活性氧/活性氮与NF-κB信号通路级联交互在肝纤维化中的作用
DOI: 10.3969/j.issn.1001-5256.2023.06.031
Research advances in the cascade interaction between reactive oxygen species/reactive nitrogen species and the NF-κB signaling pathway in liver fibrosis
-
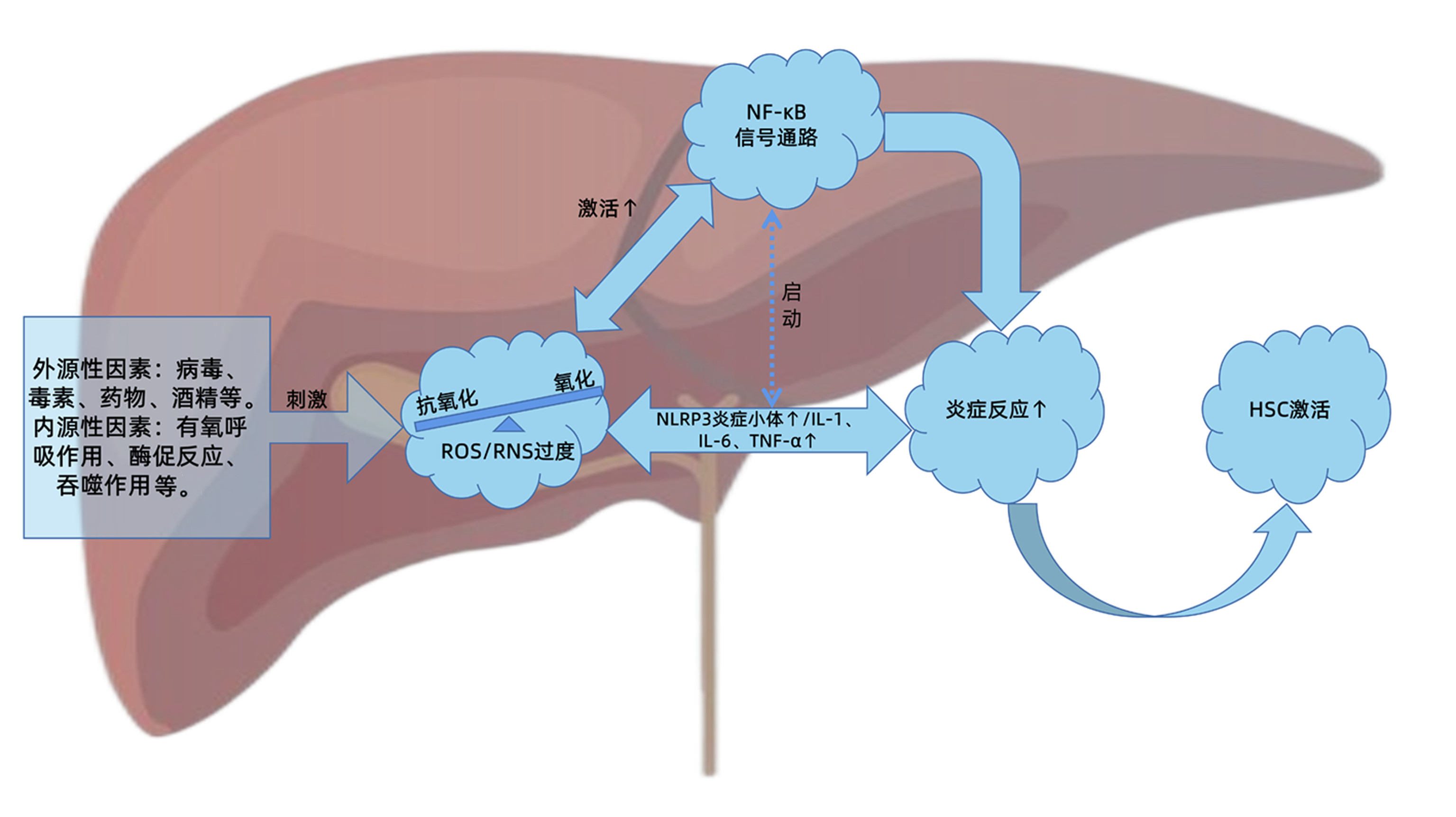
摘要: 肝纤维化是慢性肝损伤后组织修复过程中的代偿反应,也是诸多慢性肝病发展必经的病理过程。在病理状态下,肝脏氧化系统与抗氧化系统失衡,造成活性氧/活性氮产生过多或清除不足,诱导肝细胞损伤,扩大炎症反应,促进肝纤维化的发生发展。NF-κB作为炎症反应和氧化应激的主调控器,在肝纤维化进程中发挥关键作用。因此,活性氧/活性氮与NF-κB信号通路的级联交互关系对于进一步阐明肝纤维化的发病机制,探索有效的防治策略起到重要的指导作用。本文即对两者之间的交互关系及在肝纤维化进程中的重要作用进行综述和讨论,以期为肝纤维化的靶向治疗提供策略和参考。Abstract: Liver fibrosis is a compensatory response in the process of tissue repair after chronic liver injury, and it is also a necessary pathological process in the progression of a variety of chronic liver diseases. In the pathological state, the imbalance between hepatic oxidative system and antioxidant system can lead to the excessive production or insufficient clearance of reactive oxygen species (ROS)/reactive nitrogen species (RNS), which may induce the injury of hepatocytes, expand inflammatory response, and promote the development and progression of liver fibrosis. As a master regulator of oxidative stress and inflammatory response, NF-κB plays a key role in the process of liver fibrosis. Therefore, the cascade interaction between ROS/RNS and the NF-κB signaling pathway plays a guiding role in further clarifying the pathogenesis of liver fibrosis and exploring effective prevention and treatment strategies. This article reviews and discusses the interaction between ROS/RNS and the NF-κB signaling pathway and its important role in the progression of liver fibrosis, so as to provide strategies and references for targeted therapy for liver fibrosis.
-
Key words:
- Liver Fibrosis /
- Oxidative Stress /
- Reactive Oxygen Species /
- Reactive Nitrogen Species /
- NF-kappa B
-
表 1 肝纤维化时ROS/RNS与NF-κB级联交互作用相关文献回顾
Table 1. Literature review of the cross-link between ROS/RNS and NF-κB signaling pathway in liver fibrosis
实验药品 模型 影响 机制 文献 三七总皂苷 非酒精性脂肪肝大鼠 炎症反应↓;纤维化↓ NO/iNOS↓;NF-κB↓ [31] 山奈素 高脂饮食诱导肥胖小鼠 炎症损伤↓;抗氧化能力↑ TLR4↓;NF-κB↓;IκB↑;MDA↓;GSH/CAT/T-AOC/SOD↑ [32] 知母皂苷 高脂饮食诱导肥胖小鼠 炎症损伤↓;氧化应激↓;抗氧化能力↑ p-NF-κB↓;Nrf2/HO-1↑;MDA↓;T-AOC/GSH-Px↑ [33] 橙皮素 高脂饮食诱导NAFLD大鼠 炎症反应↓;氧化应激↓;纤维化↓ PI3K/AKT-Nrf2↑;ROS↓;NF-κB↓ [34] 脱落酸 TAA诱导肝纤维化小鼠 炎症反应↓;氧化应激↓;纤维化↓ 肝细胞凋亡因子(如: caspase-3;Bcl-2)↓;NF-κB↓;MDA↓;SOD/GSH↑ [35] Maresin-1 二乙基亚硝胺诱导肝纤维化大鼠 炎症反应↓;氧化应激↓;抗氧化能力↑ NF-κB↓; Nrf2↑; ROS↓; TGF-β↓ [36] 黄芩苷 TAA诱导肝硬化大鼠 炎症反应↓;氧化应激↓;纤维化↓ NF-κB/IL-6↓; NF-κB/NLRP3炎症小体↓;IL-1↓;NOX4/iNOS↓ [37] 白花丹素(PL) CCl4诱导肝纤维化大鼠 氧化应激↓;纤维化↓ NOX4↓;ROS↓;NF-κB↓;α-SMA↓;collagen Ⅲ↓ [38] 甲氧基丁香酚 人和鼠HSC(试管内)(ⅰ) CCl4诱导肝纤维化小鼠(ⅱ) 炎症损伤↓;纤维化↓ PPAR-γ↑;ROS/RNS↓;NF-κB;纤维化基因mRNA↓;HSC活化↓ [39] 缺乏无活性菱形蛋白2 (iRhom2)敲除 敲除iRhom2的L02细胞(ⅰ)“酒精-吡唑-玉米油”混合物灌胃诱导酒精性肝纤维化小鼠(ⅱ) 炎症反应↓;氧化应激↓;纤维化↓ 炎症细胞因子(如: IL-1β、TNF-α);TACE/NF-κB↓;MDA↓;OH-/SOD/GSH/T-AOC正常 [40] Shorthairpin RNA HBV感染诱导肝纤维化小鼠 炎症反应↓;氧化应激↓;纤维化↓ PPAR-γ↑;ROS/RNS↓;NF-κB↓;TGF-β↓;PRDX1↓;GSTP1↑;α-SMA↓;HSC活化↓ [41] 4-羟基-2(3H)- 苯并噁唑酮(HBOA) CCl4诱导肝纤维化大鼠 炎症反应↓;氧化应激↓;纤维化↓ TGF-β1\smad2/3\smad4\α-SMA↓;smad7↑;NF-κB↓;HSC活化↓;MDA↓;ROS↓;GSH/GSH-Px/SOD↑ [42] 注:T-AOC,总抗氧化能力;GSH-Px,谷胱甘肽过氧化物酶;PPAR-γ,过氧化物酶体增殖物激活受体γ;TACE,肿瘤坏死因子-α转换酶;PRDX1,过氧化还原酶1重组蛋白;GSTP1,谷胱甘肽S转移酶P1。↓,下降/抑制;↑,上升/激活。 -
[1] ZHANG J, WANG X, VIKASH V, et al. ROS and ROS-mediated cellular signaling[J]. Oxid Med Cell Longev, 2016, 2016: 4350965. DOI: 10.1155/2016/4350965. [2] RAMÍREZ A, VÁZQUEZ-SÁNCHEZ AY, CARRIÓN-ROBALINO N, et al. Ion channels and oxidative stress as a potential link for the diagnosis or treatment of liver diseases[J]. Oxid Med Cell Longev, 2016, 2016: 3928714. DOI: 10.1155/2016/3928714. [3] HE F, GUO FC, LI Z, et al. Myeloid-specific disruption of recombination signal binding protein Jκ ameliorates hepatic fibrosis by attenuating inflammation through cylindromatosis in mice[J]. Hepatology, 2015, 61(1): 303-314. DOI: 10.1002/hep.27394. [4] QIN TY, CHEN ZW. Physiological function of ROS in organism[J]. Life Sci Instrum, 2008, 6 (2): 12-16. DOI: 10.3969/j.issn.1671-7929.2008.02.003.秦涛余, 陈志伟. 机体内活性氧生理功能研究进展[J]. 生命科学仪器, 2008, 6(2): 12-16. DOI: 10.3969/j.issn.1671-7929.2008.02.003. [5] ZHENG RL, HUANG ZY. Free Radical Biology(The Third Edition)[M]. Beijing: Higher Education Press, 2007: 201-203.郑荣梁, 黄中洋. 自由基生物学(第三版)[M]. 北京: 高等教育出版社, 2007: 201-203. [6] NOGUEIRAS R, HABEGGER KM, CHAUDHARY N, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism[J]. Physiol Rev, 2012, 92(3): 1479-1514. DOI: 10.1152/physrev.00022.2011. [7] SÁNCHEZ-VALLE V, CHÁVEZ-TAPIA NC, URIBE M, et al. Role of oxidative stress and molecular changes in liver fibrosis: a review[J]. Curr Med Chem, 2012, 19(28): 4850-4860. DOI: 10.2174/092986712803341520. [8] WILLEMS PH, ROSSIGNOL R, DIETEREN CE, et al. Redox homeostasis and mitochondrial dynamics[J]. Cell Metab, 2015, 22(2): 207-218. DOI: 10.1016/j.cmet.2015.06.006. [9] XIA SJ, SUN T, WU JZ. Free radicals, Inflammation and Aging[J]. Pract Geriatr, 2014, 28(2): 100-103. DOI: 10.3969/j.issn.1003-9198.2014.02.004.夏世金, 孙涛, 吴俊珍. 自由基、炎症与衰老[J]. 实用老年医学, 2014, 28(2): 100-103. DOI: 10.3969/j.issn.1003-9198.2014.02.004. [10] LYU YH, WU SS, WANG ZC, et al. Research progress of traditional Chinese medicine regulating reactive oxygen species(ROS) against liver fibrosis[J]. Chin Arch Tradit Chin Med, 2021, 39(6): 117-121. DOI: 10.13193/j.issn.1673-7717.吕艳杭, 吴姗姗, 王振常, 等. 中医药调控活性氧(ROS)抗肝纤维化的研究进展[J]. 中华中医药学刊, 2021, 39(6): 117-121. DOI: 10.13193/j.issn.1673-7717. [11] GUO R, YAN M. Cellular and molecular mechanism of liver fibrosis[J/CD]. Chin J Liver Dis(Electronic Version), 2012, 4(4): 57-62.郭蓉, 阎明. 肝纤维化的细胞和分子机制研究进展[J/CD]. 中国肝脏病杂志(电子版), 2012, 4(4): 57-62. [12] ZHAO J, QI YF, YU YR. Research advances in the role of oxidative stress in the development and progression of liver fibrosis[J]. J Clin Hepatol, 2019, 35(9): 2067-2071. DOI: 10.3969/j.issn.1001-5256.2019.09.040.赵杰, 齐永芬, 鱼艳荣. 氧化应激在肝纤维化发生发展中的作用[J]. 临床肝胆病杂志, 2019, 35(9): 2067-2071. DOI: 10.3969/j.Issn.1001-5256.2019.09.040. [13] PAIK YH, KIM J, AOYAMA T, et al. Role of NADPH oxidases in liver fibrosis[J]. Antioxid Redox Signal, 2014, 20(17): 2854-2872. DOI: 10.1089/ars.2013.5619. [14] URTASUN R, CONDE DE LA ROSA L, NIETO N. Oxidative and nitrosative stress and fibrogenic response[J]. Clin Liver Dis, 2008, 12(4): 769-790, ⅷ. DOI: 10.1016/j.cld.2008.07.005. [15] SCAMBLER T, JAROSZ-GRIFFITHS HH, LARA-REYNA S, et al. ENaC-mediated sodium influx exacerbates NLRP3-dependent inflammation in cystic fibrosis[J]. Elife, 2019, 8: e49248. DOI: 10.7554/eLife.49248. [16] ZHANG K, LIN L, ZHU Y, et al. Saikosaponin d alleviates liver fibrosis by negatively regulating the ROS/NLRP3 inflammasome through activating the ERβ pathway[J]. Front Pharmacol, 2022, 13: 894981. DOI: 10.3389/fphar.2022.894981. [17] YI J, WU S, TAN S, et al. Berberine alleviates liver fibrosis through inducing ferrous redox to activate ROS-mediated hepatic stellate cells ferroptosis[J]. Cell Death Discov, 2021, 7(1): 374. DOI: 10.1038/s41420-021-00768-7. [18] GAN D, ZHANG W, HUANG C, et al. Ursolic acid ameliorates CCl4-induced liver fibrosis through the NOXs/ROS pathway[J]. J Cell Physiol, 2018, 233(10): 6799-6813. DOI: 10.1002/jcp.26541. [19] CHUNG HK, KIM YK, PARK JH, et al. The indole derivative NecroX-7 improves nonalcoholic steatohepatitis in ob/ob mice through suppression of mitochondrial ROS/RNS and inflammation[J]. Liver Int, 2015, 35(4): 1341-1353. DOI: 10.1111/liv.12741. [20] LIU YY, LI L, LI XW, et al. Research progress of microRNAs in TLR/NF-κB signaling pathway[J]. World Latest Medicine Information, 2018, 18(A5): 115-116, 119. DOI: 10.19613/j.cnki.1671-3141.2018.105.054.刘毅毅, 李林, 李小薇, 等. MicroRNAs在TLR/NF-кB信号通路中的研究进展[J]. 世界最新医学信息文摘, 2018, 18(A5): 115-116, 119. DOI: 10.19613/j.cnki.1671-3141.2018.105.054. [21] SU P, FENG SS, LI QW. Research progress of the structure and function of NF-κB and IκB in different animal groups[J]. Yi Chuan, 2016, 38(6): 523-531. DOI: 10.16288/j.yczz.15-509. [22] ZHAO YW, ZHU JN. Research progress of NF-κB signaling pathway[J]. Gansu Sci Technol, 2016, 32(21): 117-123, 112. DOI: 10.3969/j.issn.1000-0952.2016.21.048.赵运旺, 朱嘉宁. NF-κB信号通路研究进展[J]. 甘肃科技, 2016, 32(21): 117-123, 112. DOI: 10.3969/j.issn.1000-0952.2016.21.048. [23] SHEN H, SHENG L, CHEN Z, et al. Mouse hepatocyte overexpression of NF-κB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis[J]. Hepatology, 2014, 60(6): 2065-2076. DOI: 10.1002/hep.27348. [24] ZHU H, PING J, XU LM. Role of the nuclear factor - kappa B signaling pathway on the progress of hepatic fibrosis and the anti-fibroticmechanism of traditional Chinese medicine[J]. J Clin Hepatol, 2018, 34(4): 858-861. DOI: 10.3969/j.issn.1001-5256.2018.04.035.朱慧, 平键, 徐列明. NF-κB通路在肝纤维化进展和中药抗肝纤维化机制中的作用[J]. 临床肝胆病杂志, 2018, 34(4): 858-861. DOI: 10.3969/j.issn.1001-5256.2018.04.035. [25] ABBAS N, GETACHEW A, YOU K, et al. Kupffer cells mediate the recruitment of hepatic stellate cells into the localized liver damage[J]. Biochem Biophys Res Commun, 2020, 529(2): 474-479. DOI: 10.1016/j.bbrc.2020.06.041. [26] MA L, ZHAO Y, WU HY, et al. Expression of TLR4 /MyD88/NF-κB signaling pathway in cirrhosis caused by hepatitis B virus and its clinical significance[J]. Chin J Mod Med, 2021, 31(2): 72-77. DOI: 10.3969/j.issn.1005-8982.2021.21.012.马良, 赵阳, 伍华英, 等. TLR4、MyD88、NF-κB在乙型肝炎病毒肝硬化患者中的表达及其临床意义[J]. 中国现代医学杂志, 2021, 31(21): 72-77. DOI: 10.3969/j.issn.1005-8982.2021.21.012. [27] CUI DL. The roles of NF-κB in liver fibrosis[D]. Shijiazhuang: Hebei Medical University, 2010.崔东来. 抑制NF-κB诱导肝星状细胞凋亡[D]. 石家庄: 河北医科大学, 2010. [28] CHEN JY, HAO FY, LIN JQ, et al. The effect of carvedilol on the TLR4-MyD88-NF-κB signaling pathway in an animal model of liver fibrosis and its anti-liver fibrosis mechanism[J]. J Xinjiang Med Univ, 2021, 44(7): 771-776. DOI: 10.3639/j.issn.1009-5551.2021.07.002.陈建勇, 郝芳艳, 林近秋, 等. 卡维地洛对肝纤维化动物模型TLR4-MyD88-NF-κB信号通路的影响及其抗肝纤维化的作用机制[J]. 新疆医科大学学报, 2021, 44(7): 771-776. DOI: 10.3639/j.issn.1009-5551.2021.07.002. [29] ZHU J, FAN JR, PAN L, et al. Correlation of nuclear factor B expression with α-SMA and collagen Ⅲ expression in hepatic fibrosis in rats[J]. World Chin J Dig, 2012, 20(22): 2081-2085. https://www.cnki.com.cn/Article/CJFDTOTAL-XXHB201222019.htm朱净, 范建荣, 潘亮, 等. NF-κB在鼠肝纤维化组织中的表达及其与α-SMA、Ⅲ型胶原的相关性[J]. 世界华人消化杂志, 2012, 20(22): 2081-2085. https://www.cnki.com.cn/Article/CJFDTOTAL-XXHB201222019.htm [30] REEVES HL, FRIEDMAN SL. Activation of hepatic stellate cells—a key issue in liver fibrosis[J]. Front Biosci, 2002, 7: d808-d826. DOI: 10.2741/reeves. [31] FENG XY, HE PL, ZHAO W, et al. Effects of Panax notoginseng total saponins on improving nonalcoholic fatty liver disease rats and NO/iNOS/NF-κB signaling pathway[J]. Chin Tradit Patent Med, 2021, 43(1): 50-55. DOI: 10.3969/j.issn.1001-1528.2021.01.010.冯晓异, 何朋伦, 赵微, 等. 三七总皂苷对非酒精性脂肪肝大鼠的改善作用及对NO/iNOS/NF-κB通路的影响[J]. 中成药, 2021, 43(1): 50-55. DOI: 10.3969/j.issn.1001-1528.2021.01.010. [32] TANG H, ZENG Q, REN N, et al. Kaempferide improves oxidative stress and inflammation by inhibiting the TLR4/IκBα/NF-κB pathway in obese mice[J]. Iran J Basic Med Sci, 2021, 24(4): 493-498. DOI: 10.22038/ijbms.2021.52690.11892. [33] LIU F, FENG M, XING J, et al. Timosaponin alleviates oxidative stress in rats with high fat diet-induced obesity via activating Nrf2/HO-1 and inhibiting the NF-κB pathway[J]. Eur J Pharmacol, 2021, 909: 174377. DOI: 10.1016/j.ejphar.2021.174377. [34] LI J, WANG T, LIU P, et al. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD[J]. Food Funct, 2021, 12(9): 3898-3918. DOI: 10.1039/d0fo02736g. [35] CHEN X, DING C, LIU W, et al. Abscisic acid ameliorates oxidative stress, inflammation, and apoptosis in thioacetamide-induced hepatic fibrosis by regulating the NF-кB signaling pathway in mice[J]. Eur J Pharmacol, 2021, 891: 173652. DOI: 10.1016/j.ejphar.2020.173652. [36] RODRÍGUEZ MJ, SABAJ M, TOLOSA G, et al. Maresin-1 prevents liver fibrosis by targeting Nrf2 and NF-κB, reducing oxidative stress and inflammation[J]. Cells, 2021, 10(12): 3406. DOI: 10.3390/cells10123406. [37] ZAGHLOUL RA, ZAGHLOUL AM, EL-KASHEF DH. Hepatoprotective effect of Baicalin against thioacetamide-induced cirrhosis in rats: Targeting NOX4/NF-κ B/NLRP3 inflammasome signaling pathways[J]. Life Sci, 2022, 295: 120410. DOI: 10.1016/j.lfs.2022.120410. [38] CHEN Y, ZHAO C, LIU X, et al. Plumbagin ameliorates liver fibrosis via a ROS-mediated NF-кB signaling pathway in vitro and in vivo[J]. Biomed Pharmacother, 2019, 116: 108923. DOI: 10.1016/j.biopha.2019.108923. [39] de SOUZA BASSO B, HAUTE GV, ORTEGA-RIBERA M, et al. Methoxyeugenol deactivates hepatic stellate cells and attenuates liver fibrosis and inflammation through a PPAR-γ and NF-kB mechanism[J]. J Ethnopharmacol, 2021, 280: 114433. DOI: 10.1016/j.jep.2021.114433. [40] LIU Y, KUANG Q, DAI X, et al. Deficiency in Inactive Rhomboid Protein2 (iRhom2) alleviates alcoholic liver fibrosis by suppressing inflammation and oxidative stress[J]. Int J Mol Sci, 2022, 23(14): 7701. DOI: 10.3390/ijms23147701. [41] YE L, CHEN T, CAO J, et al. Short hairpin RNA attenuates liver fibrosis by regulating the PPAR-γ and NF-κB pathways in HBV induced liver fibrosis in mice[J]. Int J Oncol, 2020, 57(5): 1116-1128. DOI: 10.3892/ijo.2020.5125. [42] SUN X, HUANG X, ZHU X, et al. HBOA ameliorates CCl4-incuded liver fibrosis through inhibiting TGF-β1/Smads, NF-κB and ERK signaling pathways[J]. Biomed Pharmacother, 2019, 115: 108901. DOI: 10.1016/j.biopha.2019.108901. -



 PDF下载 ( 2107 KB)
PDF下载 ( 2107 KB)

 下载:
下载:


