胞压积(HCT)、中性粒细胞与淋巴细胞比值、血小板与淋巴细胞比值(PLR)、尿素、肌酐、格拉斯哥昏迷评分(GCS)、APACHE Ⅱ、SOFA、机械通气、急性呼吸窘迫综合征、急性肾损伤(AKI)、急性肝损伤、低血容量性休克、脓毒症、腹腔高压、腹腔出血、多器官功能障碍综合征在PICS组和非PICS组间比较差异均有统计学意义(P值均<0.05)。Lasso回归筛选的预测变量包括PLR、HCT、APACHE Ⅱ、SOFA、机械通气、AKI、低血容量性休克、腹腔高压。多因素Logistic回归显示,PLR、机械通气、AKI、低血容量性休克是SAP发生PICS的独立危险因素(OR分别为1.006、4.324、3.432、6.910,P值均<0.05)。将上述因素进行模型拟合,经bootstrap内部验证列线图模型曲线下面积为0.874(95%CI:0.822~0.925),校准曲线接近参考曲线,Hosmer-Lemeshow拟合优度检验表明该模型具有良好的拟合度(χ2=8.895,P=0.351)。临床决策曲线分析显示预测模型具有良好的临床实用性。 结论 PLR、机械通气、AKI、低血容量性休克是SAP继发PICS的独立危险因素,构建的列线图模型具有良好的区分度、校准度和临床实用性。 -
关键词:
- 胰腺炎 /
- 持续性炎症-免疫抑制-分解代谢综合征 /
- 危险因素
Abstract:Objective To investigate the influencing factors for persistent inflammation, immunosuppression, and catabolism syndrome (PICS) in patients with severe acute pancreatitis(SAP), and to establish a predictive model. Methods A retrospective analysis was performed for the clinical data of 163 patients who were admitted to the intensive care unit and the emergency intensive care unit due to SAP in The First Affiliated Hospital of Guangxi Medical University from May 2012 to May 2022, and according to the diagnostic criteria for PICS, these patients were divided into PICS group (65 SAP patients with PICS) and non-PICS group (98 SAP patients without PICS). The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U rank sum test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test or the Fisher's exact test was used for comparison of categorical data between two groups. Variance inflation factor and correlation matrix heatmap were used to evaluate multicollinearity between variables, and Lasso regression and multivariate logistic regression were used to identify independent risk factors and establish a nomogram predictive model. The receiver operating characteristic (ROC) curve, the calibration curve, and the Hosmer-Lemeshow goodness-of-fit test were used for the internal validation of the model, and the decision curve was used to evaluate the clinical practicability of the model. Results The univariate analysis showed that there were significant differences between the PICS group and the non-PICS group in mean arterial pressure, hemoglobin, hematocrit (HCT), neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio (PLR), blood urea nitrogen, creatinine, Glasgow coma score, Acute Physiology and Chronic Health Evaluation Ⅱ (APACHE Ⅱ) score, Sequential Organ Failure Assessment (SOFA) score, mechanical ventilation, acute respiratory distress syndrome, acute kidney injury (AKI), acute liver injury, hypovolemic shock, sepsis, intra-abdominal hypertension, intra-abdominal hemorrhage, and multiple organ dysfunction syndrome (all P < 0.05). The Lasso regression analysis showed that related predictive variables included PLR, HCT, APACHE Ⅱ, SOFA, mechanical ventilation, AKI, hypovolemic shock, and intra-abdominal hypertension, and the multivariate logistic regression analysis showed that PLR (odds ratio [OR]=1.006, P < 0.05), mechanical ventilation (OR=4.324, P < 0.05), AKI (OR=3.432, P < 0.05), and hypovolemic shock (OR= 6.910, P < 0.05) were independent risk factors for PICS in patients with SAP. Model fitting was performed for the above factors, and bootstrap internal validation showed that the nomogram model had an area under the ROC curve of 0.874 (95% confidence interval: 0.822-0.925); the calibration curve of the model was close to the reference curve, and the Hosmer-Lemeshow goodness-of-fit test showed that the model was well fitted (χ2=8.895, P=0.351). The decision curve analysis showed that the predictive model had good clinical practicability. Conclusion PLR, mechanical ventilation, AKI, and hypovolemic shock are independent risk factors for PICS in patients with SAP, and the nomogram model established has good discriminatory ability, calibration, and clinical practicability. -
持续性炎症-免疫抑制-分解代谢综合征(persistent inflammation, immunosuppression and catabolism syndrome,PICS) 基于“ SIRS-CARS-MARS ”免疫模型基础上进一步发展而来,机体在度过早期多器官功能障碍综合征(multiple organ dysfunction syndrome,MODS)死亡高峰后,全身炎症反应综合征(systemic inflammatory response syndrome,SIRS)及对抗性的代偿性抗炎反应综合征(compensatory anti-inflammatory response syndrome,CARS)势均力敌,机体达到相对平衡稳定状态,最终形成持续低水平炎症和严重免疫抑制[1]。最早于2012年,由Gentile等[2]提出,指患者在遭受如脓毒症、创伤、急性胰腺炎等重大疾病后期进入慢性危重症(chronic critical ill,CCI),表现出的持续炎症反应、免疫失衡与代谢紊乱,需要长期的紧急医疗支持。重症急性胰腺炎(severe acute pancreatitis,SAP)发生PICS后难以有效逆转,不仅增加医疗资源的消耗,也增加患者治疗负担。但目前临床医生对PICS的认识及相对性治疗仍相对缺乏。本研究通过分析SAP继发PICS的影响因素,构建预测模型,以期早期识别并给予针对性的治疗,为改善SAP患者预后提供帮助。
1. 资料与方法
1.1 研究对象
本研究对2012年5月—2022年5月于本院因SAP进入重症医学科、急诊重症监护室的163例患者临床资料进行回顾性分析。纳入标准:(1)SAP符合2012年亚特兰大分类标准:急性胰腺炎伴有持续性器官功能衰竭(>48 h); (2)临床资料完整。排除标准:(1)年龄≤18岁;(2)合并免疫系统缺陷性疾病;(3)合并恶性肿瘤;(4)胰腺手术术后重症监护患者。
1.2 诊断标准和分组
以SAP患者入住ICU后0~5 d的临床数据进行评估,PICS诊断标准参考相应指南及研究[2-5]: (1)ICU住院时间>14 d;(2)炎症反应:C反应蛋白(CRP)>30 mg/L;(3)免疫抑制: 总淋巴细胞计数(Lym)<0.8×109/L;(4)蛋白质高分解代谢:白蛋白<30 g/L或前白蛋白<100 g/L。将纳入患者分为2组:PICS组(65例,SAP发生PICS患者)和非PICS组(98例,SAP未发生PICS患者)。
1.3 数据采集
收集患者年龄、性别、BMI、既往史、SAP病因、入住ICU后的体温、心率、呼吸、平均动脉压、白细胞计数(WBC)、血红蛋白(Hb)、血小板计数、中性粒细胞计数(Neu)、Lym、中性粒细胞与淋巴细胞比值(NLR)、血小板与淋巴细胞比值(PLR)、红细胞压积(HCT)、CRP、血气分析pH值、氧分压(PO2)、二氧化碳分压(PCO2)、静脉血血钠(Na)、静脉血血钾(K)、总胆红素(TBil)、直接胆红素(DBil)、前白蛋白、白蛋白、尿素(BUN)、肌酐(SCr)、血淀粉酶、格拉斯哥昏迷评分(GCS)、急性生理与慢性健康评分(APACHE Ⅱ)、序贯器官衰竭估计评分(SOFA)、SAP患者进入ICU时并发症[机械通气、急性呼吸窘迫综合征(ARDS)、急性肾损伤(AKI)、急性肝损伤、低血容量性休克、脓毒症、肺部感染、腹腔高压、腹腔出血、肠瘘、MODS]、ICU住院时间、住院时间、临床结局、总费用等。
1.4 统计学方法
采用SPSS 26.0与R-4.2.1统计软件进行数据分析。符合正态分布的计量资料以x±s表示,2组间比较采用成组t检验;不符合正态分布的计量资料以M(P25~P75)表示,2组间比较采用Mann-Whitney U秩和检验。计数资料2组间比较采用χ2检验或Fisher确切概率法。P<0.05为差异有统计学意义。计算方差膨胀因子、相关系数矩阵热图评估变量多重共线性,采用Lasso回归法筛选变量,然后采用多因素Logistic回归模型筛选独立危险因素纳入最终模型。采用R-4.2.1统计软件及相关程序包绘制列线图预测模型。采用bootstrap法(抽样1 000次)进行内部验证,采用受试者工作特征曲线(ROC曲线)评估模型的区分度,校准曲线及Hosmer-Lemeshow拟合优度检验评估模型的预测精度,临床决策曲线检验模型的临床实用性。
2. 结果
2.1 一般资料比较
PICS组患者的平均动脉压显著低于非PICS组,差异具有统计学意义(P<0.05)。预后方面,PICS组的病死率、住院时间、总费用均显著高于非PICS组,差异均有统计学意义(P值均<0.05)(表 1)。
表 1 2组患者一般资料比较Table 1. Comparison of general data between the two groups项目 PICS组(n=65) 非PICS组(n=98) 统计值 P值 年龄(岁) 48.00(39.50~59.00) 47.50(37.00~59.25) Z=-0.005 0.996 性别[例(%)] χ2=0.916 0.339 男 52(80.0) 72(73.5) 女 13(20.0) 26(26.5) BMI(kg/m2) 23.94(22.00~27.55) 24.98(22.49~27.76) Z=-0.668 0.504 既往史[例(%)] 高血压 14(34.7) 34(20.9) χ2=3.255 0.071 糖尿病 8(12.3) 15(15.3) χ2=0.290 0.590 腹部疾病 27(41.5) 52(53.1) χ2=1.897 0.168 SAP病因[例(%)] 胆源性 5(7.7) 11(1.1) χ2=0.551 0.458 高脂血症性 17(26.2) 34(34.7) χ2=1.326 0.250 酒精性 12(18.5) 18(18.4) χ2<0.001 0.988 创伤性 2(3.1) 3(3.1) χ2<0.001 0.995 病因不明 29(44.6) 32(32.7) χ2=2.388 0.122 体温(℃) 37.60(37.00~38.00) 37.50(36.78~38.10) Z=-0.553 0.580 心率(次/min) 119.43±23.02 112.69±21.48 t=1.881 0.062 呼吸(次/min) 24.00(20.00~33.00) 26.00(21.50~32.00) Z=-1.064 0.287 平均动脉压(mmHg) 98.33(80.67~111.67) 104.50(91.59~113.08) Z=-2.207 0.027 预后 开放饮食时间(d) 8.00(3.50~13.50) 7.00(3.00~11.00) Z=-1.288 0.198 病死率[例(%)] 26(40.0) 16(16.3) χ2=11.450 0.001 住院时间(d) 30.00(22.00~51.00) 22.00(17.00~27.25) Z=-4.666 <0.001 总费用(万元) 22.23(12.10~39.31) 8.18(6.06~11.86) Z=-6.893 <0.001 2.2 PICS因素比较
在PICS因素中,2组患者的ICU住院时间、Lym、前白蛋白、白蛋白差异均有统计学意义(P值均<0.05)(表 2)。
表 2 2组患者PICS指标比较Table 2. Comparison of PICS factors between the two groups指标 PICS组(n=65) 非PICS组(n=98) Z值 P值 ICU住院时间(d) 22.00(16.00~42.50) 7.00(4.00~11.00) -8.862 <0.001 CRP(mg/L) 180.39(120.84~192.00) 190.67(109.04~192.00) -0.265 0.791 Lym(×109/L) 0.60(0.49~0.75) 1.07(0.72~1.39) -6.547 <0.001 前白蛋白(g/L) 83.30(52.25~104.15) 110.90(76.73~155.35) -3.488 <0.001 白蛋白(g/L) 28.30(26.10~31.25) 30.80(27.58~34.78) -3.517 <0.001 2.3 实验室指标和并发症比较
PICS组Hb、HCT、GCS评分显著低于非PICS组,NLR、PLR、BUN、SCr、APACHE Ⅱ评分、SOFA评分均高于非PICS组,差异均有统计学意义(P值均<0.05)。并发症方面,PICS组合并机械通气、ARDS、AKI、急性肝损伤、低血容量性休克、脓毒症、腹腔高压、腹腔出血、MODS均高于非PICS组,差异均有统计学意义(P值均<0.05)(表 3)。
表 3 2组患者实验室指标和并发症比较Table 3. Comparison of laboratory data and complication between the two groups指标 PICS组(n=65) 非PICS组(n=98) 统计值 P值 WBC(×109/L) 14.46(8.94~18.69) 14.72(10.45~20.09) Z=-0.857 0.391 Hb(g/L) 87.70(75.05~112.70) 106.60(83.68~126.13) Z=-2.532 0.011 血小板计数(×109/L) 176.00(117.15~237.20) 196.35(141.93~272.40) Z=-1.366 0.172 Neu(×109/L) 13.22(8.00~17.00) 12.18(8.38~17.45) Z=-0.095 0.924 NLR 21.69(11.47~30.30) 12.35(7.41~23.13) Z=-3.383 0.001 PLR 261.85(198.42~455.46) 207.28(118.19~315.79) Z=-3.210 0.001 HCT 0.26(0.22~0.36) 0.33(0.26~0.39) Z=-2.649 0.008 PH 7.44(7.36~7.48) 7.43(7.38~7.48) Z=-0.061 0.951 PO2(mmHg) 102.00(78.05~136.05) 88.50(71.00~126.03) Z=-1.161 0.246 PCO2(mmHg) 34.10(30.40~39.40) 35.55(30.00~39.00) Z=-0.278 0.781 氧合指数 253.00(198.00~332.90) 234.50(184.25~326.50) Z=-0.552 0.581 血Na(mmol/L) 138.10(133.95~143.20) 137.00(133.80~140.95) Z=-0.727 0.467 血K(mmol/L) 4.10(3.80~4.52) 4.00(3.59~4.31) Z=-1.466 0.143 TBil(μmol/L) 26.30(13.30~47.30) 20.55(12.48~35.03) Z=-1.469 0.142 DBil(μmol/L) 13.30(6.25~28.20) 9.30(5.00~18.75) Z=-1.737 0.082 BUN(mmol/L) 10.36(6.32~17.51) 6.43(4.15~10.50) Z=-3.250 0.001 SCr(μmol/L) 152.00(71.50~293.50) 73.50(55.75~141.00) Z=-2.915 0.004 血淀粉酶(U/L) 76.00(44.00~306.50) 103.00(53.75~389.75) Z=-0.935 0.350 GCS评分 15.00(13.00~15.00) 15.00(15.00~15.00) Z=-2.973 0.003 APACHE Ⅱ 13.00(10.00~16.00) 11.00(7.75~14.00) Z=-3.173 0.002 SOFA 6.00(4.00~10.00) 4.00(2.00~6.00) Z=-4.309 <0.001 并发症[例(%)] 机械通气 54(83.1) 34(34.7) χ2=17.785 <0.001 ARDS 54(83.1) 49(50.0) χ2=17.785 <0.001 AKI 40(61.5) 23(23.5) χ2=44.521 <0.001 急性肝损伤 16(24.6) 12(12.2) χ2=105.631 <0.001 低血容量性休克 15(23.1) 3(3.1) χ2=108.741 <0.001 脓毒症 37(56.9) 27(27.6) χ2=50.971 <0.001 肺部感染 63(96.9) 90(91.8) χ2=3.053 0.158 腹腔高压 34(52.3) 22(22.4) χ2=57.715 <0.001 腹腔出血 12(18.5) 6(6.1) χ2=118.409 <0.001 肠瘘 2(3.1) 2(2.0) χ2=0.175 0.524 MODS 49(75.4) 36(36.7) χ2=26.749 <0.001 2.4 多重共线性检验
计算P<0.05变量的方差膨胀因子,结果显示Hb及HCT的方差膨胀因子≥10。所有因素的相关系数矩阵热图提示Hb与HCT(r=0.99)、BUN与SCr(r=0.84)、BUN与AKI(r=0.62)、SCr与AKI(r=0.68)、机械通气与ARDS(r=0.67)、AKI与MODS(r=0.66)间均存在强相关(图 1)。
2.5 SAP发生PICS的Lasso回归分析
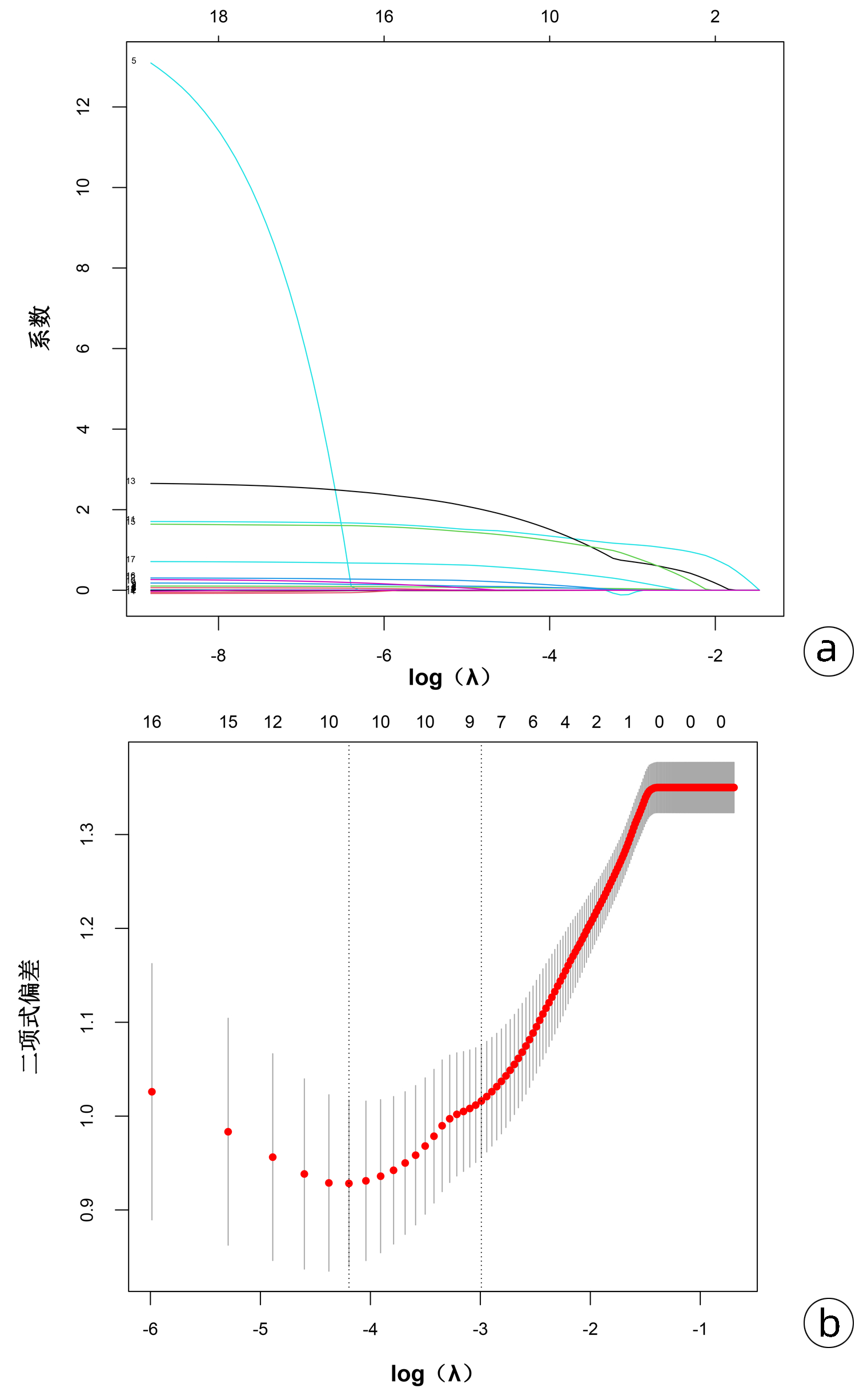
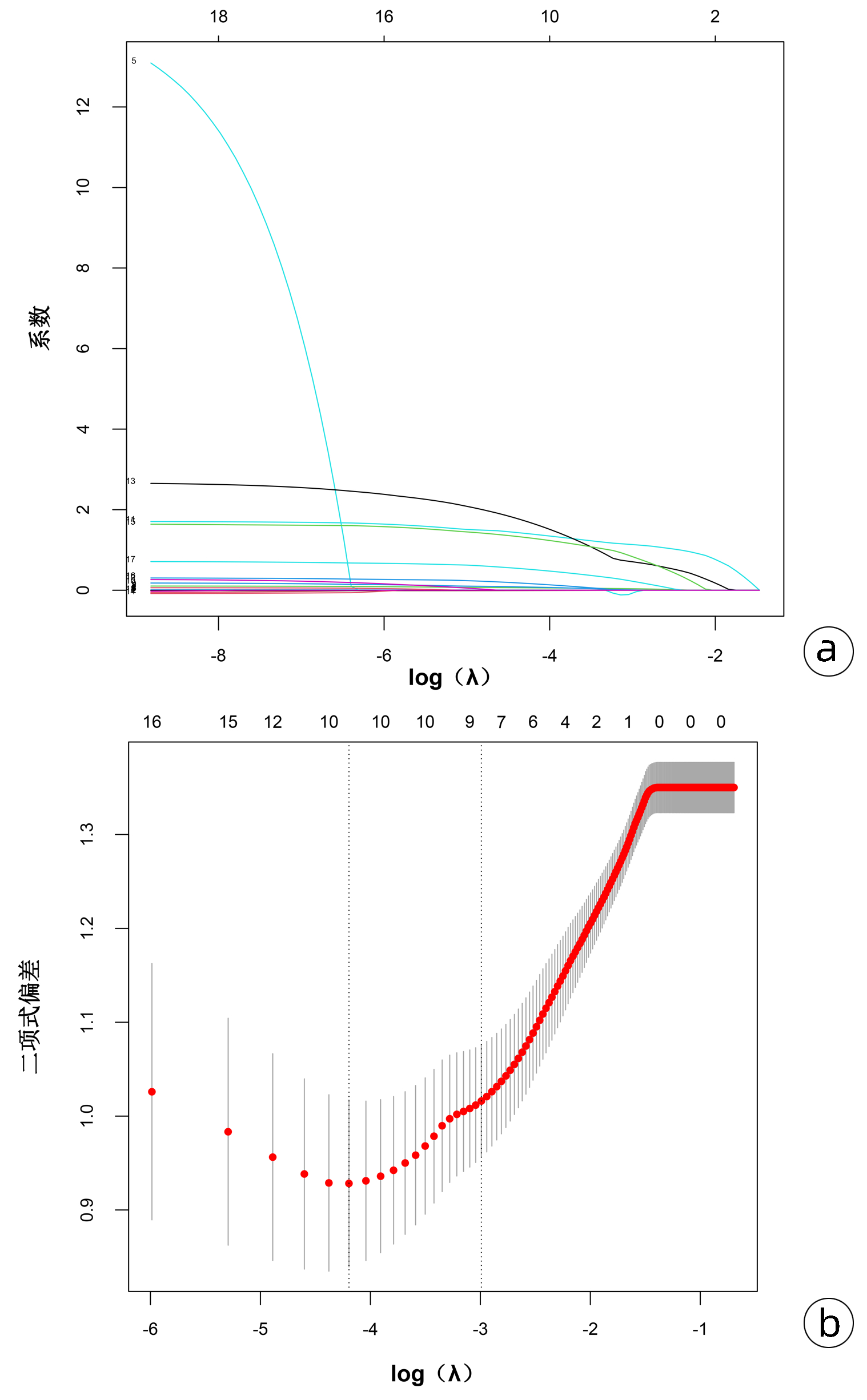
Lasso回归可通过添加惩罚系数,将非特征变量的系数压缩为0,从而限制多重共线性带来的影响,筛选高质量变量。将P<0.05的19个变量(包括平均动脉压、Hb、NLR、PLR、HCT、BUN、SCr、GCS评分、APACHE Ⅱ评分、SOFA评分、机械通气、ARDS、AKI、急性肝损伤、低血容量性休克、脓毒症、腹腔高压、腹腔出血、MODS)进行Lasso回归分析,图 2a结果提示随着惩罚系数的对数logλ增大,模型纳入的变量也在逐渐减少,图 2b结果显示了10倍交叉验证确定最优λ值的过程,二项式偏差越小说明方程的拟合效果越好,最终选择最小λ右侧的1个标准差(0.050)作为模型的最优值,此时筛选的预测变量包括PLR、HCT、APACHE Ⅱ、SOFA、机械通气、AKI、低血容量性休克、腹腔高压。
2.6 SAP发生PICS的多因素Logistic回归分析
将Lasso回归筛选出来的8个变量纳入多因素Logistic分析中,结果显示PLR、机械通气、AKI、低血容量性休克是SAP发生PICS的独立危险因素(P值均<0.05)(表 4)。
表 4 SAP患者发生PICS多因素Logistic回归分析Table 4. Multivariate logistic regression analysis of PICS in patients with SAP变量 β值 P值 OR 95%CI PLR 0.006 <0.001 1.006 1.003~1.009 HCT -2.810 0.256 0.060 0.000~7.768 APACHE Ⅱ 0.076 0.124 1.079 0.982~1.195 SOFA 0.053 0.458 1.055 0.913~1.216 机械通气 1.464 0.002 4.324 1.723~11.423 AKI 1.233 0.018 3.432 1.253~9.854 低血容量性休克 1.933 0.013 6.910 1.685~39.068 腹腔高压 0.743 0.107 2.102 0.852~5.245 2.7 列线图预测模型构建
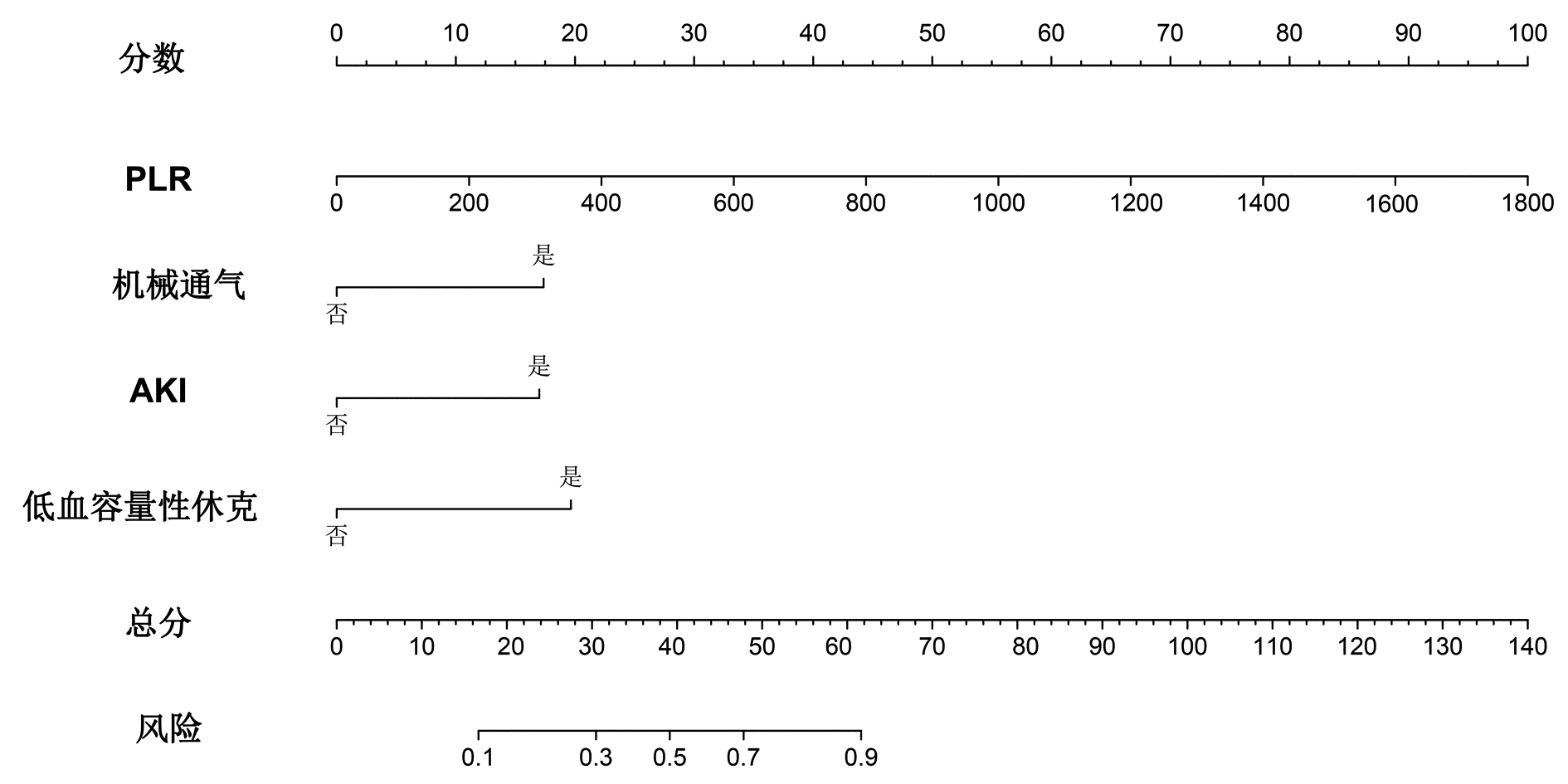
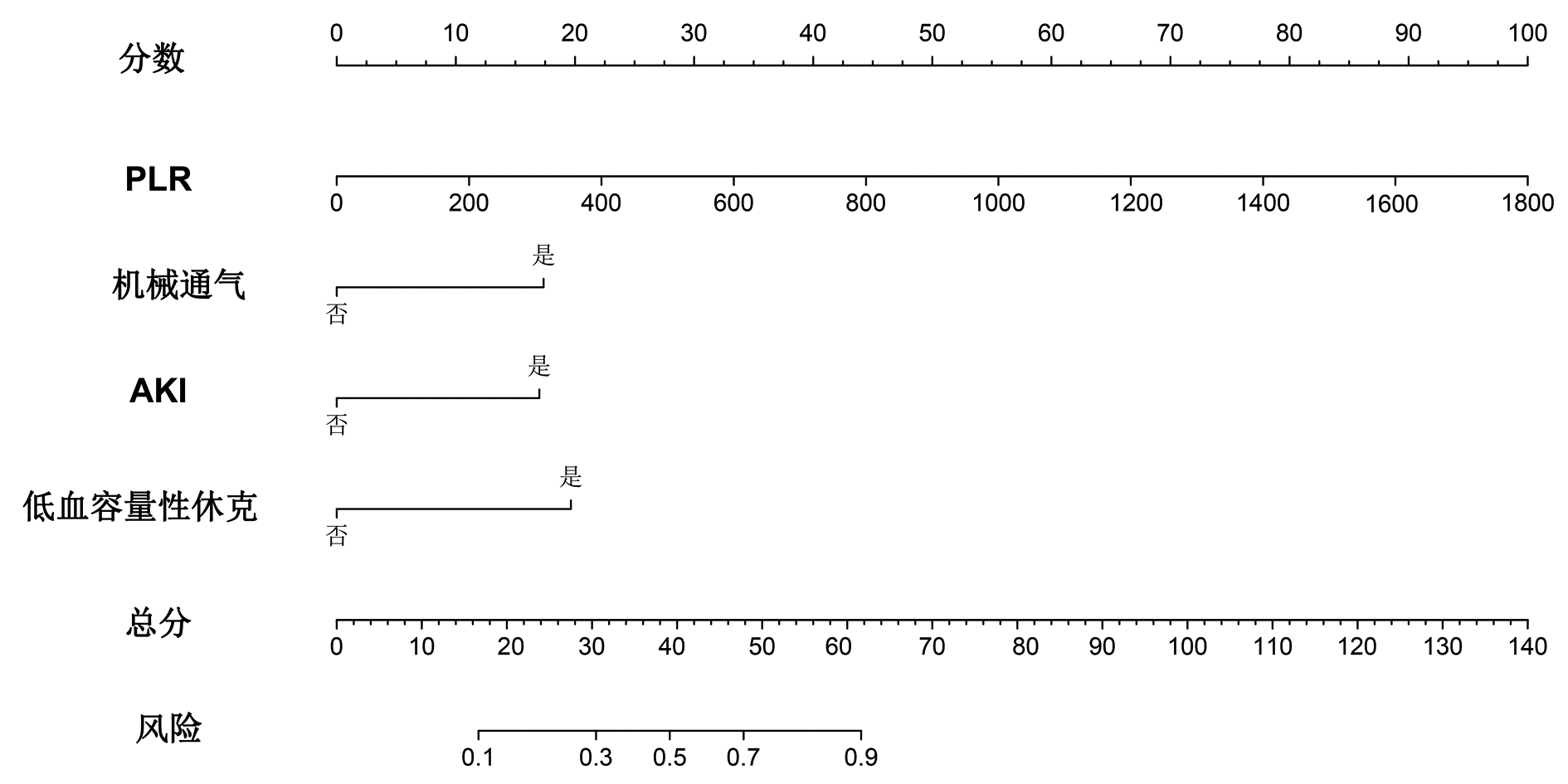
将多因素Logistic回归分析结果筛选出的变量PLR、机械通气、AKI、低血容量性休克纳入列线图预测模型,结局指标选取SAP患者PICS的发病风险,绘制列线图(图 3),根据每个风险因素所对应列线图上方的标尺,从而得到该因素的单项评分,所有风险因素评分相加得到总分,便可得到对应SAP患者PICS发生率,总分越高,SAP患者发生PICS风险的可能性越大。
2.8 预测模型的内部验证与评价
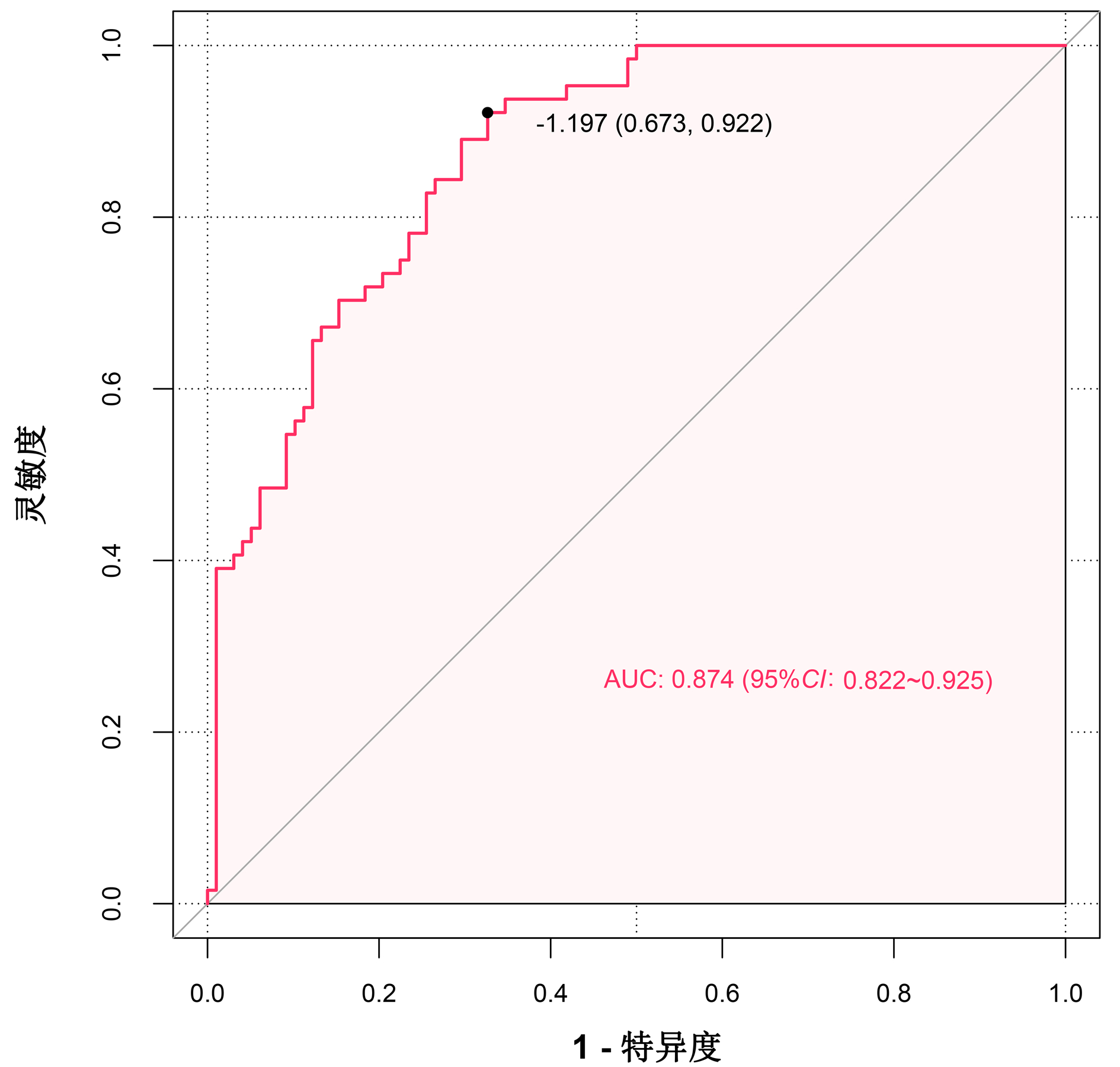
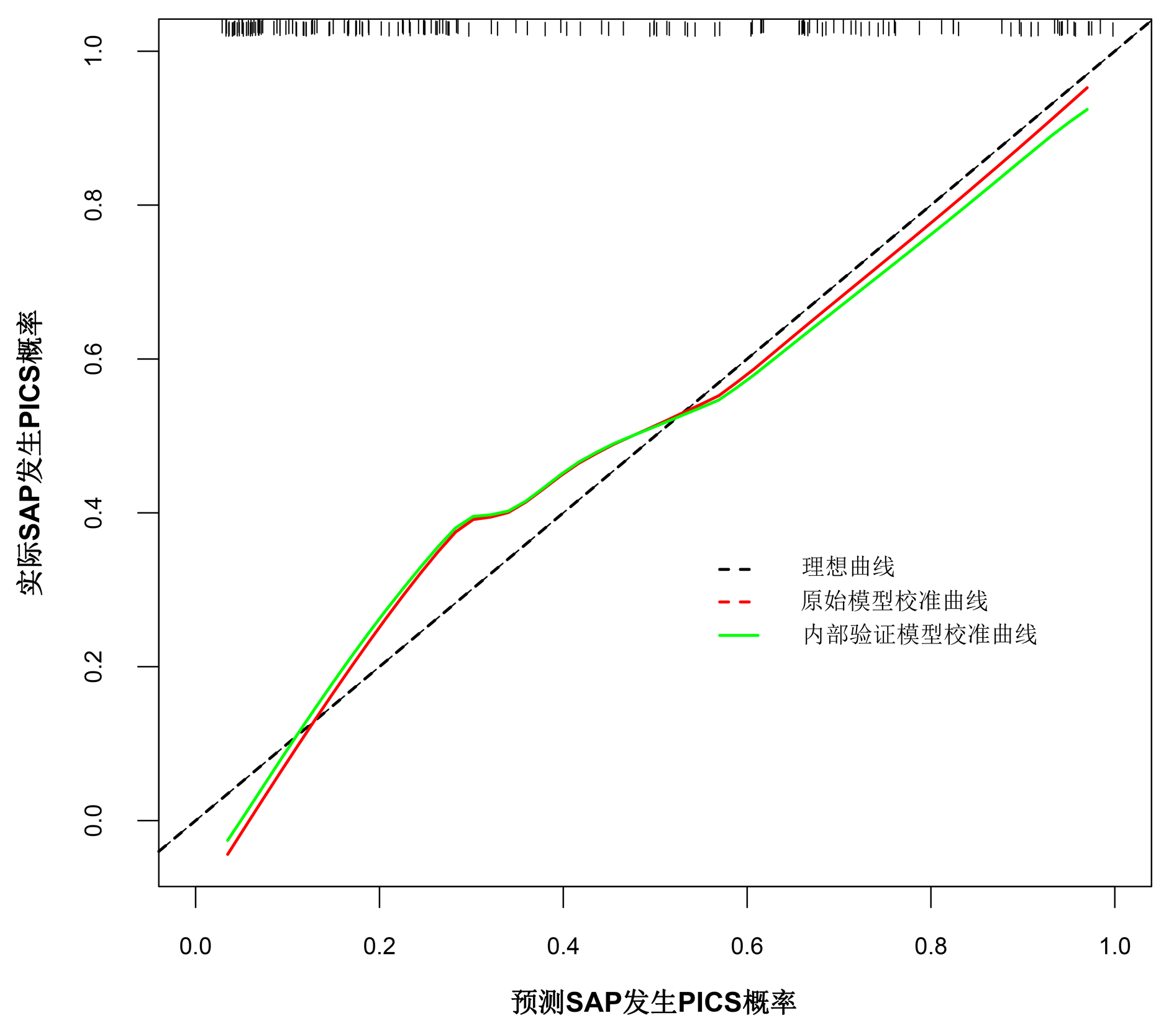
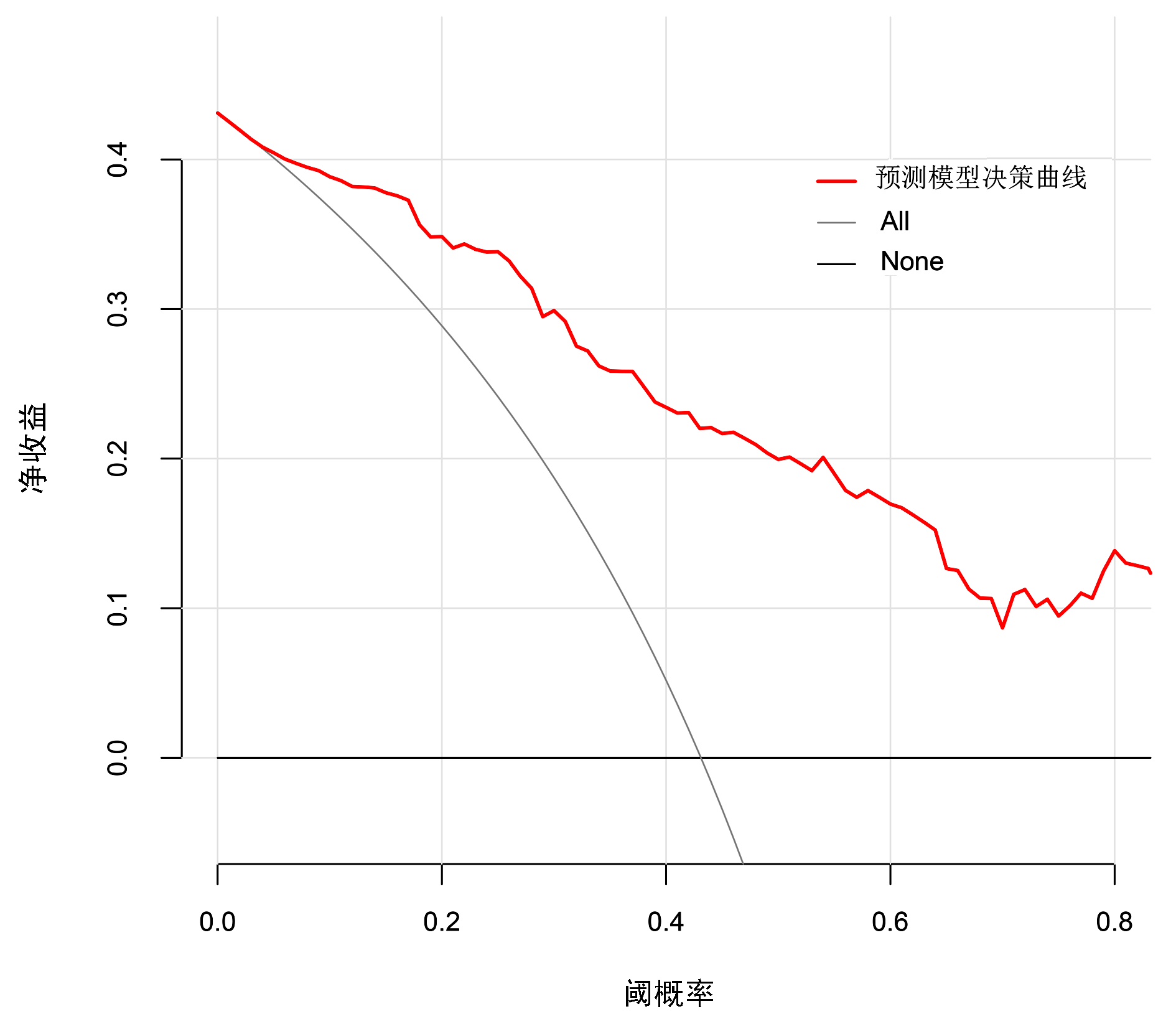
运用bootstrap法(抽样1 000次)对该预测模型进行内部验证,其ROC曲线下面积为0.874,95%CI:0.822~0.925,最佳截断值为-1.197,在该截断值下,预测模型的敏感度为0.922,特异度为0.673。表明该预测模型具有良好的区分度(图 4)。绘制模型的校准曲线,可见校准曲线接近参考曲线(图 5),Hosmer-Lemeshow拟合优度检验(χ2=8.895,P=0.351)表明该模型具有良好的拟合度。临床决策曲线分析发现,在SAP患者的阈值概率为0~0.8时,预测模型的决策曲线均高于2条极端曲线,即预测模型的净收益更高。说明该预测模型具有良好的临床实用性(图 6)。
3. 讨论
SAP患者约43.1%后期可合并PICS[6],这与本中心约39.9% SAP患者发生PICS基本相符,SAP发生时,大量胰蛋白酶激活可刺激胰腺内单核巨噬细胞及损伤的胰腺腺泡产生炎症介质,引起白细胞过度激活-炎症介质级联“瀑布效应”,导致PICS。杨蓉等[7]的研究发现重症患者发生PICS组CD3+、CD4+、CD4+/CD8+均低于非PICS组,而CD4+T淋巴细胞比例减少可引起免疫麻痹,CD4+/CD8+比例改变可出现Th1/Th2漂移,Th2细胞极化进一步表现为固有免疫与适应性免疫失衡,导致免疫抑制。SAP发生时大量细胞因子及应激相关激素释放,引起机体高分解代谢,大量能量消耗及肌肉蛋白质分解,同时促炎细胞因子等可作用于线粒体致呼吸链酶活性下降,导致能量代谢不足,加重能量供需失衡,介导代谢紊乱。
SAP胰酶释放引起局部炎症,导致血管通透性增加,凝血系统被激活,促进血小板黏附聚集,同时炎症因子作用于骨髓细胞,引起血小板产生和释放增加[8]。PLR为血小板与淋巴细胞比值,是一个能综合反映机体凝血和炎症的指标,Zhou等[9]对406例急性胰腺炎患者进行回顾性分析,结果提示PLR在预测急性胰腺炎严重程度及预后方面均有意义,本研究结果也提示PLR越高,SAP患者发生PICS风险越大。Hawkins等[10]研究发现SAP发生PICS时骨髓来源抑制性细胞(MDSC)持续增殖,同时释放大量炎症因子,炎症因子可进一步收缩肾血管,加重肾缺血缺氧,同时MDSC可浸润于肾脏,引起肾脏氧化应激及免疫抑制,直接损伤肾组织。此研究也发现AKI患者进展为慢性肾脏病是CCI发生的主要原因,本研究同样发现SAP患者入住ICU时出现AKI,发生PICS的风险增大。一项Meta分析[11]提示采用机械通气是SAP患者并发AKI的危险因素,这可能与机械通气通过调节神经体液、改变肾脏血流动力学等机制影响肾脏灌注和滤过功能有关。SAP时由于胰腺及胰周大量渗出而导致循环血容量不足,严重者可出现低血压乃至低血容量性休克,而由于血流重分布,极易发生选择性肠道缺血,若未及时进行充分的液体复苏,会引起小肠组织灌注不足、肠黏膜机械屏障受损。Efron等[5]研究表明肠道屏障受损与PICS中全身炎症及免疫抑制发生有关,同时低血容量性休克时肾脏供血不足、肾血管收缩,AKI风险增大,CCI发生风险增高。
目前PICS治疗包括以下几方面:(1)改善炎症反应。Wang等[12]研究结果表明在SAP大鼠模型中乌司他丁治疗可有效减少血清中TNFα、IL-6水平。王燕等[13]研究发现厚朴酚能够改善肠屏障功能,通过功能性阻断肠淋巴循环,降低炎症因子水平,减轻SAP肺损伤。王雪等[14]研究发现血必净注射液治疗组CD4+T淋巴细胞、CD8+T淋巴细胞、CD4+/CD8+及NKT淋巴细胞水平较对照组显著增加。此外血液净化治疗可清除炎性因子,改善单核巨噬细胞功能,提高淋巴细胞数量、维持机体免疫系统内环境稳定[15]。(2)免疫调节治疗。胸腺肽α1可诱导T淋巴细胞分化、成熟,增强单核巨噬细胞的吞噬,抑制中性粒细胞凋亡。苏和毅等[16]对68例脓毒症后发生PICS的老年患者进行研究,结果提示接受胸腺肽α1治疗组CD4+/CD8+、HLADR/CD14+水平显著高于对照组。(3)营养支持治疗。Lubbers等[17]一项双盲、随机对照研究发现富含脂肪酸、蛋白质的肠内营养剂可抑制早期炎症反应,患者体内的促炎因子TNFα、IL-6、IL-8水平显著降低,同时增强抗炎作用,免疫功能得到调节。精氨酸是正常T淋巴细胞合成所必需的氨基酸,而PICS发生后,表达精氨酸酶的MDSC增殖,精氨酸减少导致免疫抑制,补充精氨酸有助于促进淋巴细胞的增殖和成熟[18]。此外,早期锻炼康复、调节肠道菌群等在PICS患者治疗方面均有报道。
本研究基于PLR、机械通气、AKI、低血容量性休克等指标,构建了预测SAP患者发生PICS的列线图模型,该模型具有良好的区分度、校准度和临床实用性,便于入住ICU的SAP患者早期识别和预测发生PICS风险并及时干预,以期减少SAP患者发生PICS可能、降低SAP合并PICS患者的住院时间及病死率。本研究局限性在于为单中心回顾性研究,样本量相对较少,一定程度上存在选择偏倚,影响预测模型的准确性,同时未进行外部验证评估模型的泛化能力,仍需开展多中心、大样本的对照研究进一步证实本研究结果。
-
表 1 2组患者一般资料比较
Table 1. Comparison of general data between the two groups
项目 PICS组(n=65) 非PICS组(n=98) 统计值 P值 年龄(岁) 48.00(39.50~59.00) 47.50(37.00~59.25) Z=-0.005 0.996 性别[例(%)] χ2=0.916 0.339 男 52(80.0) 72(73.5) 女 13(20.0) 26(26.5) BMI(kg/m2) 23.94(22.00~27.55) 24.98(22.49~27.76) Z=-0.668 0.504 既往史[例(%)] 高血压 14(34.7) 34(20.9) χ2=3.255 0.071 糖尿病 8(12.3) 15(15.3) χ2=0.290 0.590 腹部疾病 27(41.5) 52(53.1) χ2=1.897 0.168 SAP病因[例(%)] 胆源性 5(7.7) 11(1.1) χ2=0.551 0.458 高脂血症性 17(26.2) 34(34.7) χ2=1.326 0.250 酒精性 12(18.5) 18(18.4) χ2<0.001 0.988 创伤性 2(3.1) 3(3.1) χ2<0.001 0.995 病因不明 29(44.6) 32(32.7) χ2=2.388 0.122 体温(℃) 37.60(37.00~38.00) 37.50(36.78~38.10) Z=-0.553 0.580 心率(次/min) 119.43±23.02 112.69±21.48 t=1.881 0.062 呼吸(次/min) 24.00(20.00~33.00) 26.00(21.50~32.00) Z=-1.064 0.287 平均动脉压(mmHg) 98.33(80.67~111.67) 104.50(91.59~113.08) Z=-2.207 0.027 预后 开放饮食时间(d) 8.00(3.50~13.50) 7.00(3.00~11.00) Z=-1.288 0.198 病死率[例(%)] 26(40.0) 16(16.3) χ2=11.450 0.001 住院时间(d) 30.00(22.00~51.00) 22.00(17.00~27.25) Z=-4.666 <0.001 总费用(万元) 22.23(12.10~39.31) 8.18(6.06~11.86) Z=-6.893 <0.001 表 2 2组患者PICS指标比较
Table 2. Comparison of PICS factors between the two groups
指标 PICS组(n=65) 非PICS组(n=98) Z值 P值 ICU住院时间(d) 22.00(16.00~42.50) 7.00(4.00~11.00) -8.862 <0.001 CRP(mg/L) 180.39(120.84~192.00) 190.67(109.04~192.00) -0.265 0.791 Lym(×109/L) 0.60(0.49~0.75) 1.07(0.72~1.39) -6.547 <0.001 前白蛋白(g/L) 83.30(52.25~104.15) 110.90(76.73~155.35) -3.488 <0.001 白蛋白(g/L) 28.30(26.10~31.25) 30.80(27.58~34.78) -3.517 <0.001 表 3 2组患者实验室指标和并发症比较
Table 3. Comparison of laboratory data and complication between the two groups
指标 PICS组(n=65) 非PICS组(n=98) 统计值 P值 WBC(×109/L) 14.46(8.94~18.69) 14.72(10.45~20.09) Z=-0.857 0.391 Hb(g/L) 87.70(75.05~112.70) 106.60(83.68~126.13) Z=-2.532 0.011 血小板计数(×109/L) 176.00(117.15~237.20) 196.35(141.93~272.40) Z=-1.366 0.172 Neu(×109/L) 13.22(8.00~17.00) 12.18(8.38~17.45) Z=-0.095 0.924 NLR 21.69(11.47~30.30) 12.35(7.41~23.13) Z=-3.383 0.001 PLR 261.85(198.42~455.46) 207.28(118.19~315.79) Z=-3.210 0.001 HCT 0.26(0.22~0.36) 0.33(0.26~0.39) Z=-2.649 0.008 PH 7.44(7.36~7.48) 7.43(7.38~7.48) Z=-0.061 0.951 PO2(mmHg) 102.00(78.05~136.05) 88.50(71.00~126.03) Z=-1.161 0.246 PCO2(mmHg) 34.10(30.40~39.40) 35.55(30.00~39.00) Z=-0.278 0.781 氧合指数 253.00(198.00~332.90) 234.50(184.25~326.50) Z=-0.552 0.581 血Na(mmol/L) 138.10(133.95~143.20) 137.00(133.80~140.95) Z=-0.727 0.467 血K(mmol/L) 4.10(3.80~4.52) 4.00(3.59~4.31) Z=-1.466 0.143 TBil(μmol/L) 26.30(13.30~47.30) 20.55(12.48~35.03) Z=-1.469 0.142 DBil(μmol/L) 13.30(6.25~28.20) 9.30(5.00~18.75) Z=-1.737 0.082 BUN(mmol/L) 10.36(6.32~17.51) 6.43(4.15~10.50) Z=-3.250 0.001 SCr(μmol/L) 152.00(71.50~293.50) 73.50(55.75~141.00) Z=-2.915 0.004 血淀粉酶(U/L) 76.00(44.00~306.50) 103.00(53.75~389.75) Z=-0.935 0.350 GCS评分 15.00(13.00~15.00) 15.00(15.00~15.00) Z=-2.973 0.003 APACHE Ⅱ 13.00(10.00~16.00) 11.00(7.75~14.00) Z=-3.173 0.002 SOFA 6.00(4.00~10.00) 4.00(2.00~6.00) Z=-4.309 <0.001 并发症[例(%)] 机械通气 54(83.1) 34(34.7) χ2=17.785 <0.001 ARDS 54(83.1) 49(50.0) χ2=17.785 <0.001 AKI 40(61.5) 23(23.5) χ2=44.521 <0.001 急性肝损伤 16(24.6) 12(12.2) χ2=105.631 <0.001 低血容量性休克 15(23.1) 3(3.1) χ2=108.741 <0.001 脓毒症 37(56.9) 27(27.6) χ2=50.971 <0.001 肺部感染 63(96.9) 90(91.8) χ2=3.053 0.158 腹腔高压 34(52.3) 22(22.4) χ2=57.715 <0.001 腹腔出血 12(18.5) 6(6.1) χ2=118.409 <0.001 肠瘘 2(3.1) 2(2.0) χ2=0.175 0.524 MODS 49(75.4) 36(36.7) χ2=26.749 <0.001 表 4 SAP患者发生PICS多因素Logistic回归分析
Table 4. Multivariate logistic regression analysis of PICS in patients with SAP
变量 β值 P值 OR 95%CI PLR 0.006 <0.001 1.006 1.003~1.009 HCT -2.810 0.256 0.060 0.000~7.768 APACHE Ⅱ 0.076 0.124 1.079 0.982~1.195 SOFA 0.053 0.458 1.055 0.913~1.216 机械通气 1.464 0.002 4.324 1.723~11.423 AKI 1.233 0.018 3.432 1.253~9.854 低血容量性休克 1.933 0.013 6.910 1.685~39.068 腹腔高压 0.743 0.107 2.102 0.852~5.245 -
[1] VANZANT EL, LOPEZ CM, OZRAZGAT-BASLANTI T, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma[J]. J Trauma Acute Care Surg, 2014, 76(1): 21-29. DOI: 10.1097/TA.0b013e3182ab1ab5. [2] GENTILE LF, CUENCA AG, EFRON PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care[J]. J Trauma Acute Care Surg, 2012, 72(6): 1491-1501. DOI: 10.1097/TA.0b013e318256e000. [3] MIRA JC, GENTILE LF, MATHIAS BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome[J]. Crit Care Med, 2017, 45(2): 253-262. DOI: 10.1097/CCM.0000000000002074. [4] NAKAMURA K, OGURA K, NAKANO H, et al. C-reactive protein clustering to clarify persistent inflammation, immunosuppression and catabolism syndrome[J]. Intensive Care Med, 2020, 46(3): 437-443. DOI: 10.1007/s00134-019-05851-3. [5] EFRON PA, MOHR AM, BIHORAC A, et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery[J]. Surgery, 2018, 164(2): 178-184. DOI: 10.1016/j.surg.2018.04.011. [6] XU D, XUN J. Persistentinflammation-immunosuppression-catabolism syndrome in severe acute pancreatitis[J]. J Chin Pract Diagn Ther, 2018, 32(7): 725-728. DOI: 10.13507/j.issn.1674-3474.2018.07.030.徐冬, 荀江. 重症急性胰腺炎并持续性炎症-免疫抑制-分解代谢综合征研究进展[J]. 中华实用诊断与治疗杂志, 2018, 32(7): 725-728. DOI: 10.13507/j.issn.1674-3474.2018.07.030. [7] YANG R, DU LL, ZHANG ZD, et al. Clinical observation of severe patients with persistent inflammation immunosuppression catabolism syndrome[J]. China J Emerg Resuscitation Disaster Med, 2021, 16(8): 919-921. DOI: 10.3969/j.issn.1673-6966.2021.08.021.杨蓉, 杜玲玲, 章志丹, 等. 重症患者持续性炎症-免疫抑制-分解代谢综合征的临床观察研究[J]. 中国急救复苏与灾害医学杂志, 2021, 16(8): 919-921. DOI: 10.3969/j.issn.1673-6966.2021.08.021. [8] KOUPENOVA M, CLANCY L, CORKREY HA, et al. Circulating platelets as mediators of immunity, inflammation, and thrombosis[J]. Circ Res, 2018, 122(2): 337-351. DOI: 10.1161/circresaha.117.310795. [9] ZHOU H, MEI X, HE X, et al. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: A retrospective study[J]. Medicine (Baltimore), 2019, 98(16): e15275. DOI: 10.1097/md.0000000000015275. [10] HAWKINS RB, RAYMOND SL, STORTZ JA, et al. Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome[J]. Front Immunol, 2018, 9: 1511. DOI: 10.3389/fimmu.2018.01511. [11] CHEN MY, CHEN MX, WANG MX, et al. Risk factors for acute kidney injury in severe acute pancreatitis: a Meta-analysis[J]. Chin Gen Pract, 2022, 25(30): 3834-3842. DOI: 10.12114/j.issn.1007-9572.2022.0452.陈美颖, 陈木欣, 王明欣, 等. 重症急性胰腺炎患者并发急性肾损伤危险因素的Meta分析[J]. 中国全科医学, 2022, 25(30): 3834-3842. DOI: 10.12114/j.issn.1007-9572.2022.0452. [12] WANG X, ZHUANG X, WEI R, et al. Protective effects of Acanthopanax vs. Ulinastatin against severe acute pancreatitis-induced brain injury in rats[J]. Int Immunopharmacol, 2015, 24(2): 285-298. DOI: 10.1016/j.intimp.2014.12.020. [13] WANG Y, QI WJ, ZENG YW, et al. Mechanism of action of magnolol in the treatment of acute lung injury in a rat model of severe acute pancreatitis[J]. J Clin Hepatol, 2020, 36(12): 2782-2787. DOI: 10.3969/j.issn.1001-5256.2020.12.028.王燕, 齐文杰, 曾亚薇, 等. 厚朴酚治疗重症急性胰腺炎大鼠模型并发急性肺损伤的作用机制[J]. 临床肝胆病杂志, 2020, 36(12): 2782-2787. DOI: 10.3969/j.issn.1001-5256.2020.12.028. [14] WANG X, WANG XK, CEN RF, et al. Influence of Xuebijing injection on clinical effects and cellular immune function of patients with severe acute pancreatitis[J]. Chin J New Drugs Clin Rem, 2015, 34(6): 476-479. DOI: 10.14109/j.cnki.xyylc.2015.06.016.王雪, 王先坤, 岑荣飞, 等. 血必净注射液对重症急性胰腺炎疗效及患者细胞免疫功能的影响[J]. 中国新药与临床杂志, 2015, 34(6): 476-479. DOI: 10.14109/j.cnki.xyylc.2015.06.016. [15] WANG P, LI L, LAN D, et al. Effects of hemofiltration therapy on biochemical indexes, liver and kidney function and inflammatory factors in patients with severe acute pancreatitis complicated with abdominal compartment syndrome[J]. J Clin Exp Med, 2022, 21(4): 384-388. DOI: 10.3969/j.issn.1671-4695.2022.04.014.王萍, 李乐, 兰东, 等. 血液滤过治疗对重症急性胰腺炎合并腹腔间隔室综合征患者生化指标、肝肾功能及炎症因子水平的影响[J]. 临床和实验医学杂志, 2022, 21(4): 384-388. DOI: 10.3969/j.issn.1671-4695.2022.04.014. [16] SU HY, MO ZX, CHEN R, et al. Effect of thymosin α1 on immunity, metabolism and prognosis in elderly patients with sepsis followed by persistent inflammation, immunosuppression, and catabolism syndrome[J]. J Pract Med, 2018, 34(1): 119-123. DOI: 10.3969/j.issn.1006-5725.2018.01.030.苏和毅, 莫泽珣, 陈蕊, 等. 胸腺肽α1干预治疗对脓毒症后的PICS老年患者免疫、代谢功能及预后的影响[J]. 实用医学杂志, 2018, 34(1): 119-123. DOI: 10.3969/j.issn.1006-5725.2018.01.030. [17] LUBBERS T, KOX M, DE HAAN JJ, et al. Continuous administration of enteral lipid- and protein-rich nutrition limits inflammation in a human endotoxemia model[J]. Crit Care Med, 2013, 41(5): 1258-1265. DOI: 10.1097/CCM.0b013e31827c0a17. [18] ZHU X, PRIBIS JP, RODRIGUEZ PC, et al. The central role of arginine catabolism in T-cell dysfunction and increased susceptibility to infection after physical injury[J]. Ann Surg, 2014, 259(1): 171-178. DOI: 10.1097/SLA.0b013e31828611f8. 期刊类型引用(4)
1. 孙备,白睿,隋宇航. 重症急性胰腺炎外科救援的实施与策略. 中华消化外科杂志. 2024(05): 653-657 .  百度学术
百度学术2. 徐霞,范辉,王小红. 参芪扶正液辅助治疗重症急性胰腺炎的临床效果. 中国医学创新. 2024(27): 10-14 .  百度学术
百度学术3. 郑云,涂倩倩,张阿芳,张泓. 重症急性胰腺炎并发持续炎症-免疫抑制-分解代谢综合征的列线图预测模型构建与验证. 中国急救医学. 2024(10): 890-896 .  百度学术
百度学术4. 梁文美,赵晓宇,薄世兴. 乙酰肝素酶在重症急性胰腺炎相关肺损伤中的表达及意义. 罕少疾病杂志. 2023(12): 64-66 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 3410 KB)
PDF下载 ( 3410 KB)

 下载:
下载:







 下载:
下载:



 百度学术
百度学术