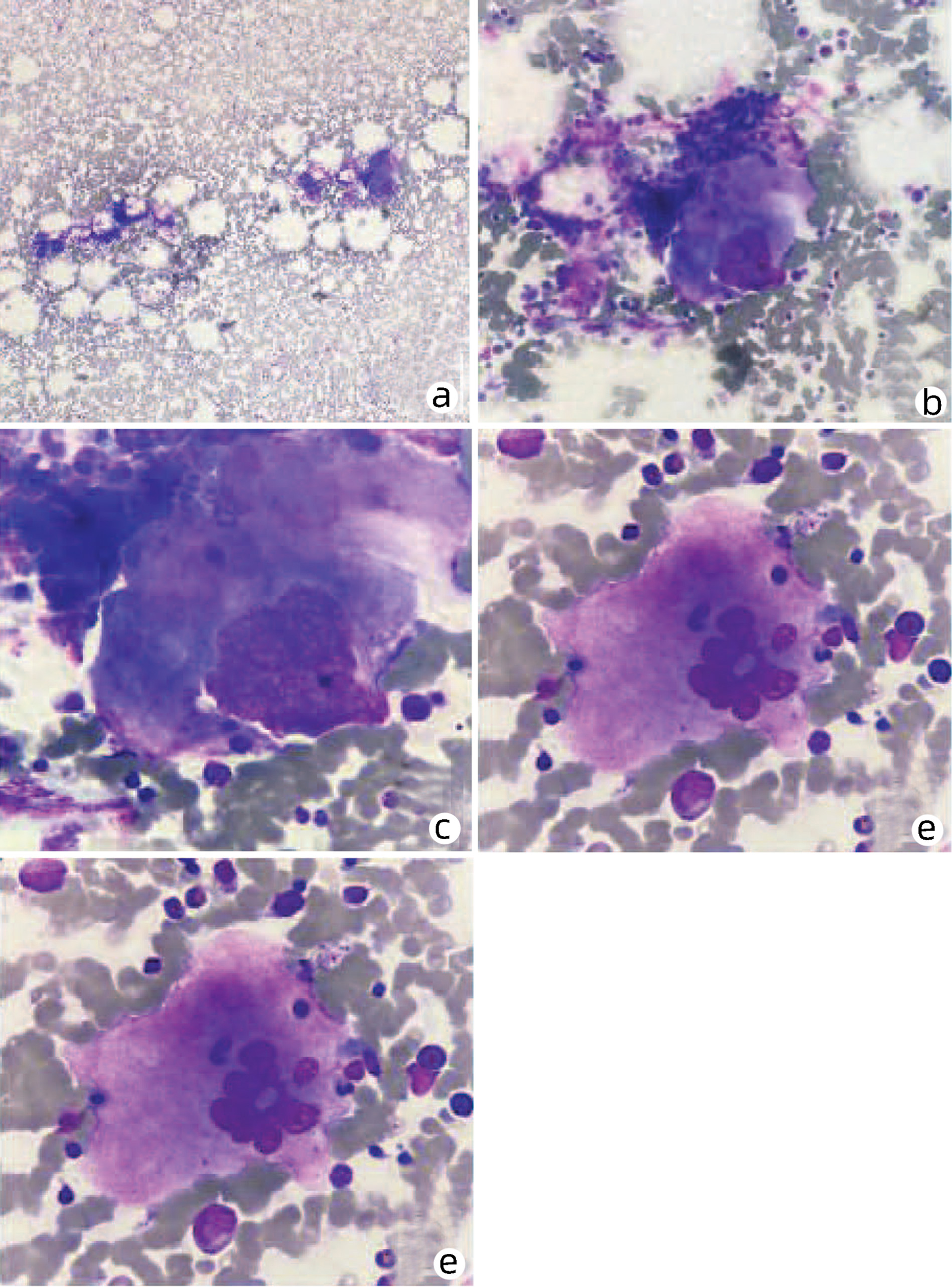
骨髓增生性疾病所致门静脉海绵样变性1例报告
DOI: 10.3969/j.issn.1001-5256.2023.06.025
伦理学声明:本例报告已获得患者/患者家属知情同意。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:韩冰负责课题设计,资料分析,撰写论文;涂传涛负责拟定写作思路,收集资料,指导撰写文章并最后定稿。
Cavernous transformation of the portal vein caused by myeloproliferative disease: A case report
-
[1] CUNNINGHAM ME, PARASTANDEH-CHEHR G, CEROCCHI O, et al. Noninvasive predictors of high-risk varices in patients with non-cirrhotic portal hypertension[J]. Can J Gastroenterol Hepatol, 2019, 2019: 1808797. DOI: 10.1155/2019/1808797. [2] KHANNA R, SARIN SK. Non-cirrhotic portal hypertension-diagnosis and management[J]. J Hepatol, 2014, 60(2): 421-441. DOI: 10.1016/j.jhep.2013.08.013. [3] SIRAMOLPIWAT S, SEIJO S, MIQUEL R, et al. Idiopathic portal hypertension: natural history and long-term outcome[J]. Hepatology, 2014, 59(6): 2276-2285. DOI: 10.1002/hep.26904. [4] ZHANG BJ, HAN GH, FAN DM. Research advances in non-cirrhotic portal hypertension[J]. J Clin Hepatol, 2016, 32(2): 245-249. DOI: 10.3969/j.issn.1001-5256.2016.02.009.张博静, 韩国宏, 樊代明. 非肝硬化性门静脉高压症的研究现状[J]. 临床肝胆病杂志, 2016, 32(2): 245-249. DOI: 10.3969/j.issn.1001-5256.2016.02.009. [5] de FRANCHIS R. Expanding consensus in portal hypertension: report of the Baveno Ⅵ Consensus Workshop: stratifying risk and individualizing care for portal hypertension[J]. J Hepatol, 2015, 63(3): 743-752. DOI: 10.1016/j.jhep.2015.05.022. [6] DELEVE LD, VALLA DC, GARCIA-TSAO G, et al. Vascular disorders of the liver[J]. Hepatology, 2009, 49(5): 1729-1764. DOI: 10.1002/hep.22772. [7] LI H, SUN PM, SUN HW, et al. Progress in clinical diagnosis and treatment of cavernous transformation of the portal vein[J]. World Chin J Dig, 2021, 29(12): 662-669. DOI: 10.11569/wcjd.v29.i12.662j.李昊, 孙培鸣, 孙宏伟, 等. 门静脉海绵样变性的临床诊疗进展[J]. 世界华人消化杂志, 2021, 29(12): 662-669. DOI: 10.11569/wcjd.v29.i12.662j. [8] RUNGJIRAJITTRANON T, OWATTANAPANICH W, UNGPRASERT P, et al. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms[J]. BMC Cancer, 2019, 19(1): 184. DOI: 10.1186/s12885-019-5387-9. [9] LEVINE RL, WADLEIGH M, COOLS J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis[J]. Cancer Cell, 2005, 7(4): 387-397. DOI: 10.1016/j.ccr.2005.03.023. [10] MCCLURE R, MAI M, LASHO T. Validation of two clinically useful assays for evaluation of JAK2V617F mutation in chronic myeloproliferative disorders[J]. Leukemia, 2006, 20(1): 168-171. DOI: 10.1038/sj.leu.2404007. [11] SUN CC, LI Y, TIAN WJ, et al. JAK2V617F mutation and TNF-α expression in myeloproliferative neoplasms and their correlation[J]. J Exp Hematol, 2014, 22(4): 1022-1026. DOI: 10.7534/j.issn.1009-2137.2014.04.025.孙聪聪, 李英, 田文君, 等. 骨髓增殖性肿瘤中JAK2V617F突变和TNF-α的表达及两者的相关性研究[J]. 中国实验血液学杂志, 2014, 22(4): 1022-1026. DOI: 10.7534/j.issn.1009-2137.2014.04.025. [12] TEFFERI A. Primary myelofibrosis: 2019 update on diagnosis, risk-stratification and management[J]. Am J Hematol, 2018, 93(12): 1551-1560. DOI: 10.1002/ajh.25230. -



 PDF下载 ( 2374 KB)
PDF下载 ( 2374 KB)


 下载:
下载: