抑制性受体TIGIT与慢性HBV感染中免疫紊乱的关系
DOI: 10.3969/j.issn.1001-5256.2023.06.026
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:周玉霞负责课题设计,撰写论文;王彩红、姚晓文、王蓉、郑晓凤参与修改论文;张久聪、于晓辉负责拟定写作思路,指导撰写文章并最后定稿。
Association of inhibitory receptor T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain with immune disorders in chronic HBV infection
-
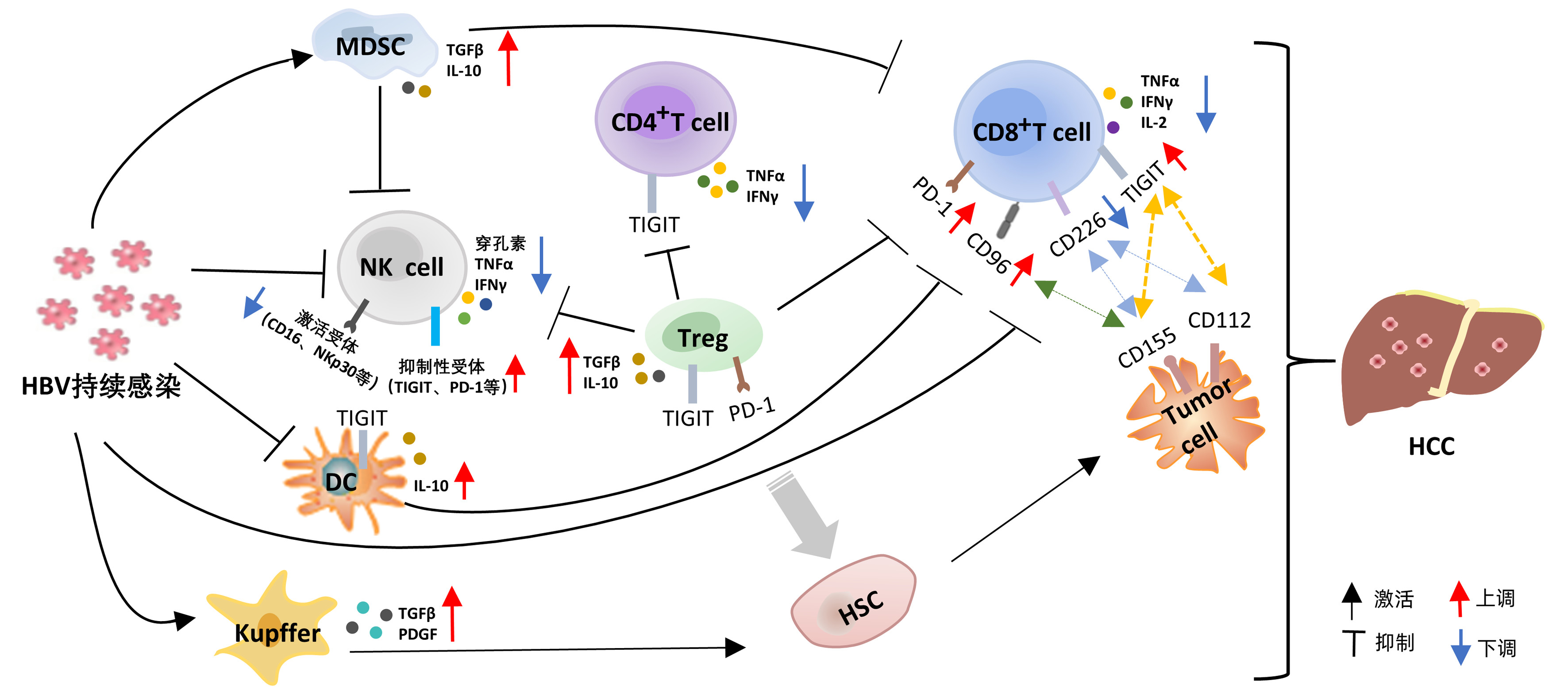
摘要: 持续HBV感染改变了天然免疫细胞和获得性免疫细胞表面受体的表达,由此引发的多种免疫紊乱,可导致免疫逃逸,最终使疾病慢性化。研究表明,抑制性受体的上调是患者免疫紊乱的主要原因,阻断抑制性受体可一定程度上恢复患者的免疫功能。T淋巴细胞免疫球蛋白和免疫受体酪氨酸抑制性基序结构域(TIGIT)是目前较为关注的一种新型抑制性受体,在NK细胞和T淋巴细胞中高水平表达。研究发现,TIGIT在慢性病毒感染中发挥重要作用,现就TIGIT与慢性HBV感染中免疫紊乱的相关性研究进展进行简要综述。Abstract: Persistent HBV infection alters the expression of receptors on the surface of innate and acquired immune cells, which may cause a variety of immune disorders and finally lead to immune escape and disease chronicity. Studies have shown that the upregulation of inhibitory receptors is the main cause of immune disorders in patients, and blocking inhibitory receptors can restore immune function to a certain extent. T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT) is a new type of inhibitory receptor attracting much attention at present, and it is highly expressed in NK cells and T cells. It has been found that TIGIT plays an important role in chronic viral infection, and this article briefly reviews the research advances in the association between TIGIT and immune disorders in chronic HBV infection.
-
Key words:
- Hepatitis B virus /
- Receptors, Immunologic /
- Immune Disorder
-
[1] MENG Z, CHEN Y, LU M. Advances in targeting the innate and adaptive immune systems to cure chronic hepatitis B virus infection[J]. Front Immunol, 2019, 10: 3127. DOI: 10.3389/fimmu.2019.03127. [2] COX MA, NECHANITZKY R, MAK TW. Check point inhibitors as therapies for infectious diseases[J]. Curr Opin Immunol, 2017, 48: 61-67. DOI: 10.1016/j.coi.2017.07.016. [3] WANG L, WANG K, ZOU ZQ. Crosstalk between innate and adaptive immunity in hepatitis B virus infection[J]. World J Hepatol, 2015, 7(30): 2980-2991. DOI: 10.4254/wjh.v7.i30.2980. [4] HOPCRAFT SE, DAMANIA B. Tumour viruses and innate immunity[J]. Philos Trans R Soc Lond B Biol Sci, 2017, 372(1732): 20160267. DOI: 10.1098/rstb.2016.0267. [5] ZHAO HJ, HU YF, HAN QJ, et al. Innate and adaptive immune escape mechanisms of hepatitis B virus[J]. World J Gastroenterol, 2022, 28(9): 881-896. DOI: 10.3748/wjg.v28.i9.881. [6] WU J, HAN M, LI J, et al. Immunopathogenesis of HBV infection[J]. Adv Exp Med Biol, 2020, 1179: 71-107. DOI: 10.1007/978-981-13-9151-4_4. [7] JIN X, YAN ZH, LU L, et al. Peripheral Immune cells exhaustion and functional impairment in patients with chronic hepatitis B[J]. Front Med (Lausanne), 2021, 8: 759292. DOI: 10.3389/fmed.2021.759292. [8] BAUDI I, KAWASHIMA K, ISOGAWA M. HBV-specific CD8+ T-cell tolerance in the liver[J]. Front Immunol, 2021, 12: 721975. DOI: 10.3389/fimmu.2021.721975. [9] FISICARO P, BARILI V, ROSSI M, et al. Pathogenetic mechanisms of T cell dysfunction in chronic HBV infection and related therapeutic approaches[J]. Front Immunol, 2020, 11: 849. DOI: 10.3389/fimmu.2020.00849. [10] ROTTE A, SAHASRANAMAN S, BUDHA N. Targeting TIGIT for immunotherapy of cancer: update on clinical development[J]. Biomedicines, 2021, 9(9): 1277. DOI: 10.3390/biomedicines9091277. [11] STANIETSKY N, ROVIS TL, GLASNER A, et al. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR[J]. Eur J Immunol, 2013, 43(8): 2138-2150. DOI: 10.1002/eji.201243072. [12] JIN HS, PARK Y. Hitting the complexity of the TIGIT-CD96-CD112R-CD226 axis for next-generation cancer immunotherapy[J]. BMB Rep, 2021, 54(1): 2-11. DOI: 10.5483/BMBRep.2021.54.1.229. [13] HUANG Z, QI G, MILLER JS, et al. CD226: An emerging role in immunologic diseases[J]. Front Cell Dev Biol, 2020, 8: 564. DOI: 10.3389/fcell.2020.00564. [14] HARJUNPÄÄ H, GUILLEREY C. TIGIT as an emerging immune checkpoint[J]. Clin Exp Immunol, 2020, 200(2): 108-119. DOI: 10.1111/cei.13407. [15] WANG J, HOU H, MAO L, et al. TIGIT signaling pathway regulates natural killer cell function in chronic hepatitis B virus infection[J]. Front Med (Lausanne), 2021, 8: 816474. DOI: 10.3389/fmed.2021.816474. [16] WEI YY, FAN J, SHAN MX, et al. TIGIT marks exhausted T cells and serves as a target for immune restoration in patients with chronic HBV infection[J]. Am J Transl Res, 2022, 14(2): 942-954. [17] LIU C, XU L, XIA C, et al. Increased proportion of functional subpopulations in circulating regulatory T cells in patients with chronic hepatitis B[J]. Hepatol Res, 2020, 50(4): 439-452. DOI: 10.1111/hepr.13472. [18] ZHANG W, SUN H, SUN R, et al. HBV immune tolerance of HBs-transgenic mice observed through parabiosis with WT mice[J]. Front Immunol, 2022, 13: 993246. DOI: 10.3389/fimmu.2022.993246. [19] LOZANO E, DOMINGUEZ-VILLAR M, KUCHROO V, et al. The TIGIT/CD226 axis regulates human T cell function[J]. J Immunol, 2012, 188(8): 3869-3875. DOI: 10.4049/jimmunol.1103627. [20] JIA L, GAO Y, HE Y, et al. HBV induced hepatocellular carcinoma and related potential immunotherapy[J]. Pharmacol Res, 2020, 159: 104992. DOI: 10.1016/j.phrs.2020.104992. [21] WU Y, HAO X, WEI H, et al. Blockade of T-cell receptor with Ig and ITIM domains elicits potent antitumor immunity in naturally occurring HBV-related HCC in mice[J]. Hepatology, 2023, 77(3): 965-981. DOI: 10.1002/hep.32715. [22] XU F, JIN T, ZHU Y, et al. Immune checkpoint therapy in liver cancer[J]. J Exp Clin Cancer Res, 2018, 37(1): 110. DOI: 10.1186/s13046-018-0777-4. [23] LIU X, LI M, WANG X, et al. PD-1+ TIGIT+ CD8+ T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma[J]. Cancer Immunol Immunother, 2019, 68(12): 2041-2054. DOI: 10.1007/s00262-019-02426-5. [24] YU L, LIU X, WANG X, et al. TIGIT+ TIM-3+ NK cells are correlated with NK cell exhaustion and disease progression in patients with hepatitis B virus-related hepatocellular carcinoma[J]. Oncoimmunology, 2021, 10(1): 1942673. DOI: 10.1080/2162402X.2021.1942673. [25] BLAZKOVA J, HUITING ED, BODDAPATI AK, et al. Correlation between TIGIT expression on CD8+ T cells and higher cytotoxic capacity[J]. J Infect Dis, 2021, 224(9): 1599-1604. DOI: 10.1093/infdis/jiab155. [26] DUAN X, LIU J, CUI J, et al. Expression of TIGIT/CD155 and correlations with clinical pathological features in human hepatocellular carcinoma[J]. Mol Med Rep, 2019, 20(4): 3773-3781. DOI: 10.3892/mmr.2019.10641. [27] AMANCHA PK, HONG JJ, ROGERS K, et al. In vivo blockade of the programmed cell death-1 pathway using soluble recombinant PD-1-Fc enhances CD4+ and CD8+ T cell responses but has limited clinical benefit[J]. J Immunol, 2013, 191(12): 6060-6070. DOI: 10.4049/jimmunol.1302044. [28] FERRANDO-MARTINEZ S, SNELL BENNETT A, LINO E, et al. Functional exhaustion of HBV-specific CD8 T cells impedes pD-L1 blockade efficacy in chronic HBV infection[J]. Front Immunol, 2021, 12: 648420. DOI: 10.3389/fimmu.2021.648420. [29] CHEW GM, FUJITA T, WEBB GM, et al. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection[J]. PLoS Pathog, 2016, 12(1): e1005349. DOI: 10.1371/journal.ppat.1005349. [30] GE Z, ZHOU G, CAMPOS CARRASCOSA L, et al. TIGIT and PD1 co-blockade restores ex vivo functions of human tumor-infiltrating CD8+ T cells in hepatocellular carcinoma[J]. Cell Mol Gastroenterol Hepatol, 2021, 12(2): 443-464. DOI: 10.1016/j.jcmgh.2021.03.003. [31] ZONG L, PENG H, SUN C, et al. Breakdown of adaptive immunotolerance induces hepatocellular carcinoma in HBsAg-tg mice[J]. Nat Commun, 2019, 10(1): 221. DOI: 10.1038/s41467-018-08096-8. -



 PDF下载 ( 2165 KB)
PDF下载 ( 2165 KB)

 下载:
下载:


