嘌呤信号在肝脏免疫调节中的作用
DOI: 10.3969/j.issn.1001-5256.2023.06.036
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:马发祥负责查阅文献,撰写文章;涂正坤负责拟定文章思路,指导撰写文章并最后定稿。
-
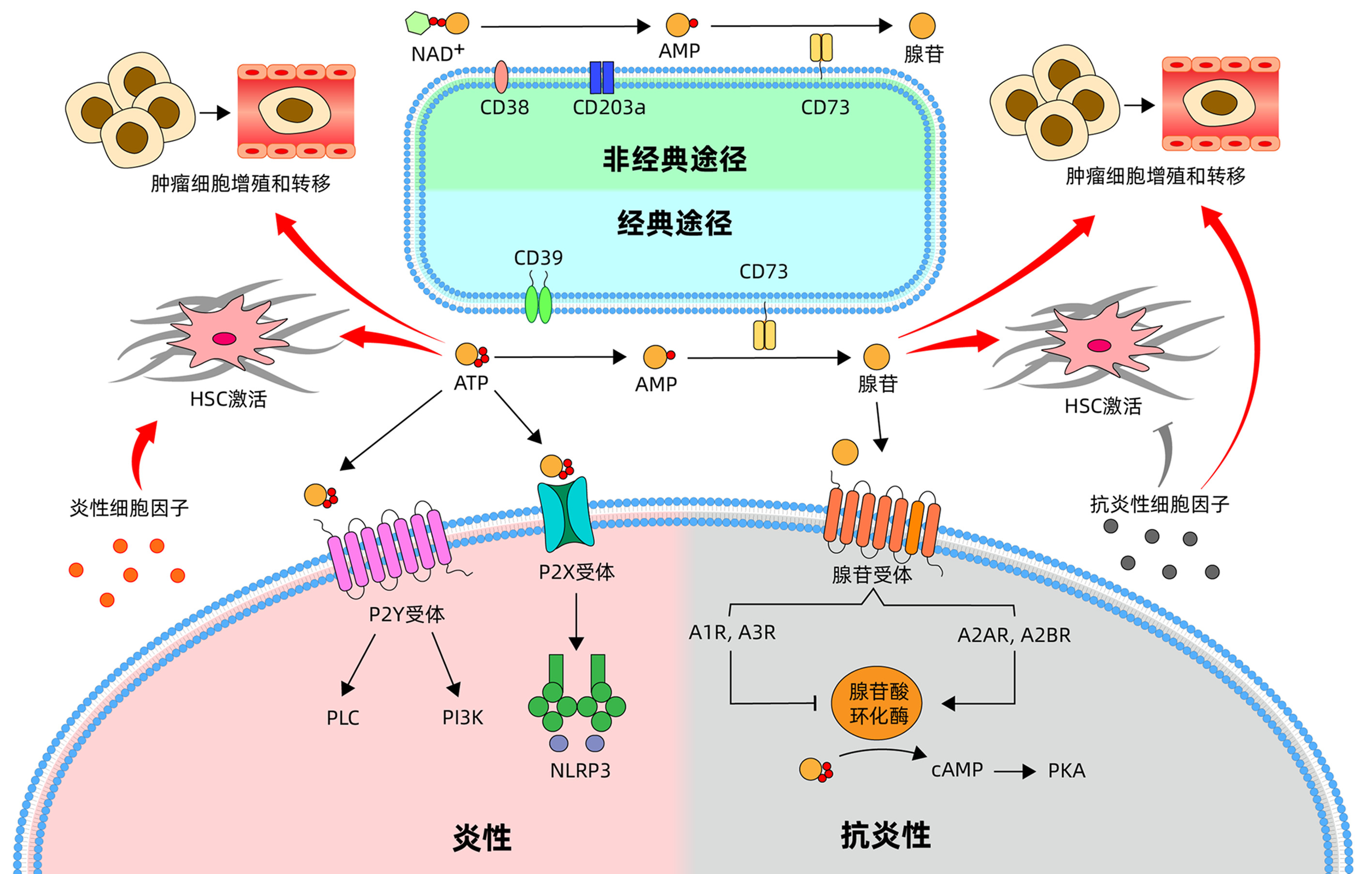
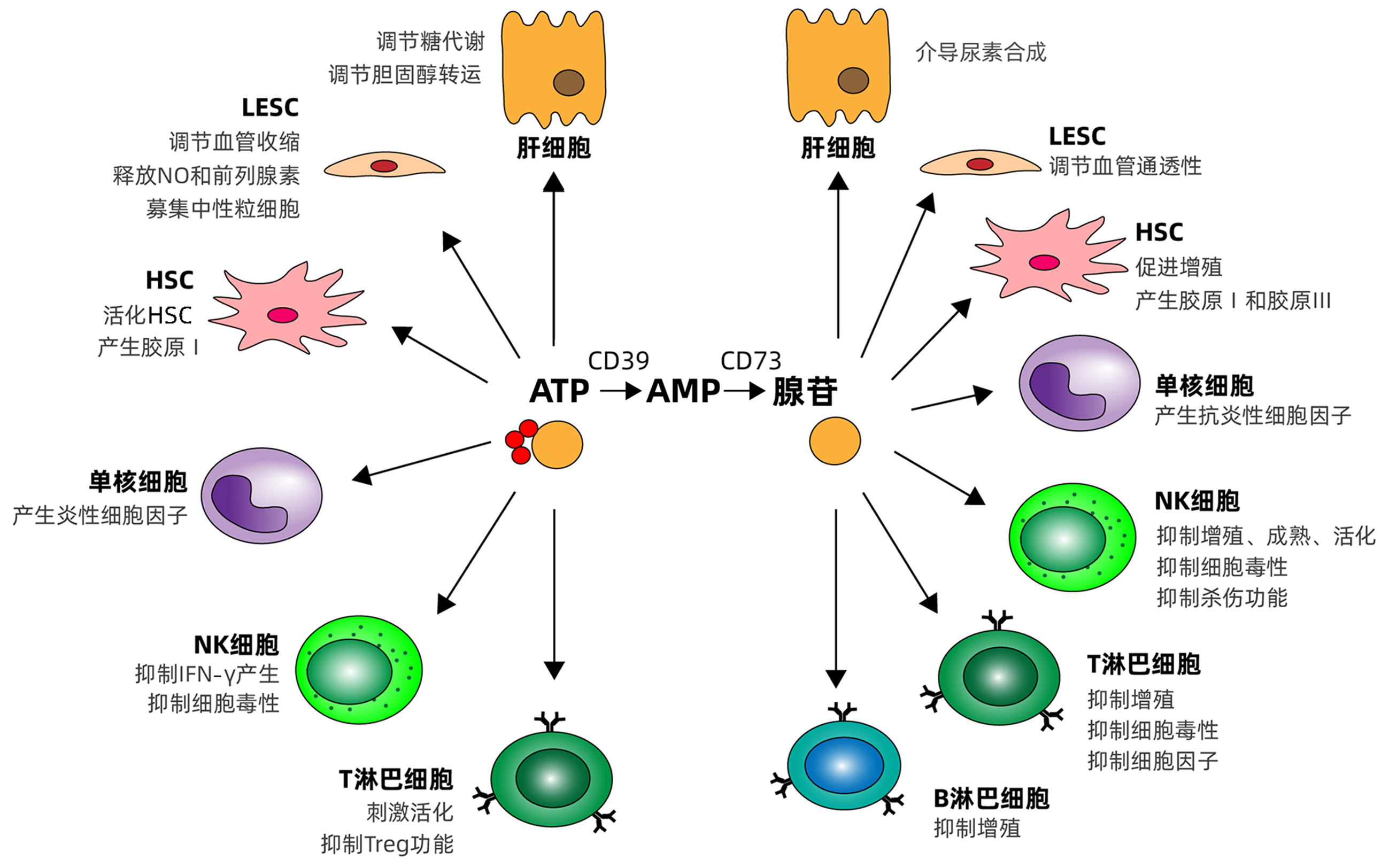
摘要: 嘌呤主要由ATP、NAD+和核酸组成,除了具有关键的细胞内功能外,NAD+和ATP及其水解产物包括ADP、AMP和腺苷,是参与生理过程和病理状况的重要细胞外信号。嘌呤信号在肝脏微环境的免疫调节中发挥重要作用。本文主要总结了嘌呤信号对肝脏免疫细胞的调控,以及嘌呤信号通过调节肝脏免疫细胞炎性和抗炎性反应影响肝脏疾病进展。Abstract: Purines are mainly composed of ATP, NAD+, and nucleic acid. In addition to their key intracellular functions, NAD+, ATP, and their hydrolyzed products (including ADP, AMP, and adenosine) are important extracellular signals involved in physiological processes and pathological conditions. Purine signaling plays an important role in immune regulation of liver microenvironment. This article mainly summarizes the regulatory effect of purine signaling on immune cells in the liver and the effect of purine signaling on the progression of liver diseases by regulating the inflammatory and anti-inflammatory responses of immune cells in the liver.
-
Key words:
- Liver Diseases /
- Adenosine Triphosphate /
- Inflammation /
- Immunomodulation
-
[1] WANG P, JIA J, ZHANG D. Purinergic signalling in liver diseases: Pathological functions and therapeutic opportunities[J]. JHEP Rep, 2020, 2(6): 100165. DOI: 10.1016/j.jhepr.2020.100165. [2] SHUAI Z, LEUNG MW, HE X, et al. Adaptive immunity in the liver[J]. Cell Mol Immunol, 2016, 13(3): 354-368. DOI: 10.1038/cmi.2016.4. [3] LINDEN J, KOCH-NOLTE F, DAHL G. Purine release, metabolism, and signaling in the inflammatory response[J]. Annu Rev Immunol, 2019, 37: 325-347. DOI: 10.1146/annurev-immunol-051116-052406. [4] DI VIRGILIO F, DAL BEN D, SARTI AC, et al. The P2X7 receptor in infection and inflammation[J]. Immunity, 2017, 47(1): 15-31. DOI: 10.1016/j.immuni.2017.06.020. [5] MÜLLER T, FAY S, VIEIRA RP, et al. The purinergic receptor subtype P2Y2 mediates chemotaxis of neutrophils and fibroblasts in fibrotic lung disease[J]. Oncotarget, 2017, 8(22): 35962-35972. DOI: 10.18632/oncotarget.16414. [6] ALBERTO AV, FARIA RX, DE MENEZES JR, et al. Role of P2 receptors as modulators of rat eosinophil recruitment in allergic inflammation[J]. PLoS One, 2016, 11(1): e0145392. DOI: 10.1371/journal.pone.0145392. [7] BOREA PA, GESSI S, MERIGHI S, et al. Pharmacology of adenosine receptors: The state of the art[J]. Physiol Rev, 2018, 98(3): 1591-1625. DOI: 10.1152/physrev.00049.2017. [8] KIRLEY TL, CRAWFORD PA, SMITH TM. The structure of the nucleoside triphosphate diphosphohydrolases (NTPDases) as revealed by mutagenic and computational modeling analyses[J]. Purinergic Signal, 2006, 2(2): 379-389. DOI: 10.1007/s11302-005-5301-6. [9] ALLARD B, LONGHI MS, ROBSON SC, et al. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets[J]. Immunol Rev, 2017, 276(1): 121-144. DOI: 10.1111/imr.12528. [10] ANTONIOLI L, PACHER P, VIZI ES, et al. CD39 and CD73 in immunity and inflammation[J]. Trends Mol Med, 2013, 19(6): 355-367. DOI: 10.1016/j.molmed.2013.03.005. [11] SYNNESTVEDT K, FURUTA GT, COMERFORD KM, et al. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia[J]. J Clin Invest, 2002, 110(7): 993-1002. DOI: 10.1172/JCI15337. [12] CHALMIN F, MIGNOT G, BRUCHARD M, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression[J]. Immunity, 2012, 36(3): 362-373. DOI: 10.1016/j.immuni.2011.12.019. [13] ELTZSCHIG HK, KÖHLER D, ECKLE T, et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection[J]. Blood, 2009, 113(1): 224-232. DOI: 10.1182/blood-2008-06-165746. [14] PIEDRA-QUINTERO ZL, WILSON Z, NAVA P, et al. CD38: An immunomodulatory molecule in inflammation and autoimmunity[J]. Front Immunol, 2020, 11: 597959. DOI: 10.3389/fimmu.2020.597959. [15] GOLDFINE ID, MADDUX BA, YOUNGREN JF, et al. The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities[J]. Endocr Rev, 2008, 29(1): 62-75. DOI: 10.1210/er.2007-0004. [16] KUBES P, JENNE C. Immune responses in the liver[J]. Annu Rev Immunol, 2018, 36: 247-277. DOI: 10.1146/annurev-immunol-051116-052415. [17] SNIDER NT, GRIGGS NW, SINGLA A, et al. CD73 (ecto-5'- nucleotidase) hepatocyte levels differ across mouse strains and contribute to mallory-denk body formation[J]. Hepatology, 2013, 58(5): 1790-1800. DOI: 10.1002/hep.26525. [18] VELÁZQUEZ-MIRANDA E, DÍAZ-MUÑOZ M, VÁZQUEZ-CUEVAS FG. Purinergic signaling in hepatic disease[J]. Purinergic Signal, 2019, 15(4): 477-489. DOI: 10.1007/s11302-019-09680-3. [19] ZOETEWIJ JP, VAN DE WATER B, DE BONT HJ, et al. The role of a purinergic P2z receptor in calcium-dependent cell killing of isolated rat hepatocytes by extracellular adenosine triphosphate[J]. Hepatology, 1996, 23(4): 858-865. DOI: 10.1002/hep.510230429. [20] DIXON CJ, HALL JF, WEBB TE, et al. Regulation of rat hepatocyte function by P2Y receptors: focus on control of glycogen phosphorylase and cyclic AMP by 2-methylthioadenosine 5'-diphosphate[J]. J Pharmacol Exp Ther, 2004, 311(1): 334-341. DOI: 10.1124/jpet.104.067744. [21] TACKETT BC, SUN H, MEI Y, et al. P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy[J]. Am J Physiol Gastrointest Liver Physiol, 2014, 307(11): G1073-G1087. DOI: 10.1152/ajpgi.00092.2014. [22] FABRE AC, MALAVAL C, BEN ADDI A, et al. P2Y13 receptor is critical for reverse cholesterol transport[J]. Hepatology, 2010, 52(4): 1477-1483. DOI: 10.1002/hep.23897. [23] GUINZBERG R, URIBE S, DÍAZ-CRUZ A, et al. In rat hepatocytes, different adenosine receptor subtypes use different secondary messengers to increase the rate of ureagenesis[J]. Life Sci, 2006, 79(4): 382-390. DOI: 10.1016/j.lfs.2006.01.021. [24] BELDI G, WU Y, SUN X, et al. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration[J]. Gastroenterology, 2008, 135(5): 1751-1760. DOI: 10.1053/j.gastro.2008.07.025. [25] ELTZSCHIG HK, THOMPSON LF, KARHAUSEN J, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism[J]. Blood, 2004, 104(13): 3986-3992. DOI: 10.1182/blood-2004-06-2066. [26] MCDONALD B, PITTMAN K, MENEZES GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation[J]. Science, 2010, 330(6002): 362-366. DOI: 10.1126/science.1195491. [27] DRANOFF JA, OGAWA M, KRUGLOV EA, et al. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells[J]. Am J Physiol Gastrointest Liver Physiol, 2004, 287(2): G417-424. DOI: 10.1152/ajpgi.00294.2003. [28] FAUSTHER M, LECKA J, SOLIMAN E, et al. Coexpression of ecto-5'-nucleotidase/CD73 with specific NTPDases differentially regulates adenosine formation in the rat liver[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302(4): G447-G459. DOI: 10.1152/ajpgi.00165.2011. [29] SOHAIL MA, HASHMI AZ, HAKIM W, et al. Adenosine induces loss of actin stress fibers and inhibits contraction in hepatic stellate cells via Rho inhibition[J]. Hepatology, 2009, 49(1): 185-194. DOI: 10.1002/hep.22589. [30] AHSAN MK, MEHAL WZ. Activation of adenosine receptor A2A increases HSC proliferation and inhibits death and senescence by down-regulation of p53 and Rb[J]. Front Pharmacol, 2014, 5: 69. DOI: 10.3389/fphar.2014.00069. [31] CHE J, CHAN ES, CRONSTEIN BN. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway[J]. Mol Pharmacol, 2007, 72(6): 1626-1636. DOI: 10.1124/mol.107.038760. [32] CHAN ES, MONTESINOS MC, FERNANDEZ P, et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis[J]. Br J Pharmacol, 2006, 148(8): 1144-1155. DOI: 10.1038/sj.bjp.0706812. [33] SAVIO L, DE ANDRADE MELLO P, FIGLIUOLO VR, et al. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury[J]. J Hepatol, 2017, 67(4): 716-726. DOI: 10.1016/j.jhep.2017.05.021. [34] BASU M, GUPTA P, DUTTA A, et al. Increased host ATP efflux and its conversion to extracellular adenosine is crucial for establishing Leishmania infection[J]. J Cell Sci, 2020, 133(7). DOI: 10.1242/jcs.239939. [35] LU JC, ZHANG PF, HUANG XY, et al. Amplification of spatially isolated adenosine pathway by tumor-macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma[J]. J Hematol Oncol, 2021, 14(1): 200. DOI: 10.1186/s13045-021-01207-x. [36] BIANCHI G, VUERICH M, PELLEGATTI P, et al. ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment[J]. Cell Death Dis, 2014, 5(3): e1135. DOI: 10.1038/cddis.2014.109. [37] VIJAYAN D, BARKAUSKAS D S, STANNARD K, et al. Selective activation of anti-CD73 mechanisms in control of primary tumors and metastases[J]. Oncoimmunology, 2017, 6(5): e1312044. DOI: 10.1080/2162402x.2017.1312044 [38] CHATTERJEE D, TUFA DM, BAEHRE H, et al. Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells[J]. Blood, 2014, 123(4): 594-595. DOI: 10.1182/blood-2013-09-524827. [39] BELDI G, BANZ Y, KROEMER A, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice[J]. Hepatology, 2010, 51(5): 1702-1711. DOI: 10.1002/hep.23510. [40] BASTID J, REGAIRAZ A, BONNEFOY N, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity[J]. Cancer Immunol Res, 2015, 3(3): 254-265. DOI: 10.1158/2326-6066.CIR-14-0018. [41] GRAUBARDT N, FAHRNER R, TROCHSLER M, et al. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis[J]. Hepatology, 2013, 57(5): 1969-1979. DOI: 10.1002/hep.26008. [42] BEAVIS PA, DIVISEKERA U, PAGET C, et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors[J]. Proc Natl Acad Sci U S A, 2013, 110(36): 14711-14716. DOI: 10.1073/pnas.1308209110. [43] YOUNG A, NGIOW SF, GAO Y, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment[J]. Cancer Res, 2018, 78(4): 1003-1016. DOI: 10.1158/0008-5472.CAN-17-2826. [44] LOKSHIN A, RASKOVALOVA T, HUANG X, et al. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells[J]. Cancer Res, 2006, 66(15): 7758-7765. DOI: 10.1158/0008-5472.CAN-06-0478. [45] RASKOVALOVA T, HUANG X, SITKOVSKY M, et al. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells[J]. J Immunol, 2005, 175(7): 4383-4391. DOI: 10.4049/jimmunol.175.7.4383. [46] NOBLE A, MEHTA H, LOVELL A, et al. IL-12 and IL-4 activate a CD39-dependent intrinsic peripheral tolerance mechanism in CD8(+) T cells[J]. Eur J Immunol, 2016, 46(6): 1438-1448. DOI: 10.1002/eji.201545939. [47] SCHNEIDER E, WINZER R, RISSIEK A, et al. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression[J]. Nat Commun, 2021, 12(1): 5911. DOI: 10.1038/s41467-021-26134-w. [48] OHTA A, OHTA A, MADASU M, et al. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments[J]. J Immunol, 2009, 183(9): 5487-5493. DOI: 10.4049/jimmunol.0901247. [49] SCHENK U, FRASCOLI M, PROIETTI M, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors[J]. Sci Signal, 2011, 4(162): ra12. DOI: 10.1126/scisignal.2001270. [50] SAZE Z, SCHULER PJ, HONG CS, et al. Adenosine production by human B cells and B cell-mediated suppression of activated T cells[J]. Blood, 2013, 122(1): 9-18. DOI: 10.1182/blood-2013-02-482406. [51] NASCIMENTO DC, VIACAVA PR, FERREIRA RG, et al. Sepsis expands a CD39+ plasmablast population that promotes immunosuppression via adenosine-mediated inhibition of macrophage antimicrobial activity[J]. Immunity, 2021, 54(9): 2024-2041. e8. DOI: 10.1016/j.immuni.2021.08.005. [52] ZHANG F, LI R, YANG Y, et al. Specific decrease in B-cell-derived extracellular vesicles enhances post-chemotherapeutic CD8+ T cell responses[J]. Immunity, 2019, 50(3): 738-750. e7. DOI: 10.1016/j.immuni.2019.01.010. [53] EYENGA P, REY B, EYENGA L, et al. Regulation of oxidative phosphorylation of liver mitochondria in sepsis[J]. Cells, 2022, 11(10): 1598. DOI: 10.3390/cells11101598. [54] MORANDINI AC, SAVIO LE, COUTINHO-SILVA R. The role of P2X7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases[J]. Biomed J, 2014, 37(4): 169-177. DOI: 10.4103/2319-4170.127803. [55] AYATA CK, GANAL SC, HOCKENJOS B, et al. Purinergic P2Y2 receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury[J]. Gastroenterology, 2012, 143(6): 1620-1629. e4. DOI: 10.1053/j.gastro.2012.08.049. [56] IDZKO M, FERRARI D, ELTZSCHIG HK. Nucleotide signalling during inflammation[J]. Nature, 2014, 509(7500): 310-317. DOI: 10.1038/nature13085. [57] COHEN HB, BRIGGS KT, MARINO JP, et al. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses[J]. Blood, 2013, 122(11): 1935-1945. DOI: 10.1182/blood-2013-04-496216. [58] NAGANUMA M, WIZNEROWICZ EB, LAPPAS CM, et al. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis[J]. J Immunol, 2006, 177(5): 2765-2769. DOI: 10.4049/jimmunol.177.5.2765. [59] CSÓKA B, HIMER L, SELMECZY Z, et al. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function[J]. FASEB J, 2008, 22(10): 3491-3499. DOI: 10.1096/fj.08-107458. [60] ELTZSCHIG HK, IBLA JC, FURUTA GT, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors[J]. J Exp Med, 2003, 198(5): 783-796. DOI: 10.1084/jem.20030891. [61] TANG Y, JIANG L, ZHENG Y, et al. Expression of CD39 on FoxP3+ T regulatory cells correlates with progression of HBV infection[J]. BMC Immunol, 2012, 13: 17. DOI: 10.1186/1471-2172-13-17. [62] RATHOD SB, DAS R, THANAPATI S, et al. Suppressive activity and altered conventional phenotype markers/mediators of regulatory T cells in patients with self-limiting hepatitis E[J]. J Viral Hepat, 2014, 21(2): 141-151. DOI: 10.1111/jvh.12125. [63] WU X, WANG Y, WANG S, et al. Purinergic P2X7 receptor mediates acetaldehyde-induced hepatic stellate cells activation via PKC-dependent GSK3β pathway[J]. Int Immunopharmacol, 2017, 43: 164-171. DOI: 10.1016/j.intimp.2016.12.017. [64] LE GUILCHER C, GARCIN I, DELLIS O, et al. The P2X4 purinergic receptor regulates hepatic myofibroblast activation during liver fibrogenesis[J]. J Hepatol, 2018, 69(3): 644-653. DOI: 10.1016/j.jhep.2018.05.020. [65] PENG Z, FERNANDEZ P, WILDER T, et al. Ecto-5'-nucleotidase (CD73) -mediated extracellular adenosine production plays a critical role in hepatic fibrosis[J]. FASEB J, 2008, 22(7): 2263-2272. DOI: 10.1096/fj.07-100685. [66] CHIANG DJ, ROYCHOWDHURY S, BUSH K, et al. Adenosine 2A receptor antagonist prevented and reversed liver fibrosis in a mouse model of ethanol-exacerbated liver fibrosis[J]. PLoS One, 2013, 8(7): e69114. DOI: 10.1371/journal.pone.0069114. [67] CONNOLLY MK, BEDROSIAN AS, MALLEN-ST CLAIR J, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha[J]. J Clin Invest, 2009, 119(11): 3213-3225. DOI: 10.1172/JCI37581. [68] GIELING RG, WALLACE K, HAN YP. Interleukin-1 participates in the progression from liver injury to fibrosis[J]. Am J Physiol Gastrointest Liver Physiol, 2009, 296(6): G1324-G1331. DOI: 10.1152/ajpgi.90564.2008. [69] LIU X, HU H, YIN JQ. Therapeutic strategies against TGF-beta signaling pathway in hepatic fibrosis[J]. Liver Int, 2006, 26(1): 8-22. DOI: 10.1111/j.1478-3231.2005.01192.x. [70] CUI Q, WANG Z, JIANG D, et al. HGF inhibits TGF-β1-induced myofibroblast differentiation and ECM deposition via MMP-2 in Achilles tendon in rat[J]. Eur J Appl Physiol, 2011, 111(7): 1457-1463. DOI: 10.1007/s00421-010-1764-4. [71] MAYNARD JP, LEE JS, SOHN BH, et al. P2X3 purinergic receptor overexpression is associated with poor recurrence-free survival in hepatocellular carcinoma patients[J]. Oncotarget, 2015, 6(38): 41162-41179. DOI: 10.18632/oncotarget.6240. [72] KHALID M, BRISSON L, TARIQ M, et al. Carcinoma-specific expression of P2Y11 receptor and its contribution in ATP-induced purinergic signalling and cell migration in human hepatocellular carcinoma cells[J]. Oncotarget, 2017, 8(23): 37278-37290. DOI: 10.18632/oncotarget.16191. [73] MA XL, SHEN MN, HU B, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis[J]. J Hematol Oncol, 2019, 12(1): 37. DOI: 10.1186/s13045-019-0724-7. -



 PDF下载 ( 2540 KB)
PDF下载 ( 2540 KB)

 下载:
下载: