肝内微环境诱导的自噬在非酒精性脂肪性肝病中的作用
DOI: 10.3969/j.issn.1001-5256.2023.06.029
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:寇萱萱负责拟定写作思路并撰写文章;张华、邓婧鑫参与相关文献的收集与整理;张建刚审校并修改论文。
Role of intrahepatic microenvironment induced-autophagy in nonalcoholic fatty liver disease
-
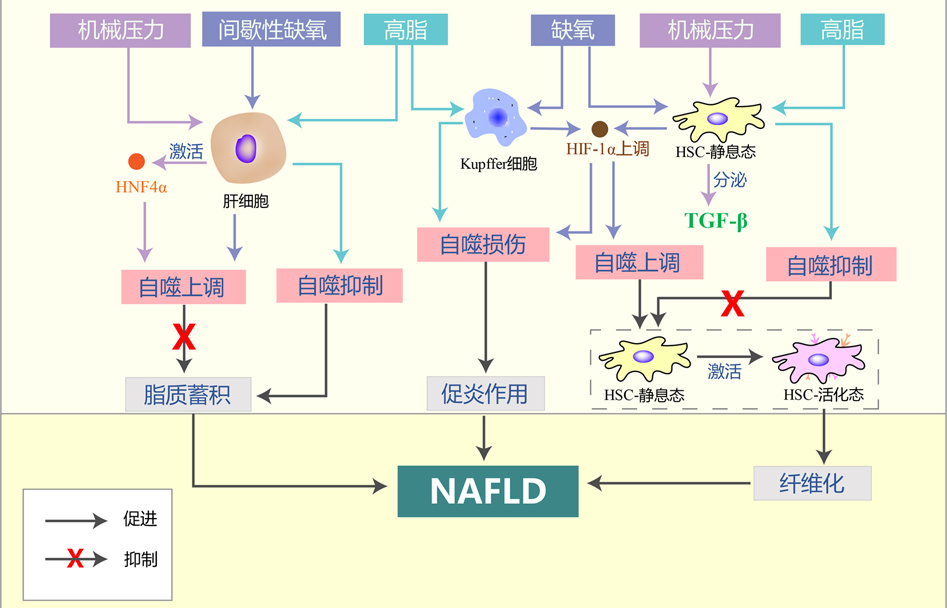
摘要: 非酒精性脂肪性肝病(NAFLD)是以肝细胞内脂质过度沉积为主要特征的一系列肝脏异常病变,也是全球范围内最常见的慢性肝病。自噬是细胞降解自身成分、参与维持器官功能及机体稳态的一种基本细胞过程,与NAFLD的进展存在密切联系。机体遭受的高脂、缺氧和压力等在肝脏内形成了细胞外微环境的异常改变,这些异常微环境可能通过诱导的肝脏细胞自噬促进NAFLD的发生发展。本文基于肝脏内的多种微环境特征,对肝细胞、Kupffer细胞、肝星状细胞等肝脏细胞的自噬在NAFLD进展中的作用和机制进行综述。Abstract: Nonalcoholic fatty liver disease (NAFLD) is a series of abnormal liver lesions mainly characterized by excessive lipid deposition in hepatocytes, and it is also the most common chronic liver disease worldwide. Autophagy is a basic cellular process in which cells degrade their own components and participate in the maintenance of organ function and body homeostasis, and it is closely associated with the progression of NAFLD. High fat, hypoxia, and stress in human body may cause abnormal changes in extracellular microenvironment in the liver, and such abnormal microenvironment may promote the development and progression of NAFLD by inducing liver cell autophagy. This article reviews the role and mechanism of autophagy of liver cells such as hepatocytes, Kupffer cells, and hepatic stellate cells in the progression of NAFLD based on various microenvironment characteristics in the liver.
-
Key words:
- Non-alcoholic Fatty Liver Disease /
- Autophagy /
- Cellular Microenvironment
-
[1] RAVIKUMAR B, SARKAR S, DAVIES JE, et al. Regulation of mammalian autophagy in physiology and pathophysiology[J]. Physiol Rev, 2010, 90(4): 1383-1435. DOI: 10.1152/physrev.00030.2009. [2] FILALI-MOUNCEF Y, HUNTER C, ROCCIO F, et al. The ménage à trois of autophagy, lipid droplets and liver disease [J]. Autophagy, 2022, 18(1): 50-72. DOI: 10.1080/15548627.2021.1895658. [3] WANG L, KLIONSKY DJ, SHEN HM. The emerging mechanisms and functions of microautophagy[J]. Nat Rev Mol Cell Biol, 2023, 24(3): 186-203. DOI: 10.1038/s41580-022-00529-z. [4] KIRKIN V, ROGOV VV. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway[J]. Mol Cell, 2019, 76(2): 268-285. DOI: 10.1016/j.molcel.2019.09.005. [5] TONG M, SAITO T, ZHAI P, et al. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy[J]. Circ Res, 2019, 124(9): 1360-1371. DOI: 10.1161/CIRCRESAHA.118.314607. [6] FENG X, ZHANG H, MENG L, et al. Hypoxia-induced acetylation of PAK1 enhances autophagy and promotes brain tumorigenesis via phosphorylating ATG5[J]. Autophagy, 2021, 17(3): 723-742. DOI: 10.1080/15548627.2020.1731266. [7] CZAJA MJ, DING WX, DONOHUE TM Jr, et al. Functions of autophagy in normal and diseased liver[J]. Autophagy, 2013, 9(8): 1131-1158. DOI: 10.4161/auto.25063. [8] SOUZA MR, DINIZ MDE F, MEDEIROS-FILHO JE, et al. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease[J]. Arq Gastroenterol, 2012, 49(1): 89-96. DOI: 10.1590/s0004-28032012000100015. [9] OTA H, FUJITA Y, YAMAUCHI M, et al. Relationship between intermittent hypoxia and type 2 diabetes in sleep apnea syndrome[J]. Int J Mol Sci, 2019, 20(19): 4756. DOI: 10.3390/ijms20194756. [10] MILIĆ S, LULIĆ D, ŠTIMAC D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations[J]. World J Gastroenterol, 2014, 20(28): 9330-9337. DOI: 10.3748/wjg.v20.i28.9330. [11] LIAN CY, ZHAI ZZ, LI ZF, et al. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms[J]. Chem Biol Interact, 2020, 330: 109199. DOI: 10.1016/j.cbi.2020.109199. [12] RODRIGUES SG, MONTANI M, GUIXÉ-MUNTET S, et al. Patients with signs of advanced liver disease and clinically significant portal hypertension do not necessarily have cirrhosis[J]. Clin Gastroenterol Hepatol, 2019, 17(10): 2101-2109. e1. DOI: 10.1016/j.cgh.2018.12.038. [13] MENDES FD, SUZUKI A, SANDERSON SO, et al. Prevalence and indicators of portal hypertension in patients with nonalcoholic fatty liver disease[J]. Clin Gastroenterol Hepatol, 2012, 10(9): 1028-1033. e2. DOI: 10.1016/j.cgh.2012.05.008. [14] LONG Y, NIU Y, LIANG K, et al. Mechanical communication in fibrosis progression[J]. Trends Cell Biol, 2022, 32(1): 70-90. DOI: 10.1016/j.tcb.2021.10.002. [15] FU Y, ZHANG N, TANG W, et al. Chronic intermittent hypoxia contributes to non-alcoholic steatohepatitis progression in patients with obesity[J]. Hepatol Int, 2022, 16(4): 824-834. DOI: 10.1007/s12072-022-10347-2. [16] VIGLINO D, JULLIAN-DESAYES I, MINOVES M, et al. Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease[J]. Eur Respir J, 2017, 49(6): 1601923. DOI: 10.1183/13993003.01923-2016. [17] PIGUET AC, STROKA D, ZIMMERMANN A, et al. Hypoxia aggravates non-alcoholic steatohepatitis in mice lacking hepatocellular PTEN[J]. Clin Sci (Lond), 2009, 118(6): 401-410. DOI: 10.1042/CS20090313. [18] SINGH R, KAUSHIK S, WANG Y, et al. Autophagy regulates lipid metabolism[J]. Nature, 2009, 458(7242): 1131-1135. DOI: 10.1038/nature07976. [19] DONG S, JIA C, ZHANG S, et al. The REGγ proteasome regulates hepatic lipid metabolism through inhibition of autophagy[J]. Cell Metab, 2013, 18(3): 380-391. DOI: 10.1016/j.cmet.2013.08.012. [20] LIU K, ZHAO E, ILYAS G, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization[J]. Autophagy, 2015, 11(2): 271-284. DOI: 10.1080/15548627.2015.1009787. [21] MOORE MP, CUNNINGHAM RP, MEERS GM, et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD[J]. Hepatology, 2022, 76(5): 1452-1465. DOI: 10.1002/hep.32324. [22] YAMADA T, MURATA D, ADACHI Y, et al. Mitochondrial stasis reveals p62-mediated ubiquitination in parkin-independent mitophagy and mitigates nonalcoholic fatty liver disease[J]. Cell Metab, 2018, 28(4): 588-604. e5. DOI: 10.1016/j.cmet.2018.06.014. [23] LI R, XIN T, LI D, et al. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy[J]. Redox Biol, 2018, 18: 229-243. DOI: 10.1016/j.redox.2018.07.011. [24] ZHOU T, CHANG L, LUO Y, et al. Mst1 inhibition attenuates non-alcoholic fatty liver disease via reversing Parkin-related mitophagy[J]. Redox Biol, 2019, 21: 101120. DOI: 10.1016/j.redox.2019.101120. [25] MEDERACKE I, HSU CC, TROEGER JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology[J]. Nat Commun, 2013, 4: 2823. DOI: 10.1038/ncomms3823. [26] BLANER WS, O'BYRNE SM, WONGSIRIROJ N, et al. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage[J]. Biochim Biophys Acta, 2009, 1791(6): 467-473. DOI: 10.1016/j.bbalip.2008.11.001. [27] HONG Y, LI S, WANG J, et al. In vitro inhibition of hepatic stellate cell activation by the autophagy-related lipid droplet protein ATG2A[J]. Sci Rep, 2018, 8(1): 9232. DOI: 10.1038/s41598-018-27686-6. [28] THOEN LF, GUIMARÃES EL, DOLLÉ L, et al. A role for autophagy during hepatic stellate cell activation[J]. J Hepatol, 2011, 55(6): 1353-1360. DOI: 10.1016/j.jhep.2011.07.010. [29] KIM JS, NITTA T, MOHUCZY D, et al. Impaired autophagy: A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes[J]. Hepatology, 2008, 47(5): 1725-1736. DOI: 10.1002/hep.22187. [30] WANG D, SI D, LI G, et al. Dysregulated autophagic activity induced in response to chronic intermittent hypoxia contributes to the pathogenesis of NAFLD[J]. Front Physiol, 2022, 13: 941706. DOI: 10.3389/fphys.2022.941706. [31] LU Y, BIAN J, KAN H, et al. Intermittent hypoxia preconditioning protects WRL68 cells against oxidative injury: Involvement of the PINK1/Parkin-mediated mitophagy regulated by nuclear respiratory factor 1[J]. Mitochondrion, 2021, 59: 113-122. DOI: 10.1016/j.mito.2021.04.012. [32] CUI F, HU HF, GUO J, et al. The effect of autophagy on chronic intermittent hypobaric hypoxia ameliorating liver damage in metabolic syndrome rats[J]. Front Physiol, 2020, 11: 13. DOI: 10.3389/fphys.2020.00013. [33] ZHAN L, HUANG C, MENG XM, et al. Hypoxia-inducible factor-1alpha in hepatic fibrosis: A promising therapeutic target[J]. Biochimie, 2015, 108: 1-7. DOI: 10.1016/j.biochi.2014.10.013. [34] NATH B, SZABO G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases[J]. Hepatology, 2012, 55(2): 622-633. DOI: 10.1002/hep.25497. [35] WANG X, de CARVALHO RIBEIRO M, IRACHETA-VELLVE A, et al. Macrophage-specific hypoxia-inducible factor-1α contributes to impaired autophagic flux in nonalcoholic steatohepatitis[J]. Hepatology, 2019, 69(2): 545-563. DOI: 10.1002/hep.30215. [36] ZHANG X, HUANG C, LI X, et al. HFD and HFD-provoked hepatic hypoxia act as reciprocal causation for NAFLD via HIF-independent signaling[J]. BMC Gastroenterol, 2020, 20(1): 366. DOI: 10.1186/s12876-020-01515-5. [37] DENG J, HUANG Q, WANG Y, et al. Hypoxia-inducible factor-1alpha regulates autophagy to activate hepatic stellate cells[J]. Biochem Biophys Res Commun, 2014, 454(2): 328-334. DOI: 10.1016/j.bbrc.2014.10.076. [38] JIN Y, BAI Y, NI H, et al. Activation of autophagy through calcium-dependent AMPK/mTOR and PKCθ pathway causes activation of rat hepatic stellate cells under hypoxic stress[J]. FEBS Lett, 2016, 590(5): 672-682. DOI: 10.1002/1873-3468.12090. [39] MUELLER S. Does pressure cause liver cirrhosis? The sinusoidal pressure hypothesis[J]. World J Gastroenterol, 2016, 22(48): 10482-10501. DOI: 10.3748/wjg.v22.i48.10482. [40] BAFFY G. Origins of portal hypertension in nonalcoholic fatty liver disease[J]. Dig Dis Sci, 2018, 63(3): 563-576. DOI: 10.1007/s10620-017-4903-5. [41] DUPONT N, CODOGNO P. Autophagy transduces physical constraints into biological responses[J]. Int J Biochem Cell Biol, 2016, 79: 419-426. DOI: 10.1016/j.biocel.2016.08.021. [42] CLAUDE-TAUPIN A, CODOGNO P, DUPONT N. Links between autophagy and tissue mechanics[J]. J Cell Sci, 2021, 134(17): jcs258589. DOI: 10.1242/jcs.258589. [43] YANG J, LIANG J, ZHENG Y, et al. Hepatic polarization accelerated by mechanical compaction involves HNF4 activation[J]. Biomed Res Int, 2020, 2020: 8016306. DOI: 10.1155/2020/8016306. [44] LEE DH, PARK SH, AHN J, et al. Mir214-3p and Hnf4a/Hnf4α reciprocally regulate Ulk1 expression and autophagy in nonalcoholic hepatic steatosis[J]. Autophagy, 2021, 17(9): 2415-2431. DOI: 10.1080/15548627.2020.1827779. [45] SERA T, SUMII T, FUJITA R, et al. Effect of shear stress on the migration of hepatic stellate cells[J]. In Vitro Cell Dev Biol Anim, 2018, 54(1): 11-22. DOI: 10.1007/s11626-017-0202-x. [46] SAKATA R, UENO T, NAKAMURA T, et al. Mechanical stretch induces TGF-beta synthesis in hepatic stellate cells[J]. Eur J Clin Invest, 2004, 34(2): 129-136. DOI: 10.1111/j.1365-2362.2004.01302.x. [47] CASTAGNARO S, CHRISAM M, CESCON M, et al. Extracellular collagen VI has prosurvival and autophagy instructive properties in mouse fibroblasts[J]. Front Physiol, 2018, 9: 1129. DOI: 10.3389/fphys.2018.01129. [48] TOTARO A, ZHUANG Q, PANCIERA T, et al. Cell phenotypic plasticity requires autophagic flux driven by YAP/TAZ mechanotransduction[J]. Proc Natl Acad Sci U S A, 2019, 116(36): 17848-17857. DOI: 10.1073/pnas.1908228116. [49] LEE YA, NOON LA, AKAT KM, et al. Autophagy is a gatekeeper of hepatic differentiation and carcinogenesis by controlling the degradation of Yap[J]. Nat Commun, 2018, 9(1): 4962. DOI: 10.1038/s41467-018-07338-z. -



 PDF下载 ( 1858 KB)
PDF下载 ( 1858 KB)

 下载:
下载:


